Hong Kong Med J 2014;20:265.e3–5 | Number 3, June 2014
DOI: 10.12809/hkmj134019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PICTORIAL MEDICINE
Pulmonary tuberculosis complicating asbestosis
YF Shea, MRCP (UK), FHKAM (Medicine)1;
Janice JK Ip, MB, BS, FRCR2
1 Department of Medicine,
Queen Mary Hospital, The University of Hong Kong, Pokfulam, Hong
Kong
2 Department of Radiology,
Queen Mary Hospital, The University of Hong Kong, Pokfulam, Hong
Kong
Corresponding author: Dr YF Shea (elphashea@gmail.com)
An 87-year-old man who previously worked in
shipyard with asbestosis was admitted in November 2012 because of
fever of unknown origin. He presented with fever on-and-off for 2
months and cough. On physical examination, there was no cervical
lymphadenopathy or hepatosplenomegaly and the chest was clear.
Complete blood picture, and liver and renal function tests
remained unremarkable. Chest X-ray (CXR) and computed tomography
(CT) of the thorax yielded calcified pleural plaques,
diaphragmatic calcification, diffuse centrilobular nodules, and
interstitial septal thickening (Fig 1). Sputum and urine cultures were
negative. Further investigations included smears and cultures for
acid-fast bacilli and testing for Mycobacterium tuberculosis
(MTB) by polymerase chain reaction of sputum, urine, and
bronchoalveolar larvage samples, all of which were negative.
Searches for aspergillus antigen, cryptococcal antigen, the
Weil-Felix test, the Widal test, nasopharyngeal aspirate for
influenza and mycoplasma, urine examination for legionella
antigen, automminue profiling, and tests for tumour markers, human
immunodeficiency virus, and sputum cytology were all
non-contributory. The patient’s C-reactive protein was elevated to
3.39 mg/dL (reference range, <0.76 mg/dL). His fever had
persisted on-and-off for 2 months despite multiple courses of
broad-spectrum antibiotics. Positron emission tomography (PET)–CT
yielded pronounced micronodules, especially over both upper lobes
(Fig 1) and patchy ground glass opacities
over both lower lobes with increased 18-fluorodeoxyglucose (18FDG)
uptake (maximum standardised uptake values of up to 4; Figs
2 and 3). The absence of pulmonary masses, mediastinal or hilar
lymphadenopathy or nodular thickening of interlobular septa and
any bronchovascular bundle made malignancy or lymphangitis
carcinomatosis unlikely. Based on the radiology, the patient was
diagnosed to have pulmonary MTB for which anti-MTB treatment was
initiated. He developed sudden cardiac arrest 1 day later, and
failed resuscitation. Sputum and urine sample culture results
available after the patient’s demise grew MTB.
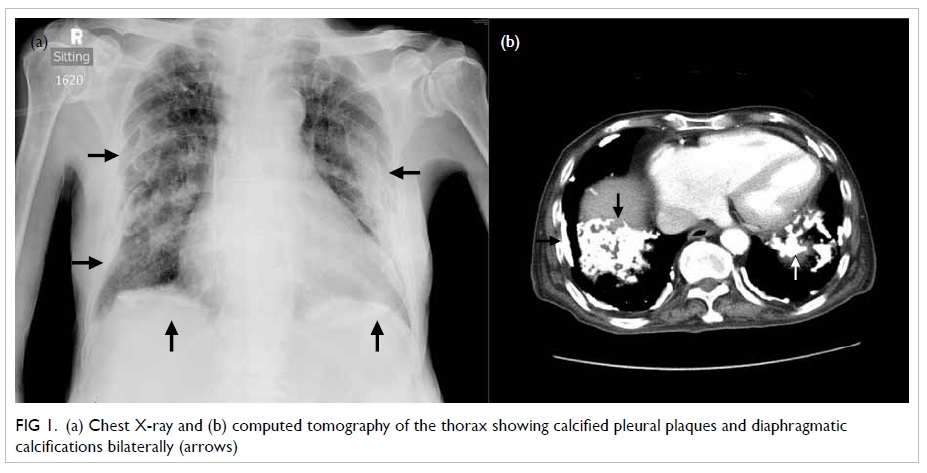
Figure 1. (a) Chest X-ray and (b) computed tomography of the thorax showing calcified pleural plaques and diaphragmatic calcifications bilaterally (arrows)
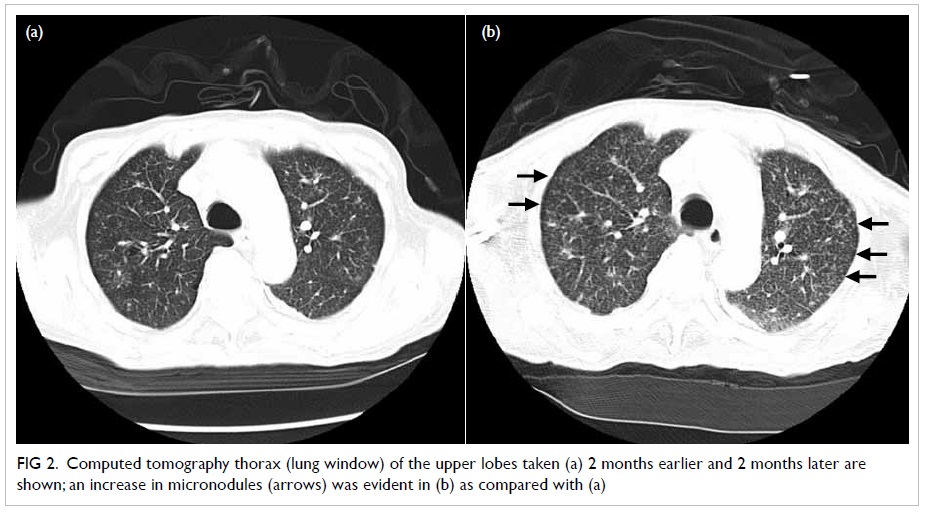
