© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Prevalence and severity of asthma among school
children in Hong Kong
James Wesley CH Cheng, MB, BS, FHKAM (Paediatrics)1; YP Tsang, MB, BS, FHKAM (Paediatrics)1; YY Lam, MB, BS, FHKAM (Paediatrics)1; Ashleigh KY Chu, MB, BS, MRCPCH1; Christina SY Ng, BSc2; Celia HY Chan, BSSc, PhD3; YL Fung, MAP, PhD2; Priscilla SY Chau, BSc2; David CK Luk, MB, BS, FHKAM (Paediatrics)1
1 Department of Paediatrics and Adolescent Medicine, United Christian Hospital, Hong Kong SAR, China
2 Department of Social Work and Social Administration, The University of Hong Kong, Hong Kong SAR, China
3 Department of Social Work, Melbourne School of Health Sciences, Faculty of Medicine, Dentistry, and Health Sciences, The University of Melbourne, Melbourne, Australia
Corresponding author: Dr James Wesley CH Cheng (cch278@ha.org.hk)
Abstract
Introduction: This study presents contemporary
epidemiological data regarding the prevalence and
severity of asthma and wheezing among children in
Hong Kong, which provides an update to the results
of the International Study of Asthma and Allergies
in Childhood (ISAAC) conducted in 1994-1995 and
2001-2003.
Methods: This cross-sectional investigation was
based on the ISAAC study protocol. Responses
from 1100 children aged 6 to 7 years (Primary 1-2)
and 1048 children aged 13 to 14 years (Secondary
2-3) in Hong Kong between September 2020 and
August 2021 were analysed. Sex differences within
each age-group were assessed using Chi squared and
independent t tests. Demographic information was
entered into hierarchical logistic regression models
to identify potential predictive factors associated
with asthma severity. Annual change in prevalence
was calculated via division of the prevalence by
the number of years between surveys. Logistic
regression modelling was conducted to identify risk
factors associated with asthma severity.
Results: The prevalences of current wheezing were
6.19% and 4.97% in the primary and secondary school
groups, respectively. The prevalences of asthma ever
were 5.55% and 6.12%, whereas those of wheezing ever were 20.38% and 12.05%, in the primary and
secondary school groups, respectively.
Conclusion: Asthma severity and prevalence have
decreased in Hong Kong since 1994-1995. A follow-up
study will explore the protective and risk factors
contributing to these trends.
New knowledge added by this study
- Epidemiological data for asthma and wheezing among Hong Kong children have not been updated in 25 years.
- Our cross-sectional study, based on the International Study of Asthma and Allergies in Childhood protocol, showed that the prevalences of asthma ever were 5.55% and 6.12% in the primary and secondary school groups, respectively; the corresponding prevalences of current wheezing were 6.19% and 4.97%.
- This study revealed decreases in asthma severity and prevalence among Hong Kong children since 1994-1995.
- These findings provide essential epidemiological data for research and policy-making in Hong Kong and Asia.
- Understanding the prevalence and severity of asthma will help estimate the associated budget for taking care of this group of patients.
Introduction
The International Study of Asthma and Allergies
in Childhood (ISAAC) is considered a landmark
international investigation of the global burden of
three major atopic diseases—asthma, allergic rhinitis,
and eczema1 2 3 4—utilising standardised methodology
to enable accurate estimation of prevalence trends
in allergic diseases. Phase One examined the
prevalence and severity of the three diseases in 1994-1995, Phase Two investigated the corresponding
risk factors, and Phase Three constituted a follow-up
study examining global prevalence and severity
trends in 2001-2003.1 2 4 The Global Asthma Network
updated global burden information using data from
27 Global Asthma Network Centres in 14 countries
in 2017-2020,3 but Hong Kong data were excluded from that study.
Asthma remains a major medical burden responsible for nearly 500 000 deaths in 20175
and substantial direct, indirect, and intangible
costs,6 ranging from mortality and hospitalisations
to school- or work-related loss (in the form of
absenteeism from school or loss of working days),
and quality of life impairments. Thus, accurate and
current asthma prevalence data are essential for
guiding public health initiatives and formulating
healthcare policies. Recent estimates suggest global
asthma prevalences of 9.1% in children, 11.0% in
adolescents, and 6.6% in adults7; asthma is often
cited as the most common chronic disease in
children.6
Trends regarding the three major atopic
diseases have considerably varied among countries
and regions since 1994-1995. Asthma has shown
the greatest variation, peaking in some countries
but continuing to increase in others, especially
developing countries.2 3 4 5 6 8 9 10 11 12 13 14 15 16 Most studies of asthma
prevalence were conducted before the coronavirus
disease 2019 (COVID-19) pandemic, and statistics
considerably differ even within the same region.
In 2019, a large-scale study examining the global
adult and paediatric prevalences of asthma in
>200 territories showed a 24% decrease in age-standardised
point prevalence in most countries5;
however, increases were observed in Oman, Saudi
Arabia, and Vietnam.5 This discrepancy highlights
the need for further investigation of the risk and
protective factors for asthma in modern societies.
Hong Kong is an urban-centric port city in southeast China with a unique blend of Chinese and
international influences, which have led to trends
that considerably differ from those of neighbouring
regions.17 Cultural practices17 18 19 and urbanisation
(eg, cooking methods, joss stick burning, and high
population density in urban areas)9 17 20 21 increase the
complexity involved in estimating asthma prevalence
within Hong Kong based on data from neighbouring
regions. This study presents contemporary
epidemiological data regarding the prevalence and
severity of asthma and wheezing among children in
Hong Kong, which provides an update to the results
of the ISAAC conducted in 1994-1995 and 2001-2003.
Methods
Study design
This cross-sectional study, based on the ISAAC study
protocol,2 was performed using traditional Chinese
versions of the validated ISSAC measurements.22
Participants
In total, 2148 children aged 6 to 7 years or 13 to
14 years residing in Hong Kong were recruited
from primary and secondary schools between
September 2020 and August 2021. Inclusion criteria
for children were enrolment in Primary 1-2 (Grades
1-2) or Secondary 2-3 (Grades 8-9) in Hong Kong
and parental provision of informed consent to
participate. The primary caregiver of the student (for
the primary school group) or the student himself/herself (for the secondary school group) was asked
to complete questionnaires in accordance with the
ISAAC protocol. Only written questionnaires were
used, considering reports of high agreement with
their video counterparts.23 24 Inclusion criteria for
parents were: (1) being either the father or mother
of the child, (2) bearing primary responsibility for
the child’s care for ≥6 months, and (3) provision of
informed consent to participate. Exclusion criteria
for parents and children were the presence of a
learning disability or organic disorder that would
impair the ability to understand and respond to the
questionnaires, and inability to understand Chinese.
