DOI: 10.12809/hkmj177137
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE
Hong Kong Institute of Allergy and Hong Kong Society
for Paediatric Immunology Allergy & Infectious Diseases joint
consensus statement 2018 on vaccination in egg-allergic patients
Gilbert T Chua, MB, BS, MRCPCH1; Philip
H Li, MRes (Med), MRCP2; Marco HK Ho, MD, FRCPCH1;
Ellen Lai, BPharm, MClinPharm3; Vivian Ngai, BPharm,
MClinPharm; Felix YS Yau, MRCP, FHKAM (Paediatrics)4; Mike YW
Kwan, FHKAM (Paediatrics), FHKCPaed5; TF Leung, MD, FRCPCH6;
TH Lee, ScD, FRCP7
1 Department of Paediatrics and
Adolescent Medicine, Queen Mary Hospital, Pokfulam, Hong Kong
2 Division of Rheumatology and Clinical
Immunology, Department of Medicine, Queen Mary Hospital, Pokfulam, Hong
Kong
3 Department of Pharmacy, Queen Mary
Hospital, Pokfulam, Hong Kong
4 Department of Paediatrics and
Adolescent Medicine, Queen Elizabeth Hospital, Jordan, Hong Kong
5 Department of Paediatrics and
Adolescent Medicine, Princess Margaret Hospital, Laichikok, Hong Kong
6 Department of Paediatrics, The Chinese
University of Hong Kong, Prince of Wales Hospital, Shatin, Hong Kong
7 Allergy Centre, Hong Kong Sanatorium
and Hospital, Happy Valley, Hong Kong
Corresponding author: Dr TH Lee (takhong.lee@hksh.com)
Abstract
Vaccination of egg-allergic individuals has been
a historical concern, particularly for influenza and
measles-mumps-rubella-varicella vaccines that are developed in chicken
egg embryos or chicken cell fibroblasts. The egg proteins in these
vaccines were believed to trigger an immediate allergic reaction in
egg-allergic individuals. However, recently published international
guidelines have updated their recommendations and now state that these
vaccines can be safely administered to egg-allergic individuals. This
joint consensus statement by the Hong Kong Institute of Allergy and the
Hong Kong Society for Paediatric Immunology Allergy & Infectious
Diseases summarises the updates and provides recommendations for local
general practitioners and paediatricians.
Background
Vaccination is an important and effective method to
develop active immunity against certain pathogens. It helps to prevent or
reduce the risks of developing certain infectious diseases as well as
moderating disease severity. However, the administration of certain
vaccines, including influenza, measles-mumps- rubella (MMR),
measles-mumps-rubellavaricella (MMR-V) and yellow fever vaccines, has
historically been relatively, if not absolutely, contra-indicated in
egg-allergic individuals. This is because these vaccines are developed in
chicken egg embryos or chicken cell fibroblasts, raising the concern that
egg proteins (notably ovalbumin) in these vaccines may trigger an
immediate allergic reaction in egg-allergic individuals. As a result,
previous vaccination guidelines and vaccine product information have
recommended avoidance of influenza and MMR or MMR-V vaccines in
individuals with a history of anaphylactic reaction to egg exposure.
Local epidemiological studies have shown that
0.4%-0.7% of Hong Kong children were reported by their parents to have had
an adverse reaction to intake of a hen’s egg.1
2 No local data for the adult
population are available. However, it is important to differentiate
between adverse reactions and genuine egg allergy, especially when
deciding the need for vaccine avoidance. A recent United Kingdom
multi-centre study found that more than a third of patients with suspected
egg allergy who were referred to a tertiary allergy centre for vaccination
were not actually egg allergic, and all were vaccinated successfully.3
Despite the paucity of evidence, there remains some
concern that administration of vaccines that could contain egg proteins,
notably ovalbumin, might cause allergic reactions in egg-allergic
subjects. The Centre for Health Protection recommends that mildly
egg-allergic individuals can safely receive inactivated influenza vaccine
in a primary care setting. However, those with confirmed or suspected egg
allergy who have experienced severe reactions should be seen by an
allergist/immunologist for evaluation of their egg allergy prior to
administration of inactivated influenza vaccine.4
Recently published international guidelines have
updated their recommendations regarding the administration of vaccines to
egg-allergic individuals. This joint consensus statement by the Hong Kong
Institute of Allergy and the Hong Kong Society for Paediatric Immunology
Allergy & Infectious Diseases summarises recent updates and provides
recommendations for local general practitioners and paediatricians. For
practical reasons, this guideline will only cover influenza and MMR/MMR-V
vaccines.
