Tumour-induced osteomalacia
Hong Kong Med J 2014 Aug;20(4):350.e1–2
DOI: 10.12809/hkmj133981
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PICTORIAL MEDICINE
Tumour-induced osteomalacia
Anjali A Bhatt, MD1; Suma S Mathews, MS, DLO2; Anusha Kumari, MD3; Thomas V Paul, MD, PhD1
1 Department of Endocrinology, Diabetes and Metabolism, Christian Medical College, Vellore 632 004, India
2 Department of ENT, Christian Medical College, Vellore 632 004, India
3 Department of General Pathology, Christian Medical College, Vellore 632 004, India
Corresponding author: Dr Thomas V Paul (thomasvpaul@yahoo.com)
Tumour-induced osteomalacia (also known as
oncogenic osteomalacia) is an uncommon condition.
The fibroblast growth factor 23 (FGF-23), a
polypeptide secreted by mesenchymal tumours,
causes phosphaturia, which in turn results in
defective mineralisation. In addition, FGF-23 causes suppression of the enzyme 1α-hydroxylase located
in the proximal convoluted tubules of kidneys and
responsible for final activation of vitamin D (from
25-hydroxyvitamin D to 1,25-dihydroxyvitamin D).1
Once the tumour causing the osteomalacia is found,
its excision usually results in complete remission of
the bone disorder.2 Herein we report on a patient
who presented to us with the features of oncogenic
osteomalacia.
A 32-year-old woman born of non-consanguineous
parents complained of gradually
increasing proximal muscle weakness of both lower
limbs over 1 year in February 2012. She also had pain
in both hips while walking, which restricted her daily
activities. She had no history of any chronic gastro-intestinal
illness, and was not taking medication
which might affect bone metabolism. Nor was
there a family history of any similar illness. Clinical
examination revealed severe proximal muscle
weakness in both lower limbs. Active movements
like external rotation and abduction at the both
hips were painful. Otherwise the examination was
unremarkable.
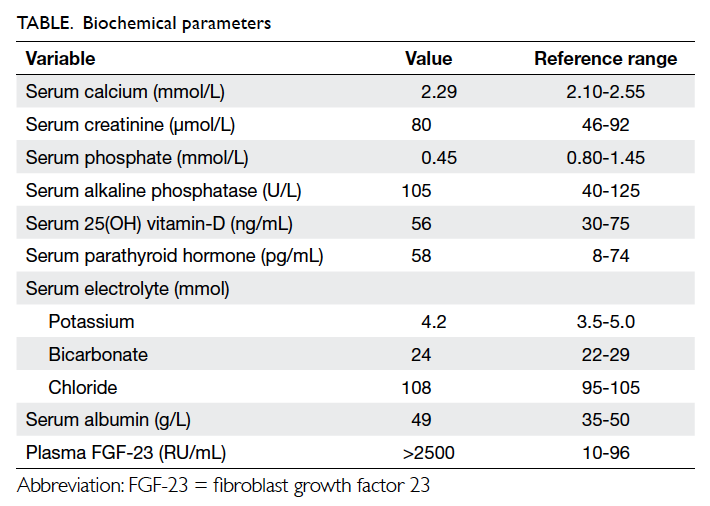
Biochemical evaluation revealed hypophosphataemia
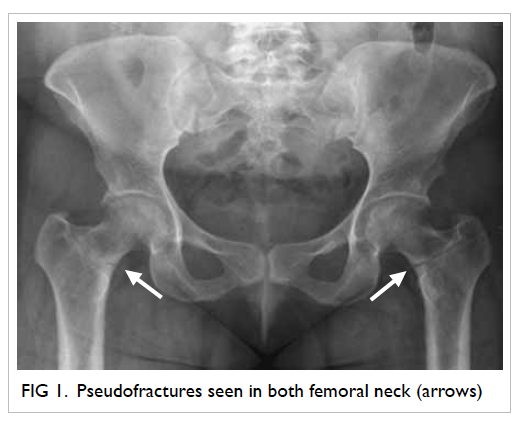
with phosphaturia (Table). Radiology
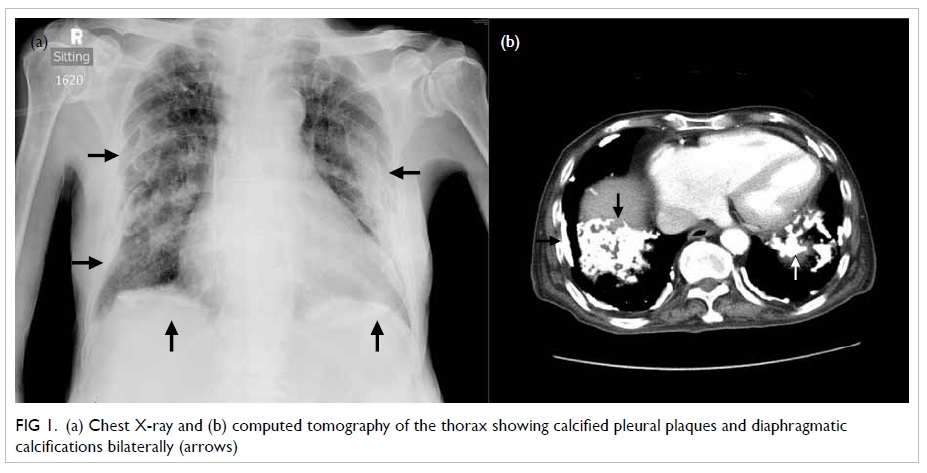
of the hips showed bilateral femoral neck pseudofractures (Fig 1). These abnormalities favoured a
diagnosis of hypophosphataemic osteomalacia.