Additionally, children attending Special Educational
Needs schools were excluded.
Sampling
All schools in Hong Kong (n=900) were invited
by phone and mail to participate in the survey.
Contact persons were identified in each school. The
investigators made at least three attempts per school
(by phone, mail and/or email) to obtain responses
from participating students. A target sample size
of 1000 to 3000 participants was established, in
accordance with the ISAAC study protocol.2
Measurements
Questions were adopted from the ISAAC study
protocol, and disease definitions were based on
the original ISAAC Phase Three questionnaire and
handbook.2 ‘Current severe asthma’ was defined as
affirmative responses to one or more of the following
items in the past 12 months: (1) ≥4 wheezing
episodes, (2) woken from sleep by wheezing ≥1
night per week, or (3) limitation of speech during
wheezing. Other questions and their definitions
adhered to the standardised ISAAC Coding and
Data Transfer Manual2 22 to ensure comparability
with previous studies utilising the ISAAC protocol.
Statistical analysis
Descriptive statistics, including means and standard
deviations, were used for continuous variables;
frequency distributions were used for categorical
variables. Sex differences within each age-group
were assessed using Chi squared and independent
t tests. Demographic information was entered into
hierarchical logistic regression models to identify
potential predictive factors associated with asthma
severity. Annual change in prevalence was calculated
via division of the prevalence by the number of years
between surveys. Logistic regression modelling
was conducted to identify risk factors associated
with asthma severity. Potential risk factors included
parent gender, parent education, and household
monthly income. All analyses were performed using
SPSS software (Windows version 26.0; IBM Corp,
Armonk [NY], United States). P values <0.05 were
considered statistically significant.
Results
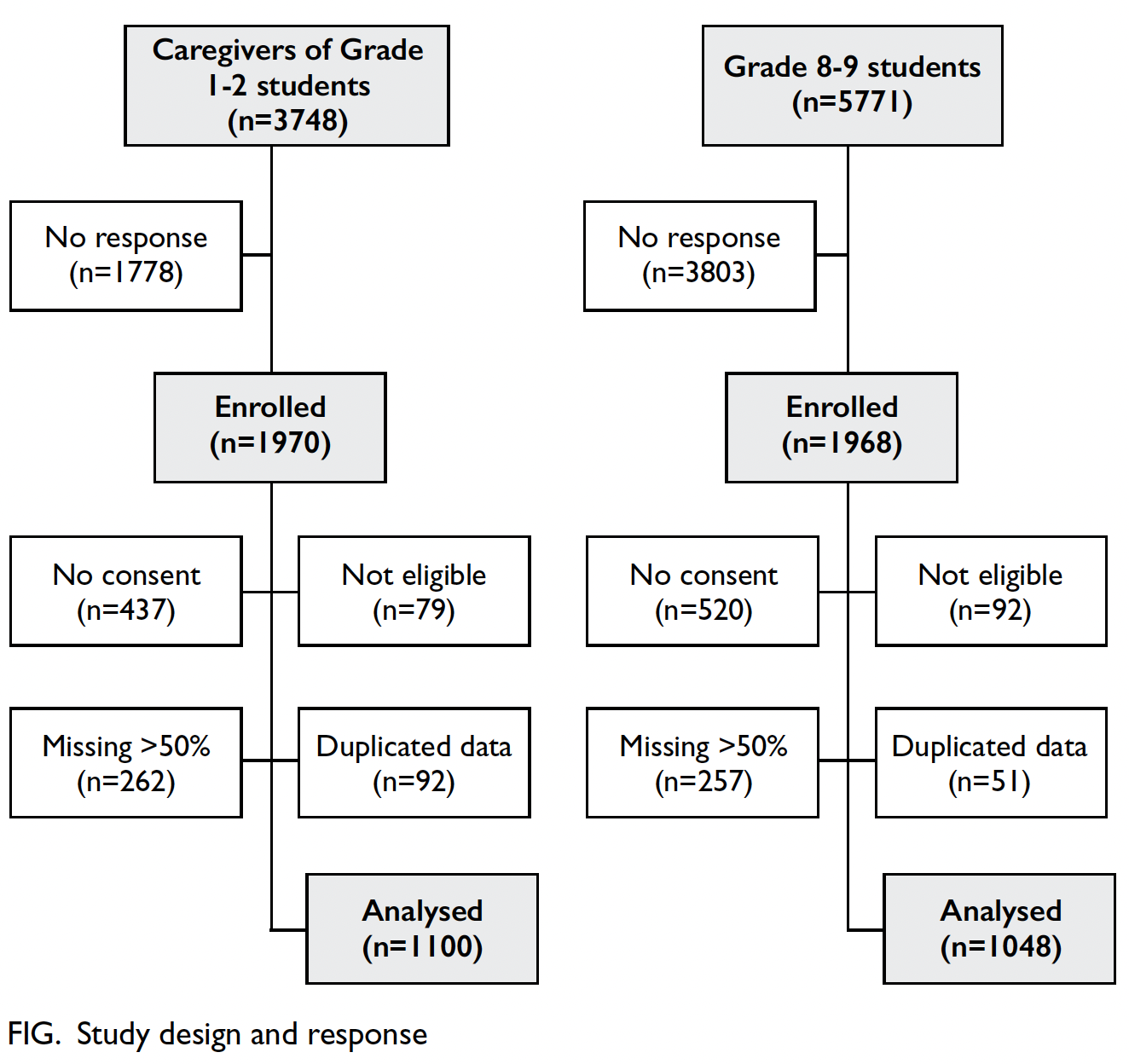
Demographic characteristics
Nineteen primary schools and 25 secondary schools
accepted the invitation to participate, representing
3748 and 5771 eligible students in the respective
groups. Overall, 1970 (52.6%) questionnaires were
returned for the primary school group, whereas
1968 (34.1%) questionnaires were returned for
the secondary school group; 1100 and 1048
questionnaires were analysed in the respective groups
after exclusion due to invalid consent, incomplete
data, or ineligibility (Fig). Boys comprised 62.51%
and 42.64% of the primary and secondary school
groups, respectively (Tables 1 and 2). The mean ages
were 7.02 years (standard deviation [SD]=0.76) in
the primary school group and 14.09 years (SD=0.89)
in the secondary school group.