Yellow fever vaccine is less commonly administered
and is commonly propagated in hens’ eggs. Specialist evaluation is
recommended prior to vaccination for evaluation of suspected egg allergies
with vaccine skin testing or consideration for desensitisation.3 An egg-free yellow fever formulation is available as an
alternative.
The Q fever vaccine is not available in Hong Kong
and therefore is not covered in this guideline.
Influenza vaccine
Influenza vaccination is well known to be effective
in preventing infections caused by influenza viruses and in reducing the
risk of developing complications. We reviewed the product information
recommendations of Vaxigrip (Sanofi Pasteur SA, Lyon, France), Fluarix
Tetra (GlaxoSmithKline Biologicals, Dresden, Germany), and FluQuadri
(Sanofi Pasteur SA, Lyon, France). All recommended that patients with egg
or chicken protein hypersensitivity are contra-indicated to receive their
vaccines. However, upon direct communication with the respective
pharmaceutical companies, all of them were reported to contain <0.1
ug/mL of ovalbumin in their vaccines. Therefore, we disagree with their
recommendations.
Moneret-Vautrin et al5
reported that only 1% of egg-allergic patients would develop allergic
reactions at a threshold as low as 1 mg. As the quantity of ovalbumin in
influenza vaccines is ≤1 μg/dose, such a level of egg protein in influenza
vaccines is very unlikely to trigger an allergic response in this group of
patients. Thus, despite the product information recommendations and the
trace amounts of ovalbumin present in these influenza vaccines, they
should be safe for egg-allergic individuals, including those with a
history of anaphylaxis to egg proteins.
Our view is supported by numerous international
guidelines on administration of influenza vaccines to egg-allergic
individuals, summarised in the Table.6 7 8 9 10 11 12
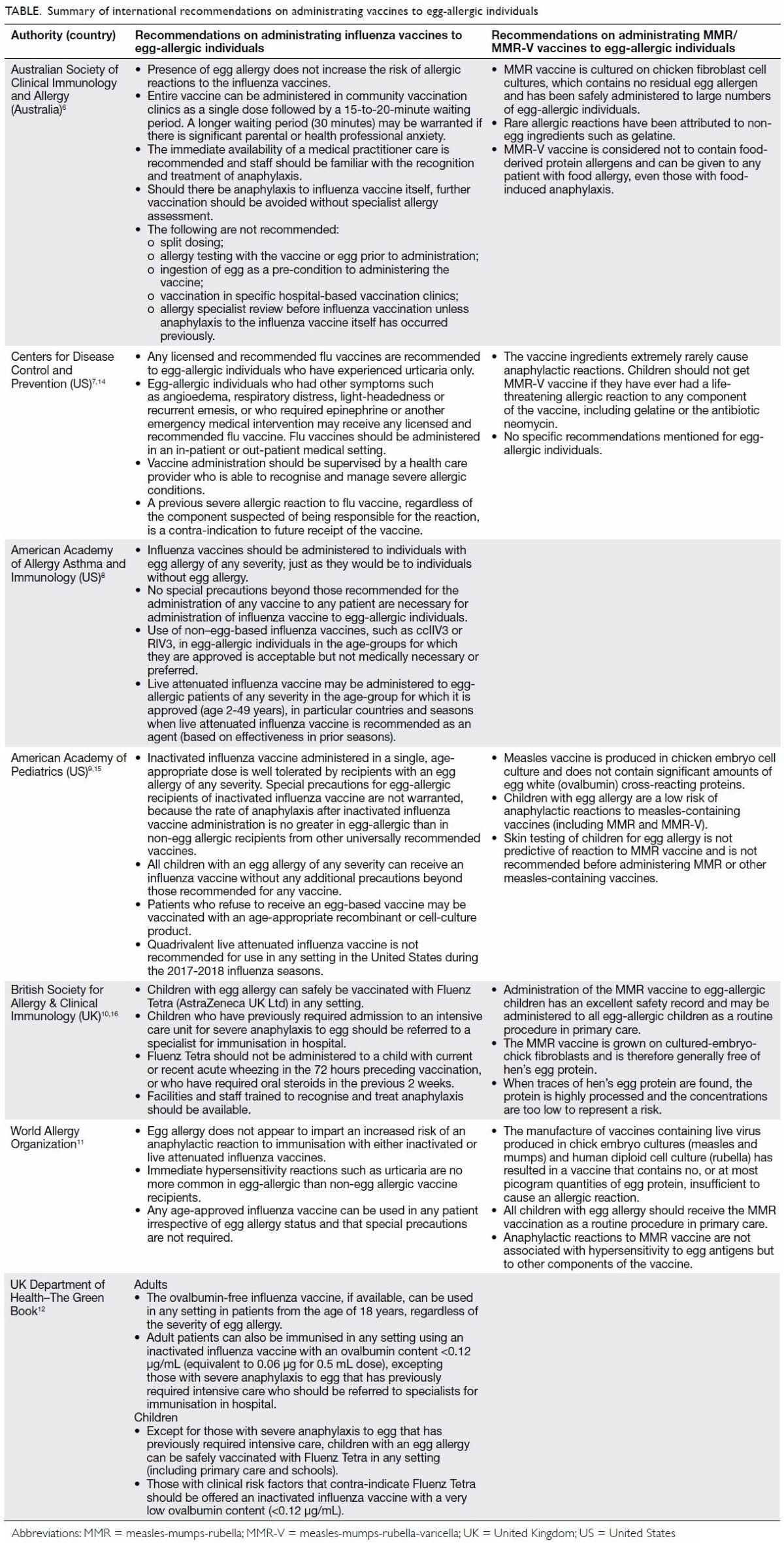
Table. Summary of international recommendations on administrating vaccines to egg-allergic individuals