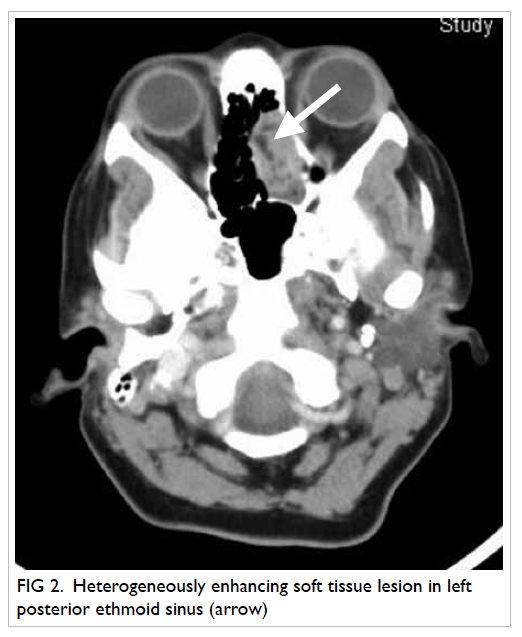
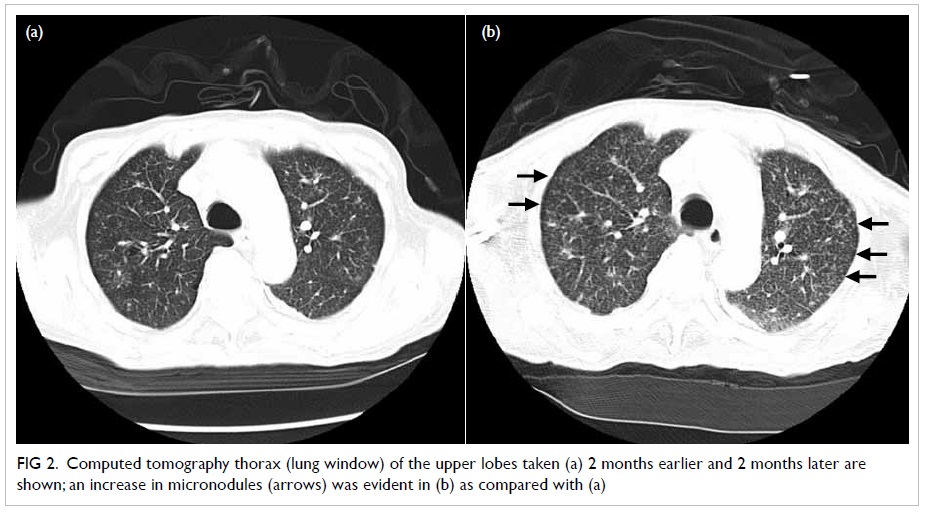
Further workup yielded a high FGF-23 level and
computed tomography of the paranasal sinuses
showed a soft tissue tumour in the left ethmoidal
sinus (Fig 2).
The patient was treated with phosphate
supplements and underwent complete excision of
the left ethmoidal sinus mass. Histopathological
examination confirmed the diagnosis of a phosphaturic mesenchymal tumour. The patient
remained stable after surgery. Two months later
she was asymptomatic, by which time her muscle
weakness had resolved markedly. Respective serial
serum phosphate concentrations were 0.90, 1.00, and
1.35 mmol/L at 1, 2 and 4 weeks after surgery. After
surgery, her plasma FGF-23 levels were undetectable.