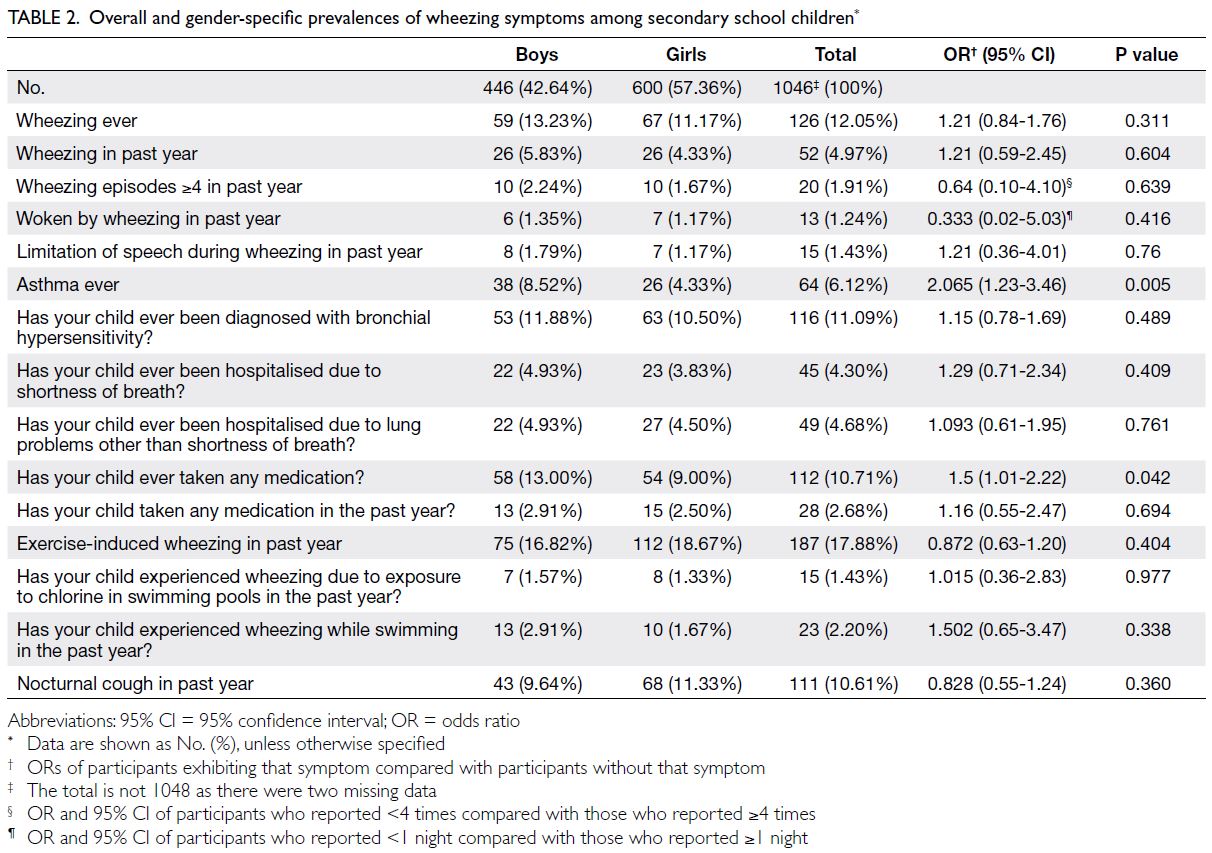
Table 2. Overall and gender-specific prevalences of wheezing symptoms among secondary school children
Asthma symptoms
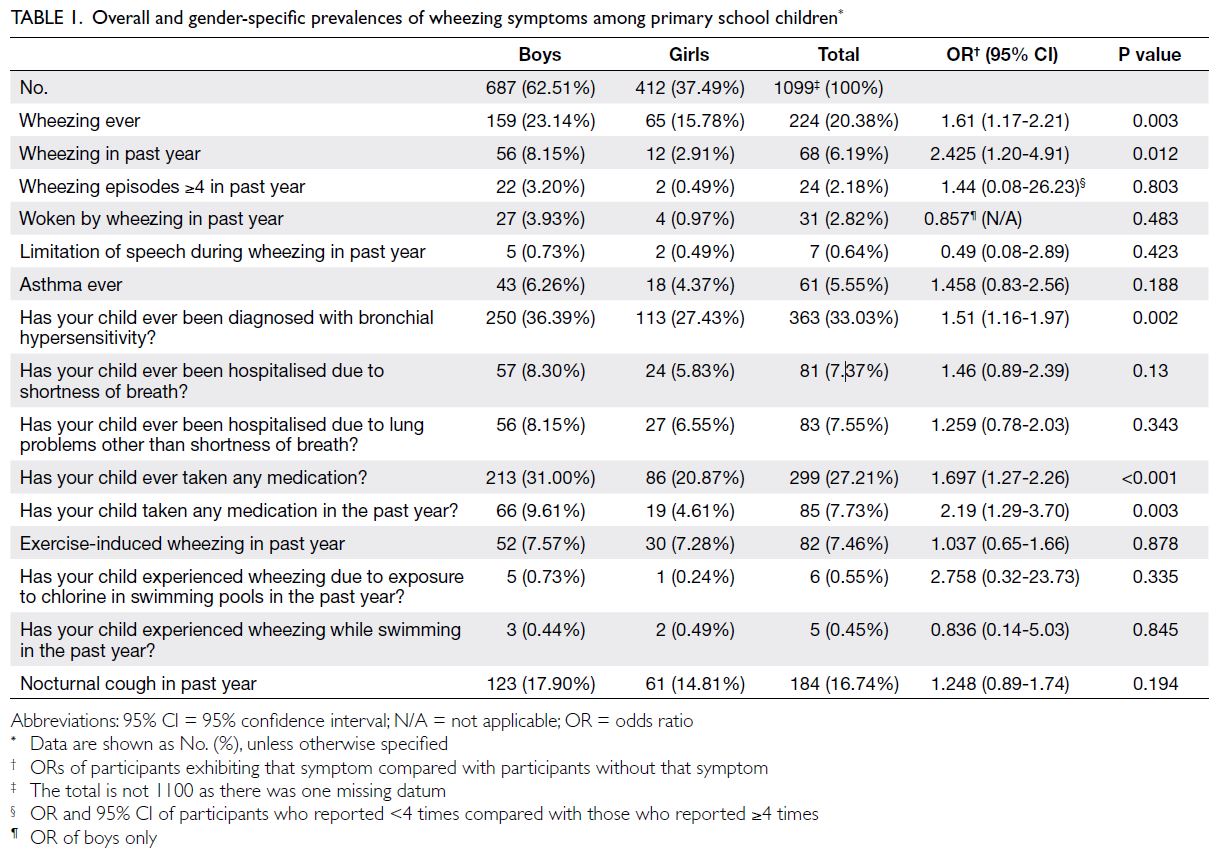
The estimated prevalences of current wheezing
were 6.19% and 4.97% in the primary school and
secondary school groups, respectively. The respective prevalences of exercise-induced wheezing and night
cough were 7.46% and 16.74% in the primary school
group and 17.88% and 10.61% in the secondary
school group (Tables 1 and 2).