Measles-mumps-rubella and
measles-mumps-rubella-varicella vaccines
The MMR and MMR-V vaccines are safe and effective
in preventing mumps, measles, rubella, and varicella. The vaccination
schedule in Hong Kong recommends that the first dose be administered at
age 1 year and the second dose at Primary 1 (age 5-6 years).13 We reviewed the product information recommendations
of two MMR-V vaccines available in Hong Kong: Priorix-Tetra
(GlaxoSmithKline plc [GSK], Brentford, UK) and ProQuad (Merck & Co,
Inc, Kenilworth [NJ], US). The manufacturers of both of these products
recommend that patients with severe allergic reactions after egg ingestion
should take extra precaution when receiving the vaccines. However, in
direct communication with the manufacturers, GSK replied that
Priorix-Tetra may contain traces of egg protein but the amount is not
measured in the final product. In contrast, Merck replied that internal
analysis was done for ProQuad for its egg protein content; however, they
refused to disclose the information as they consider it proprietary. We
disagree with their recommendations. The Table summarises international recommendations for
administration of MMR/MMR-V vaccines to egg-allergic individuals.6 11 14 15 16 It is recommended that all patients, including those
with suspected or confirmed egg allergy, should receive the MMR/MMR-V
vaccination as a matter of routine in primary care, as the vaccine does
not contain egg allergen.
Recommendations of the Hong Kong Institute of Allergy
and the Hong Kong Society for Paediatric Immunology Allergy &
Infectious Diseases
1. All patients with suspected or confirmed egg
allergy should receive the MMR/MMR-V vaccination as a matter of routine in
primary care.
2. Influenza vaccines can be safely administered, and are recommended, for disease prevention in egg-allergic individuals. They are recommended to be administered in an out-patient or ambulatory setting.