Figure 2. Computed tomography thorax (lung window) of the upper lobes taken (a) 2 months earlier and 2 months later are shown; an increase in micronodules (arrows) was evident in (b) as compared with (a)
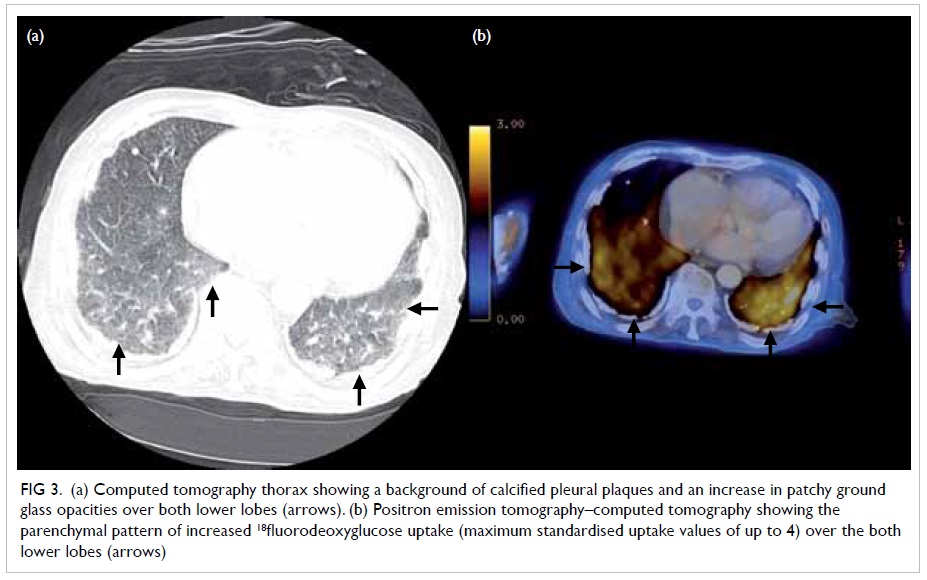
Figure 3. (a) Computed tomography thorax showing a background of calcified pleural plaques and an increase in patchy ground glass opacities over both lower lobes (arrows). (b) Positron emission tomography–computed tomography showing the parenchymal pattern of increased 18fluorodeoxyglucose uptake (maximum standardised uptake values of up to 4) over the both lower lobes (arrows)
Asbestosis is caused by the inhalation of
asbestos which was once used as an electrical and thermal
insulator. Asbestos causes fibrosis of the pleura and lung, as
well as malignant mesothelioma, and PET-CT is a well-known means
of picking up this complication as a linear area of intense 18FDG
uptake surrounding the lungs.1
Fever of unknown origin can be due to infection, malignancy,
autoimmune conditions, or drugs. Tuberculosis was one of the most
common infectious causes. One of the diagnostic difficulties in
our patient was the presence of interstitial lung disease making
the interpretation of CXR and CT images challenging. Soussan et al2 has classified the PET
appearance of pulmonary MTB into lung and lymphatic patterns and
demonstrated improved diagnostic accuracy after taking account of
the other specific CT changes such as upper lobe consolidation
with cavitations or multiple ill-defined micronodules surrounding
a cavity. Findings pertaining to our patient fitted well into
previously described lung pattern of increased 18FDG
uptake in MTB. These included predominant lung parenchymal
involvement and together with an interval excess of micronodules,
especially over the both upper lobes, which led us to make a
radiological diagnosis of MTB. The increased in 18FDG
uptake is caused by local accumulation of inflammatory cells.3 Thus, PET-CT is useful in identifying an active
pulmonary tuberculoma in the absence of initial microbiological
proof. Monitoring the response to anti-MTB treatment can also
provide early evidence of drug-resistant MTB.3 4 The
earlier performance of PET-CT and institution of anti-MTB
treatment may have changed the clinical outcome of our patient.
References
1. Alavi A, Gupta N, Alberini JL,
et al. Positron emission tomography imaging in nonmalignant
thoracic disorders. Semin Nucl Med 2002;32:293-321. CrossRef
2. Soussan M, Brillet PY, Mekinian
A, et al. Patterns of pulmonary tuberculosis on FDG-PET/CT. Eur J
Radiol 2012;81:2872-6. CrossRef
3. Kosterink JG. Positron emission
tomography in the diagnosis and treatment management of
tuberculosis. Curr Pharm Des 2011;17:2875-80. CrossRef
4. Demura Y, Tsuchida T, Uesaka D,
et al. Usefulness of 18F-fluorodeoxyglucose positron emission
tomography for diagnosing disease activity and monitoring
therapeutic response in patients with pulmonary mycobacteriosis.
Eur J Nucl Med Mol Imaging 2009;36:632-9. CrossRef