The prevalences of asthma ever were 5.55%
and 6.12% in the primary school and secondary
school groups, respectively; significantly more
boys reported asthma ever in the secondary school
group. The prevalences of bronchial hypersensitivity
ever were 33.03% and 11.09% in the primary and
secondary school groups, respectively (Tables 1, 2, 3).
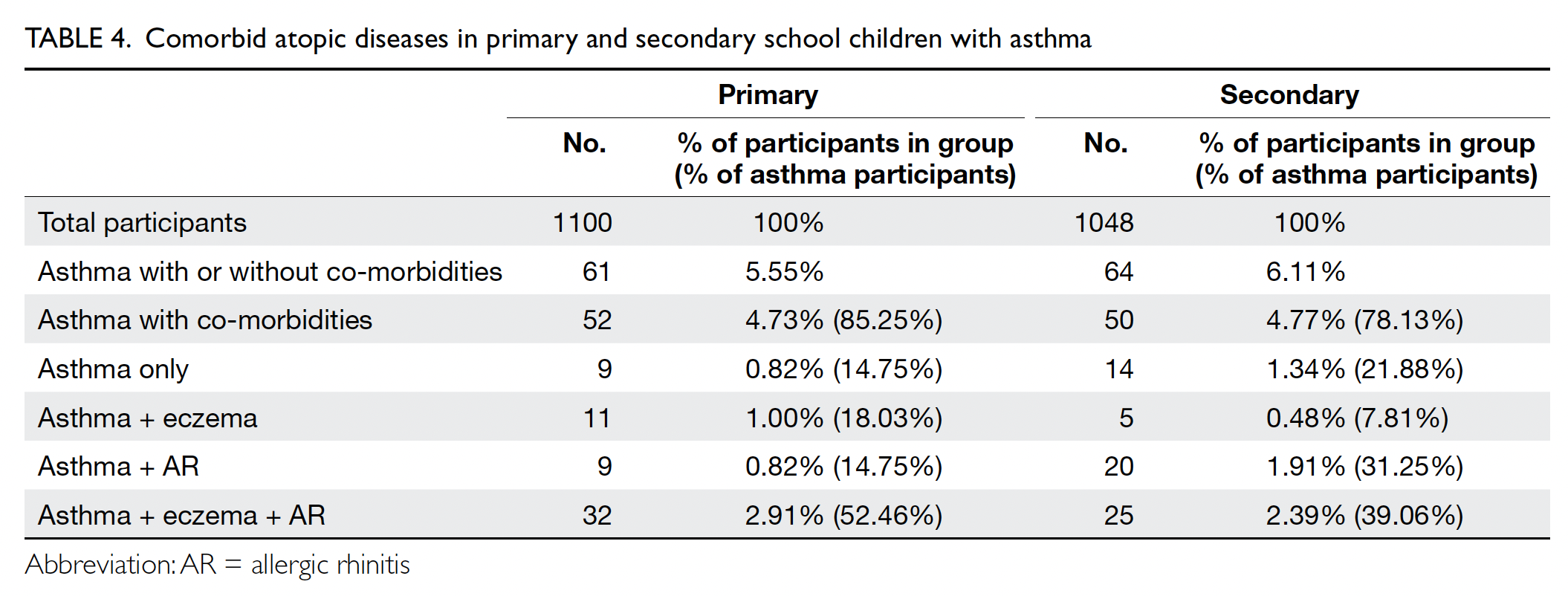
In total, 85.25% and 78.13% of primary and secondary
school participants with asthma exhibited comorbid
eczema and/or allergic rhinitis, respectively (Table 4)
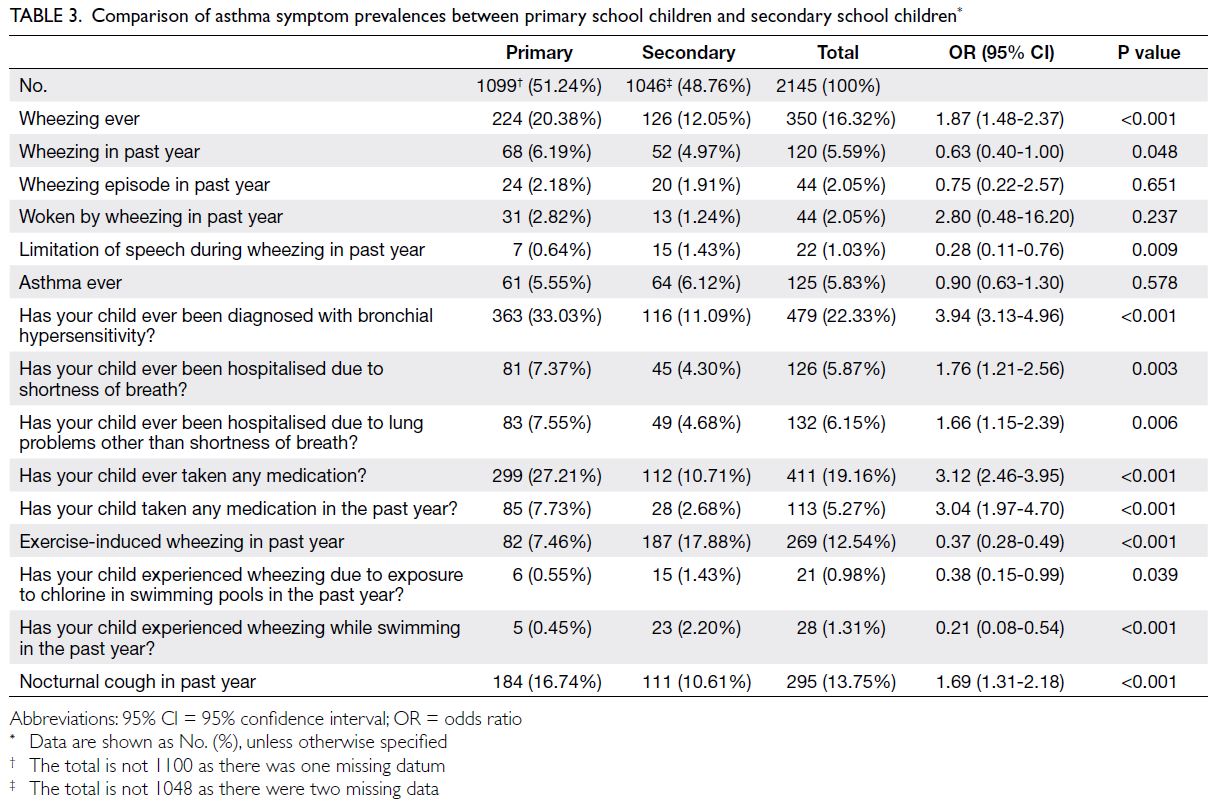
Table 3. Comparison of asthma symptom prevalences between primary school children and secondary school children
Indicators of severe asthma were also examined.
The incidences of ≥4 wheezing episodes in the past
year were 2.18% (boys vs girls: 3.20% vs 0.49%) in the
primary school group and 1.91% (boys vs girls: 2.24%
vs 1.67%) in the secondary school group. Severe
wheezing that limited speech in the past year was
reported by 0.64% and 1.43% of participants in the
primary and secondary school groups, respectively.
Waking from sleep due to wheezing in the past
year occurred in 2.82% and 1.24% of participants
in the primary and secondary school groups,
respectively. Hospitalisation due to shortness of
breath was reported by 7.37% (boys vs girls: 8.30%
vs 5.83%) and 4.30% (boys vs girls: 4.93% vs 3.83%)
of participants in the primary and secondary school groups, respectively (Tables 1 and 2). The mean ages
of wheezing onset in the primary and secondary
school groups were 2.37 years (SD=1.52) and 7.16
years (SD=4.38), respectively.
Comparison with the 1994-1995 and 2000-2001 data in Hong Kong