3. Only those patients who have previously required admission to an intensive care unit for severe anaphylaxis to egg should be referred to an allergist for further evaluation prior to influenza vaccination.
4. Should there be any significant concerns from patients, parents or health care professionals, health care professionals who are capable of recognising signs and symptoms of an allergic reaction can provide 15 to 30 minutes of monitoring following vaccination.
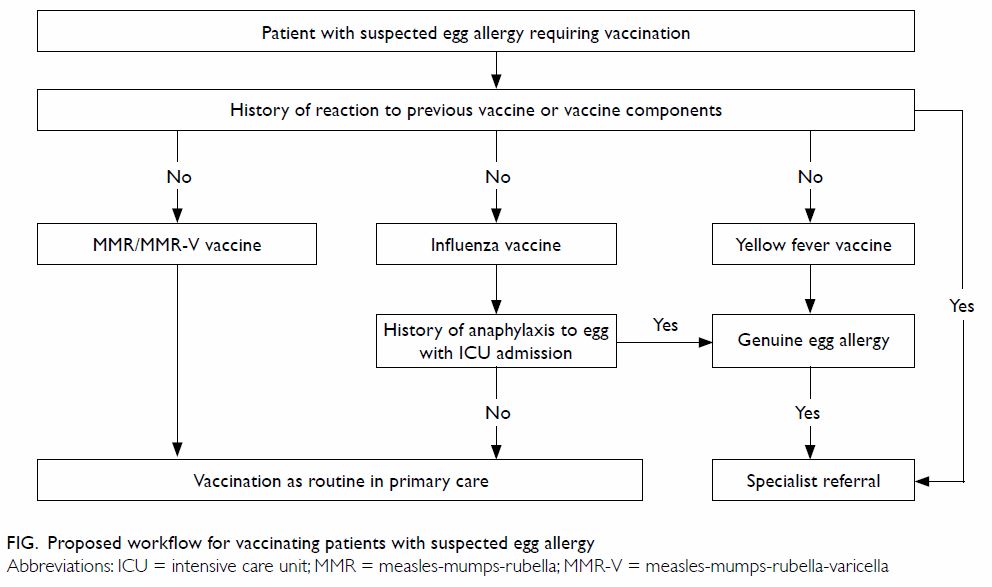
5. Specialist evaluation is recommended prior to yellow fever vaccination in egg-allergic individuals (Fig).
6. Individuals who have developed or are suspected to have developed an allergic reaction to the vaccine or other vaccine components (such as gelatine or neomycin) should not undergo further vaccination with these products. Referral to an allergy specialist for further evaluation can be considered (Fig).
7. A significant number of suspected egg-allergic patients may be misdiagnosed, so referral to an allergist for evaluation may be considered.
2. Influenza vaccines can be safely administered, and are recommended, for disease prevention in egg-allergic individuals. They are recommended to be administered in an out-patient or ambulatory setting.
3. Only those patients who have previously required admission to an intensive care unit for severe anaphylaxis to egg should be referred to an allergist for further evaluation prior to influenza vaccination.
4. Should there be any significant concerns from patients, parents or health care professionals, health care professionals who are capable of recognising signs and symptoms of an allergic reaction can provide 15 to 30 minutes of monitoring following vaccination.
5. Specialist evaluation is recommended prior to yellow fever vaccination in egg-allergic individuals (Fig).
6. Individuals who have developed or are suspected to have developed an allergic reaction to the vaccine or other vaccine components (such as gelatine or neomycin) should not undergo further vaccination with these products. Referral to an allergy specialist for further evaluation can be considered (Fig).
7. A significant number of suspected egg-allergic patients may be misdiagnosed, so referral to an allergist for evaluation may be considered.
Author contributions
GT Chua and PH Li drafted the main text of the
article, including the tables and figures. E Lai and V Ngai offered their
expert opinion as clinical pharmacists and contacted pharmaceutical
companies regarding the contents of the vaccines. MHK Ho, MYW Kwan, FYS
Yau, TF Leung, and TH Lee contributed to the concept, analysis, and
critical revision of the article.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Declaration
All authors have disclosed no conflicts of
interest. All authors had full access to the data, contributed to the
study, approved the final version for publication, and take responsibility
for its accuracy and integrity.