Phosphaturic mesenchymal tumours are rare
mesenchymal tumours and mostly comprised of
a single histological entity with mixed connective
tissue (designated PMT-MCT). Such tumours can
occur in the soft tissue or bone.3 Most PMT-MCTs
are histologically and clinically benign with rare
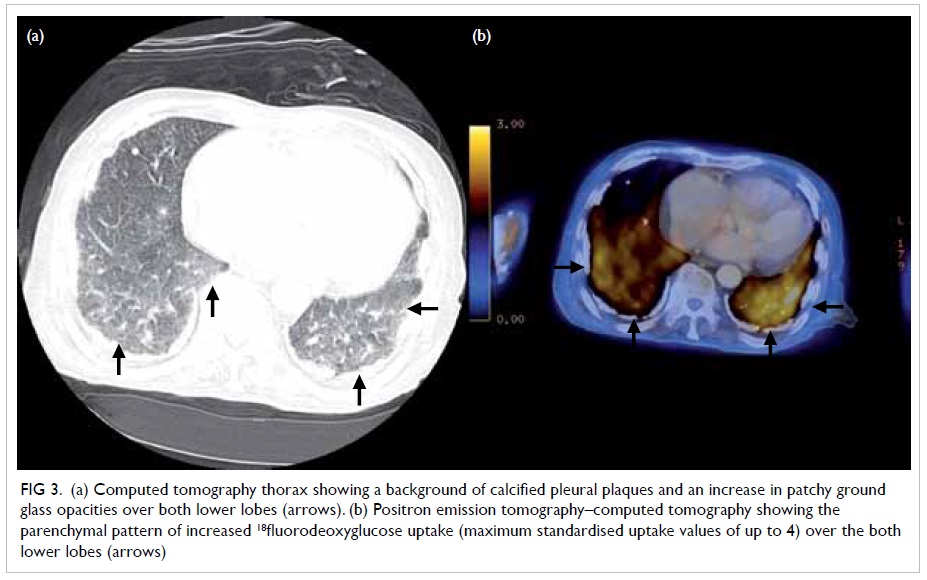
instances of malignancy. Microscopically, PMT-MCTs
are variable in appearance, usually being
composed of small, bland round-to-spindle cells embedded in a vascular to myxochondroid matrix
with variable amounts of mature adipose tissue (Fig
3). This matrix calcifies in an unusual ‘grungy’ fashion,
inciting an osteoclast-rich and fibrohistiocytic
response. A very prominent feature of PMT-MCT is
its elaborate intrinsic microvasculature. Malignant
PMT-MCTs resemble undifferentiated pleomorphic
sarcomas or fibrosarcomas.3 After complete excision
of the tumour, most patients improve dramatically4
and become symptomatically, biochemically, and
radiologically better. Such results should prompt
the physicians to search for such treatable and
potentially curable causes, whenever they encounter
hypophosphataemic osteomalacia.
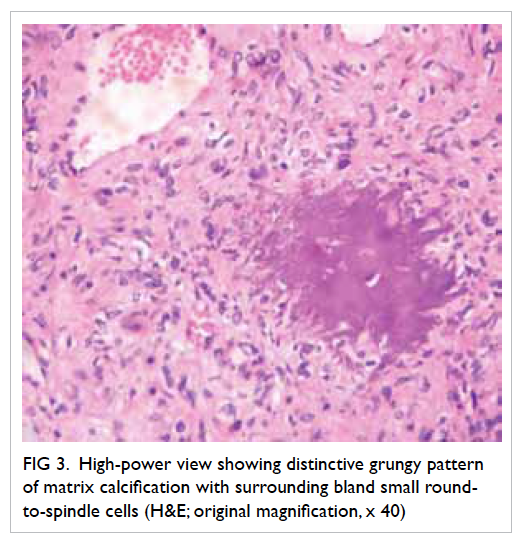
Figure 3. High-power view showing distinctive grungy pattern of matrix calcification with surrounding bland small round-to-spindle cells (H&E; original magnification, x 40)
References
1. Chokyu I, Ishibashi K, Goto T, Ohata K. Oncogenic
osteomalacia associated with mesenchymal tumor in
the middle cranial fossa: a case report. J Med Case Rep
2012;6:181. CrossRef
2. Komínek P, Stárek I, Geierová M, Matoušek P, Zeleník K. Phosphaturic mesenchymal tumour of the sinonasal area:
case report and review of the literature. Head Neck Oncol
2011;3:16. CrossRef
3. Folpe AL, Fanburg-Smith JC, Billings SD, et al. Most
osteomalacia-associated mesenchymal tumors are a
single histopathologic entity: an analysis of 32 cases and a
comprehensive review of the literature. Am J Surg Pathol
2004;28:1-30. CrossRef
4. Chong WH, Molinolo AA, Chen CC, Collins MT. Tumor-induced
osteomalacia. Endocr Relat Cancer 2011;18:R53-77. CrossRef