Compared with the previous Hong Kong ISAAC
data,1 25 26 27 the prevalence of wheezing ever increased
from 16.80%26 in 1994-1995 to 20.38% in the primary
school group but decreased from 20%25 to 12.05%
in the secondary school group. Conversely, the
prevalence of asthma ever decreased from 7.80%26 to
5.55% in the primary school group and from 11%26 to
6.12% in the secondary school group. The prevalence
of wheezing in the past year decreased from 9.20%
in 1994-199526 and 9.40% in 2000-200127 to 6.19% in
our study (2020-2021) in the primary school group;
it decreased from 12% in 1994-1995 to 4.97% in our
study in the secondary school group. The annual
changes in wheezing prevalence were -0.16% and
-0.27% in the primary and secondary school groups,
respectively. The aforementioned indicators of severe asthma, including ≥4 wheezing episodes in the past
year and severe wheezing that limited speech in the
past year, decreased in prevalence compared with
the 1994-1995 figures. However, the prevalence of
waking from sleep due to wheezing in the past year
decreased in the primary group and increased in the
secondary school group (Table 5).
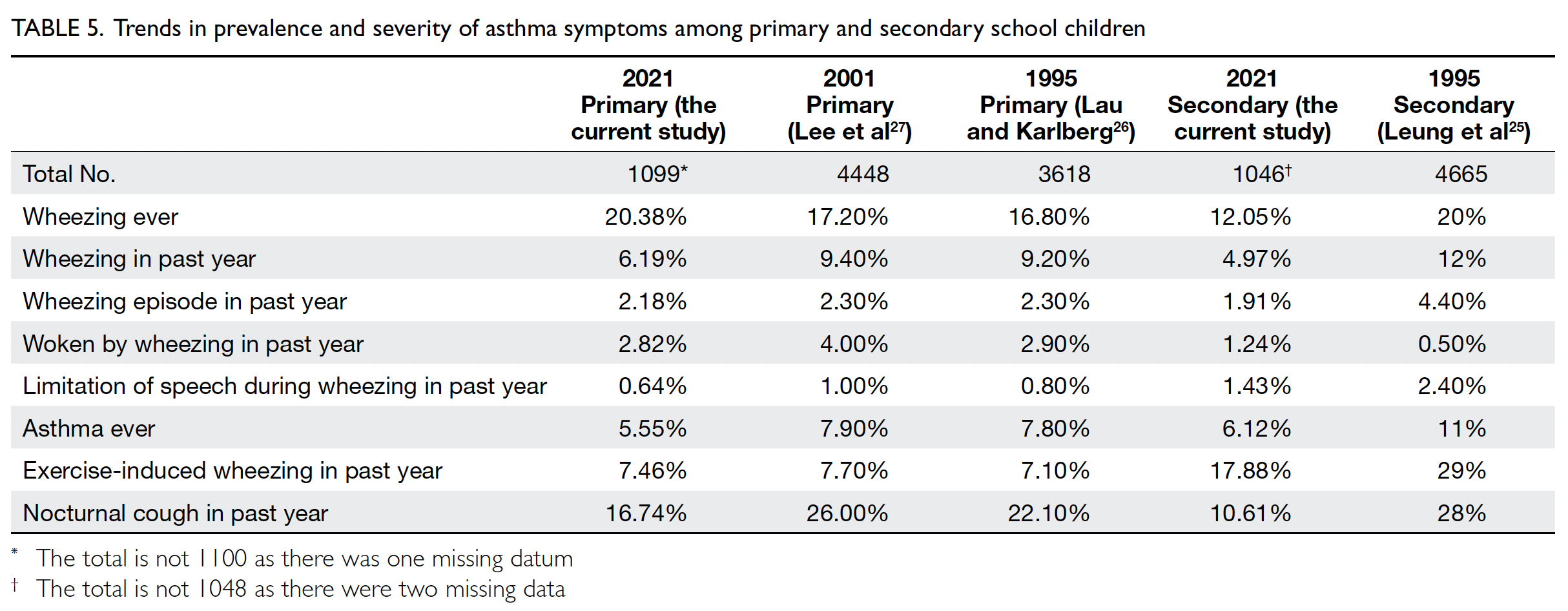
Table 5. Trends in prevalence and severity of asthma symptoms among primary and secondary school children
Overall, both the prevalence and severity of
asthma declined compared with the 1994-1995
data. We observed a statistically significant male
predominance for wheezing ever (P=0.003), current
wheezing (P=0.012), and bronchial hypersensitivity
diagnosis (P=0.002) in the primary school group
(Table 1), as well as for asthma ever in the secondary school group (P=0.005) [Table 2].