References
1. Leung TF, Yung E, Wong YS, Lam CW, Wong
GW. Parent-reported adverse food reactions in Hong Kong Chinese
pre-schoolers: epidemiology, clinical spectrum and risk factors. Pediatr
Allergy Immunol 2009;20:339-46. Crossref
2. Ho MH, Lee SL, Wong WH, Ip P, Lau YL.
Prevalence of self-reported food allergy in Hong Kong children and teens—a
population survey. Asian Pac J Allergy Immunol 2012;30:275-84.
3. Li PH, Wagner A, Rutkowski R, Rutkowski
K. Vaccine allergy: a decade of experience from 2 large UK allergy
centers. Ann Allergy Asthma Immunol 2017;118:729-31. Crossref
4. Centre for Health Protection, Department
of Health, Hong Kong SAR Government. Frequently asked question on seasonal
influenza vaccine 2015/16. Q14. Who should not receive inactivated
seasonal influenza vaccination? Available from:
http://www.chp.gov.hk/en/view_content/26837.html. Accessed 20 Nov 2017.
5. Moneret-Vautrin DA, Kanny G. Update on
threshold doses of food allergens: implications for patients and the food
industry. Curr Opin Allergy Clin Immunol 2004;4:215-9. Crossref
6. ASCIA Guidelines—Vaccination of the
Egg-allergic Individual. Australian Society of Clinical Immunology and
Allergy; 2017. Crossref
7. Centers for Disease Control and
Prevention, Department of Health & Human Service, USA Government. Flu
vaccine and people with egg allergies. Available from:
https://www.cdc.gov/flu/protect/vaccine/egg-allergies.htm. Accessed 20 Nov
2017.
8. Greenhawt M, Turner PJ, Kelso JM.
Administration of influenza vaccines to egg-allergic recipients: a
practice parameter update 2017. Ann Allergy Asthma Immunol 2018;120:49-52.
Crossref
9. Committee on Infectious Diseases.
Recommendations for prevention and control of influenza in children,
2017-2018. Pediatrics 2017;140:e20172550. Crossref
10. British Society for Allergy &
Clinical Immunology Paediatric Committee. 2015/16 Influenza vaccine
recommendations for children with egg allergy. Available from:
http://www.bsaci.org/Guidelines/Flu%20jab%20egg%20allergic%20kids.pdf.
Accessed 20 Nov 2017.
11. Dreskin SC, Halsey NA, Kelso JM, et
al. International Consensus (ICON): allergic reactions to vaccines. World
Allergy Organ J 2016;9:32. Crossref
12. United Kingdom government. The Green
Book. 2014. Available from:
https://www.gov.uk/government/collections/immunisation-against-infectious-disease-thegreen-book.
Accessed 20 Nov 2017.
13. Family Health Service. Department of
Health. Hong Kong SAR Government. Hong Kong childhood immunization
programme. Available from:
http://www.fhs.gov.hk/english/main_ser/child_health/child_health_recommend.html.
Accessed 20 Nov 2017.
14. Centers for Disease Control and
Prevention. Vaccine safety—measles, mumps, rubella, and varicella vaccine.
Available from:
https://www.cdc.gov/vaccinesafety/vaccines/mmrv-vaccine.html. Accessed 20
Nov 2017.
15. American Academy of Pediatrics.
Measles. In: Kimberlin DW, Brady MY, Hackson MA, editors. Red Books: 2015
Report of the Committee on Infectious Diseases. 30th ed. Elk Grove
Village, IL: American Academy of Pediatrics; 2015.
16. British Society for Allergy &
Clinical Immunology. BSACI recommendations for combined measles, mumps and
rubella (MMR) vaccination in egg-allergic children. 2007. Available from:
http://www.bsaci.org/guidelines/mmreggrecommendations.pdf. Accessed 20 Nov
2017.