In the logistic regression model, demographic
characteristics, including parent gender, household
income, and parent education, were not significantly
predictive of wheezing episodes in the past year.
Hierarchical logistic regression using demographic
characteristics along with concomitant current
eczema and rhinitis also did not show significantly
predictive effects for wheezing episodes in the past
year.
Discussion
This is the first study since 2001 to investigate trends
in asthma prevalence and severity among school
children in Hong Kong. The global variability in
these trends is complicated by the lack of consensus
regarding exact definitions of asthma entities,
the heterogeneity of the disease itself, changes
in community awareness, the absence of a ‘gold
standard’ diagnostic test, and the non-specific
nature of symptoms shared with other diagnoses.13
This study showed a decrease in asthma prevalence,
consistent with findings from the neighbouring
region of Taiwan.11
Global trends in asthma prevalence and
severity
The 2020 study by Asher et al4 investigating
international symptom trends showed annual
current asthma prevalence increases of 0.06% in
the secondary school group (from 13.2% to 13.7%)
and 0.13% in the primary school group (from
11.1% to 11.6%), and increases in the prevalence of asthma ever by 0.18% and 0.28% in the primary and
secondary school groups, respectively. However,
trends varied across regions; in general, asthma
prevalence decreased in higher-income regions
but increased in low- and middle-income regions.
Considering that Hong Kong exhibits higher levels
of income and gross domestic product per capita,
it is unsurprising that the local asthma prevalences
showed a decreasing trend.
A decrease in asthma severity was also observed
in most regions, reflected by the three indicators
of severe asthma in our study and parameters
such as asthma-related hospital admissions and
mortality. These findings suggest that, regardless
of overall asthma trends, milder forms of asthma
have become more prevalent in recent years.7 28
Our study revealed similar trends in terms of fewer
severe asthmatic exacerbations and flares. However,
according to the ISAAC protocol, centres with 1000
to 2999 participants are considered appropriate for
comparisons of prevalence, but not severity, with
centres in other regions.2
Age
Safiri et al5 identified the highest asthma prevalence
among 5- to 9-year-olds, which then decreased and
remained stable until adulthood. A proportion of
young children with wheezing may have ‘transient
wheezing’ that does not progress to asthma.4 This
notion is consistent with our finding of less frequent
symptoms in the past year among older children.5
The prevailing view is that approximately 50% of preschool children with wheezing will progress to
asthma by the time of primary school entry.4 The
prevalences of 12.05% and 6.12% for wheezing ever
and asthma ever observed in our secondary school
group are consistent with this view.
Sex
Our results showed a male predominance for asthma
and wheezing in the primary school group, consistent with existing literature.29 Various mechanisms have
been proposed to explain the post-pubertal shift
towards female predominance due to sex hormone
changes, genetic and epigenetic differences, co-morbidities,
and socio-economic factors.14 The
significantly older age of wheezing onset reported
in the secondary school group, along with the lower
prevalence of wheezing ever in the secondary school
group, may be attributable to recall bias due to the
use of questionnaires, a limitation noted in other
studies.30
Co-morbidities and exposure
Co-morbidities with other atopic diseases were
common among our asthma participants; most
participants in both groups had concomitant atopic
dermatitis, allergic rhinitis, and asthma, consistent
with previous reports.8 16 Otherwise, our study did
not identify relevant demographic characteristics
or risk factors through statistical analysis that could
predict wheezing episodes. Many studies have
investigated various factors potentially associated
with asthma diagnosis, wheezing, exercise-induced
symptoms, and nocturnal cough. These factors
include, but are not limited to, family history,
recurrent respiratory infections, early-life severe
respiratory syncytial virus infection, exposure to
cigarette smoke, exposure to pets, incense burning,
maternal education level, sex, race, vaccination rates,
humidity, air pollution index (particulate matter) and
exposure (particularly nitrogen dioxide and sulphur
dioxide), exposure to indoor mould, farm residence,
exposure to indoor endotoxins, socio-economic
status, obesity, open fire cooking, nutritional levels,
neonatal antibiotic use, delivery mode, urban living
environment, psychosocial environment (including
maternal stress), current paracetamol use, maternal
antibiotic use during pregnancy, maternal vitamin D consumption during pregnancy, maternal weight
gain during pregnancy, maternal paracetamol use
during pregnancy, proton-pump inhibitor and
H2-receptor antagonist use, and new immigrant
status.2 3 4 5 6 8 9 10 11 12 13 14 15 29 The next phase of our study will
examine these predictive or protective factors; it will
also explore risk factors unique to our population,
including cultural perceptions,18 feeding and weaning
practices,19 31 joss stick burning,15 and exposure to
traditional Chinese herbal medicine.32 33
Strengths and limitations
Study design and population demographics
Due to its survey approach, this study has limitations
of recall bias and cross-sectional design. The
estimations of symptom prevalence also lack
objective confirmation through medical assessments
and objective tests, which could lead to over- or
underestimation of the asthmatic population;
this aspect is further complicated by diagnostic
discrepancies among medical professionals across
regions and time periods.4 34 Although our study
population exceeded 1000 students per group, it was
smaller than the original Hong Kong ISAAC studies,
which included >300026 and 400025 participants,
respectively. The paediatric population size has also
significantly changed, according to the Hong Kong
Population Census data: 86 000 13- to 14-year-olds
in 1994 compared with 61 800 in 2021.35 The male-to-female ratio of 1.67 and 0.74 in the primary and secondary school groups, respectively, differed
from the 2021 census figures (1.063 and 1.067,
respectively)35; therefore, our findings may not reflect
the true prevalence and severity of asthma in the
Hong Kong paediatric population, which represents
a key limitation of the study. Although the ISAAC
protocol acknowledges the impracticality of video
questionnaires due to logistic or technical factors,2 exclusive use of the written questionnaire may have
impacted our results; notably, a study has shown that
video and written questionnaires are comparable.23
The relatively large population size and the use of
validated and standardised questionnaires may
partially mitigate these limitations.
Response rates
In this study, response rates were lower than in
the previous ISAAC studies (where rates >80%
for most centres),36 likely due to ‘survey fatigue’
and challenges in motivating participants during
the COVID-19 pandemic. Similarly low response
rates were observed in studies requesting parental
completion of home questionnaires.37 Although no
significant differences in airway disease or symptom
prevalences have been identified between non-responders
and responders,38 the lower response rates may have introduced selection bias into our
study.
Physician practices
The increased prevalence of wheezing ever in
the primary school group, decreased prevalence
of wheezing ever in the secondary school group,
decreased prevalences of asthma ever in both
groups, and decreased symptom severity all reflect
enhanced awareness and modified diagnostic
practices among the general population and medical
professionals.4 11 13 Doctors are less likely to classify
patients as ‘asthmatic’ without collecting a thorough
clinical history and performing diagnostic testing,
leading to a larger difference in the prevalences of
wheezing and asthma.11 13
Coronavirus disease 2019 policies
The local COVID-19 policy, commonly known as the
mask mandate, along with social isolation, increased
awareness of infection control, changes in drug
compliance, and enhanced hygiene practices, may
have significantly reduced triggers for infectious and
allergic airway diseases.39 40 However, mechanisms
linking COVID-19 to severe asthma risk have also
been proposed.41 These policies and social practices
may explain the overall decreases in asthma
prevalence and severity observed in our study. The
timing of the study coincided with the COVID-19
pandemic, where the modified local health practices
may have influenced trends concerning current
asthma and airway symptoms.
Conclusion
This study provides an essential update regarding
the prevalences of asthma and other respiratory
symptoms among school children in Hong Kong.
Our findings indicate overall decreasing trends
in asthma severity and prevalence. A follow-up study will explore the protective and risk factors
contributing to these trends.
Author contributions
Concept or design: JWCH Cheng, YP Tsang, YY Lam, AKY Chu, CHY Chan, YL Fung, PSY Chau, DCK Luk.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: JWCH Cheng, CSY Ng.
Critical revision of the manuscript for important intellectual content: JWCH Cheng, CSY Ng, CHY Chan, YL Fung.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: JWCH Cheng, CSY Ng.
Critical revision of the manuscript for important intellectual content: JWCH Cheng, CSY Ng, CHY Chan, YL Fung.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors thank all the children and their families for their participation in this research.
Funding/support
This research received no specific grant from any funding
agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This research was approved by the Human Research Ethics
Committee of The University of Hong Kong, Hong Kong (Ref No.: EA2002007). All study participants provided written
consent for publication of their data which were de-identified
in this article.
References
1. Asher MI, Montefort S, Björkstén B, et al. Worldwide time
trends in the prevalence of symptoms of asthma, allergic
rhinoconjunctivitis, and eczema in childhood: ISAAC
Phases One and Three repeat multicountry cross-sectional
surveys. Lancet 2006;368:733-43. Crossref
2. Asher MI, Keil U, Anderson HR, et al. International Study
of Asthma and Allergies in Childhood (ISAAC): rationale
and methods. Eur Respir J 1995;8:483-91. Crossref
3. Asher MI, Rutter CE, Bissell K, et al. Worldwide trends in
the burden of asthma symptoms in school-aged children:
Global Asthma Network Phase I cross-sectional study.
Lancet 2021;398:1569-80. Crossref
4. Asher MI, García-Marcos L, Pearce NE, Strachan DP.
Trends in worldwide asthma prevalence. Eur Respir J
2020;56:2002094. Crossref
5. Safiri S, Carson-Chahhoud K, Karamzad N, et al.
Prevalence, deaths, and disability-adjusted life-years due
to asthma and its attributable risk factors in 204 countries
and territories, 1990-2019. Chest 2022;161:318-29. Crossref
6. Ferrante G, La Grutta S. The burden of pediatric asthma.
Front Pediatr 2018;6:186. Crossref
7. The Global Asthma Report 2022 [editorial]. Int J Tuberc
Lung Dis 2022;26(Suppl 1):1-104 Crossref
8. Kansen HM, Le TM, Uiterwaal C, et al. Prevalence and
predictors of uncontrolled asthma in children referred
for asthma and other atopic diseases. J Asthma Allergy
2020;13:67-75. Crossref
9. Rodriguez A, Brickley E, Rodrigues L, Normansell RA,
Barreto M, Cooper PJ. Urbanisation and asthma in low-income
and middle-income countries: a systematic review
of the urban–rural differences in asthma prevalence.
Thorax 2019;74:1020-30.Crossref
10. Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE,
Celedón JC. Risk and protective factors for childhood
asthma: what is the evidence? J Allergy Clin Immunol Pract
2016;4:1111-22. Crossref
11. Chen WY, Lin CW, Lee J, Chen PS, Tsai HJ, Wang JY.
Decreasing ten-year (2008-2018) trends of the prevalence
of childhood asthma and air pollution in Southern Taiwan.
World Allergy Organ J 2021;14:100538. Crossref
12. Akinbami LJ, Simon AE, Rossen LM. Changing trends in
asthma prevalence among children. Pediatrics 2016;137:1-7. Crossref
13. Sears MR. Trends in the prevalence of asthma. Chest 2014;145:219-25. Crossref
14. Chowdhury NU, Guntur VP, Newcomb DC, Wechsler ME.
Sex and gender in asthma. Eur Respir Rev 2021;30:210067. Crossref
15. Lin TC, Krishnaswamy G, Chi DS. Incense smoke: clinical,
structural and molecular effects on airway disease. Clin
Mol Allergy 2008;6:3. Crossref
16. Arasi S, Porcaro F, Cutrera R, Fiocchi AG. Severe asthma
and allergy: a pediatric perspective. Front Pediatr 2019;7:28. Crossref
17. Wong GW, Ko FW, Hui DS, et al. Factors associated with
difference in prevalence of asthma in children from three
cities in China: multicentre epidemiological survey. BMJ
2004;329:486. Crossref
18. Leo HL, Wang A, Gong M, Clark N. Cultural perceptions
on identification and treatment of childhood asthma in the
US and China. J Allergy Clin Immunol 2006;117 Suppl:S53. Crossref
19. Mihrshahi S, Ampon R, Webb K, et al. The association
between infant feeding practices and subsequent atopy
among children with a family history of asthma. Clin Exp
Allergy 2007;37:671-9. Crossref
20. Wong GW, Brunekreef B, Ellwood P, et al. Cooking fuels
and prevalence of asthma: a global analysis of phase three
of the International Study of Asthma and Allergies in
Childhood (ISAAC). Lancet Respir Med 2013;1:386-94. Crossref
21. Asher MI. Urbanisation, asthma and allergies. Thorax 2011;66:1025-6. Crossref
22. Chan HH, Pei A, Van Krevel C, Wong GW, Lai CK.
Validation of the Chinese translated version of ISAAC core
questions for atopic eczema. Clin Exp Allergy 2001;31:903-7. Crossref
23. Lai CK, Chan JK, Chan A, et al. Comparison of the ISAAC
video questionnaire (AVQ3.0) with the ISAAC written
questionnaire for estimating asthma associated with
bronchial hyperreactivity. Clin Exp Allergy 1997;27:540-5. Crossref
24. Becerir T, Akcay A, Duksal F, Ergin A, Becerir C, Guler
N. Prevalence of asthma, local risk factors and agreement
between written and video questionnaires among Turkish
adolescents. Allergol Immunopathol (Madr) 2014;42:594-602. Crossref
25. Leung R, Wong G, Lau J, et al. Prevalence of asthma and
allergy in Hong Kong schoolchildren: an ISAAC study. Eur Respir J 1997;10:354-60. Crossref
26. Lau YL, Karlberg J. Prevalence and risk factors of childhood
asthma, rhinitis and eczema in Hong Kong. J Paediatr
Child Health 1998;34:47-52. Crossref
27. Lee SL, Wong W, Lau YL. Increasing prevalence of allergic
rhinitis but not asthma among children in Hong Kong
from 1995 to 2001 (Phase 3 International Study of Asthma
and Allergies in Childhood). Pediatr Allergy Immunol
2004;15:72-8. Crossref
28. Ebmeier S, Thayabaran D, Braithwaite I, Bénamara C,
Weatherall M, Beasley R. Trends in international asthma
mortality: analysis of data from the WHO Mortality
Database from 46 countries (1993-2012). Lancet
2017;390:935-45. Crossref
29. Estela DB, Arturo B, Nayely RN, et al. Have asthma
symptoms in Mexico changed in the past 15 years? Time
trends from the International Study of Asthma and
Allergies in Childhood to the Global Asthma Network.
Allergol Immunopathol (Madr) 2021;49:1-10. Crossref
30. Strachan DP. The prevalence and natural history of wheezing
in early childhood. J R Coll Gen Pract 1985;35:182-4.
31. Yung J, Yuen JW, Ou Y, Loke AY. Factors associated with
atopy in toddlers: a case-control study. Int J Environ Res
Public Health 2015;12:2501-20. Crossref
32. Li XM. Traditional Chinese herbal remedies for asthma
and food allergy. J Allergy Clin Immunol 2007;120:25-31. Crossref
33. Li XM, Brown L. Efficacy and mechanisms of action of
traditional Chinese medicines for treating asthma and
allergy. J Allergy Clin Immunol 2009;123:297-306; quiz
307-8. Crossref
34. Hederos CA, Hasselgren M, Hedlin G, Bornehag CG.
Comparison of clinically diagnosed asthma with parental
assessment of children’s asthma in a questionnaire. Pediatr
Allergy Immunol 2007;18:135-41. Crossref
35. Census and Statistics Department, Hong Kong SAR
Government. Population estimates. Table 110-01002:
Population by sex and age. Available from: https://www.censtatd.gov.hk/en/web_table.html?id=110-01002#. Accessed 14 Oct 2024.
36. Worldwide variations in the prevalence of asthma
symptoms: the International Study of Asthma and Allergies
in Childhood (ISAAC). Eur Respir J 1998;12:315-35. Crossref
37. Ellwood P, Ellwood E, Rutter C, et al. Global Asthma
Network Phase I surveillance: geographical coverage and
response rates. J Clin Med 2020;9:3688. Crossref
38. Rönmark EP, Ekerljung L, Lötvall J, Torén K, Rönmark E,
Lundbäck B. Large scale questionnaire survey on
respiratory health in Sweden: effects of late- and nonresponse.
Respir Med 2009;103:1807-15. Crossref
39. Izquierdo-Domínguez A, Rojas-Lechuga MJ, Alobid I.
Management of allergic diseases during COVID-19
outbreak. Curr Allergy Asthma Rep 2021;21:8. Crossref
40. Carr TF, Kraft M. Asthma and atopy in COVID-19: 2021
updates. J Allergy Clin Immunol 2022;149:562-4. Crossref
41. Hosoki K, Chakraborty A, Sur S. Molecular mechanisms
and epidemiology of COVID-19 from an allergist’s
perspective. J Allergy Clin Immunol 2020;146:285-99. Crossref