EDITORIAL
Mind the gap in kidney care: translating what we know into what we do
Valerie A Luyckx, MD1,2,3 #; Katherine R Tuttle, MD4,5 #; Dina Abdellatif, MD6 †; Ricardo Correa-Rotter, MD7 †; Winston WS Fung, MBBChir (Cantab), FRCP (Lond)8; Agnès Haris, MD, PhD9 †; LL Hsiao, MD2 †; Makram Khalife, BA10 ‡; Latha A Kumaraswami, BA11 †; Fiona Loud, BA10 ‡; Vasundhara Raghavan, BA10 ‡; Stefanos Roumeliotis, MD12; Marianella Sierra, BA10 ‡; Ifeoma Ulasi, MD13 †; Bill Wang, BA10 ‡; SF Lui, MD14 †; Vassilios Liakopoulos, MD, PhD12 †; Alessandro Balducci, MD15 †; for the World Kidney Day Joint Steering Committee
1 Department of Public and Global Health, Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland
2 Renal Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, United States
3 Department of Paediatrics and Child Health, University of Cape Town, Cape Town, South Africa
4 Providence Medical Research Center, Providence Inland Northwest Health, Spokane, United States
5 Nephrology Division, Department of Medicine, University of Washington, Seattle, United States
6 Department of Nephrology, Cairo University Hospital, Cairo, Egypt
7 Department of Nephrology and Mineral Metabolism, National Medical Science and Nutrition Institute Salvador Zubiran, Mexico City, Mexico
8 Department of Medicine and Therapeutics, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong SAR, China
9 Nephrology Department, Péterfy Hospital, Budapest, Hungary
10 International Society of Nephrology Patient Liaison Advisory Group
11 Tamilnad Kidney Research Foundation, Chennai, India
12 2nd Department of Nephrology, AHEPA University Hospital Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece
13 Department of Medicine, College of Medicine, University of Nigeria, Ituku-Ozalla, Enugu, Nigeria
14 Division of Health System, Policy and Management, The Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong, Hong Kong SAR, China
15 Italian Kidney Foundation, Rome, Italy
# Equal contribution
† Members of the World Kidney Day Joint Steering Committee
‡ Patient representatives of the Patient Liaison Advisory Group of the International Society of Nephrology
 Full paper in PDF
Full paper in PDF
Abstract
Historically, it takes an average of 17 years to move
new treatments from clinical evidence to daily
practice. Given the highly effective treatments now
available to prevent or delay kidney disease onset
and progression, this is far too long. The time is
now to narrow the gap between what we know and
what we do. Clear guidelines exist for the prevention
and management of common risk factors for kidney
disease, such as hypertension and diabetes, but only
a fraction of people worldwide with these conditions
are diagnosed, and even fewer receive appropriate
treatment. Similarly, the vast majority of people living
with kidney disease are unaware of their condition, because in the early stages it is often silent. Even among
diagnosed patients, many do not receive appropriate
treatment for kidney disease. Considering the serious
consequences of kidney disease progression, kidney
failure, or death, it is imperative to initiate treatments
early and appropriately. Opportunities to diagnose
and treat kidney disease early must be maximised,
starting at the primary care level. Many systematic
barriers exist, encompassing patient, clinician, health
system, and societal factors. To preserve and improve
kidney health for everyone everywhere, each of these
barriers must be acknowledged so that sustainable
solutions are developed and implemented without
further delay.
At least 1 in 10 people worldwide is living with a
kidney disease.
1 According to the Global Burden of
Disease study, >3.1 million deaths were attributed to
kidney dysfunction in 2019, making it the seventh
leading risk factor for mortality worldwide (
Fig 1 and
online supplementary Fig).
2 However, global mortality from all kidney diseases may actually
range from 5 to 11 million per year if the mortality
rate also includes estimated lives lost from acute
kidney injury and lack of access to renal replacement
therapy for kidney failure (KF), especially in lower-resource
settings.
3 These high global mortality rates reflect disparities in prevention, early detection,
diagnosis, and treatment of chronic kidney disease
(CKD).
4 Mortality rates from CKD are especially
high in some regions, particularly Central Latin
America and Oceania (islands of the South Pacific
Ocean), highlighting the need for urgent action.
5
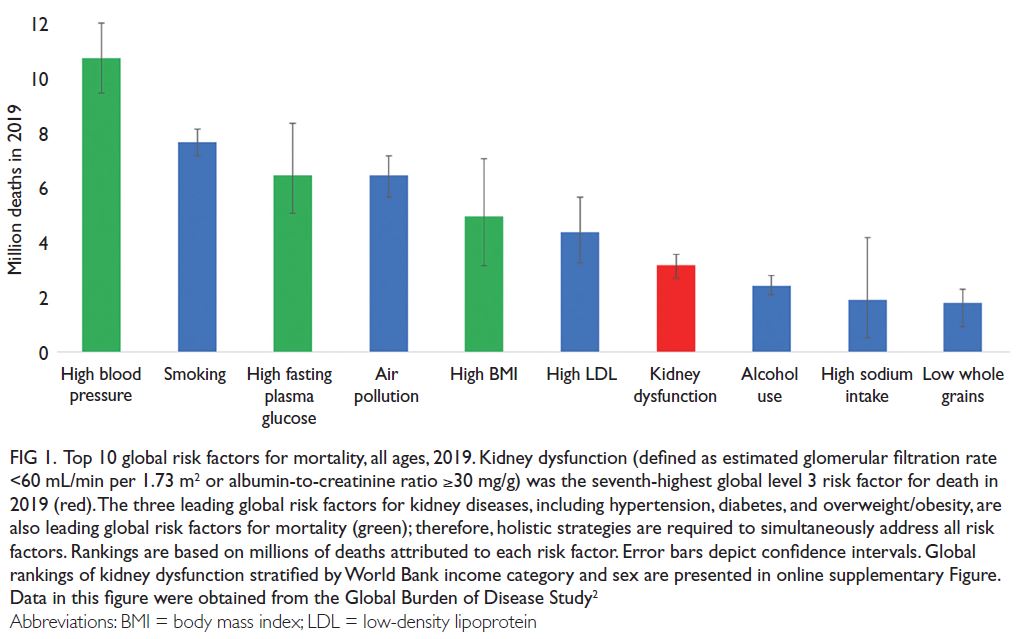 Figure 1.
Figure 1. Top 10 global risk factors for mortality, all ages, 2019. Kidney dysfunction (defined as estimated glomerular filtration rate <60 mL/min per 1.73 m
2 or albumin-to-creatinine ratio ≥30 mg/g) was the seventh-highest global level 3 risk factor for death in
2019 (red). The three leading global risk factors for kidney diseases, including hypertension, diabetes, and overweight/obesity, are
also leading global risk factors for mortality (green); therefore, holistic strategies are required to simultaneously address all risk
factors. Rankings are based on millions of deaths attributed to each risk factor. Error bars depict confidence intervals. Global
rankings of kidney dysfunction stratified by World Bank income category and sex are presented in online supplementary Figure.
Data in this figure were obtained from the Global Burden of Disease Study
2
Chronic kidney disease also represents
a substantial global economic burden, with
exponentially increasing costs as CKD progresses,
due to the costs of dialysis and transplantation, as
well as multiple co-morbidities and complications
that accumulate over time.
6 7 In the United
States, Medicare fee-for-service spending for all
beneficiaries with CKD was US$86.1 billion in 2021
(22.6% of total expenditures).
8 Data from many
lower-resource settings, where most healthcare
spending comprises out-of-pocket costs, are absent.
A recent study in Vietnam showed that the perpatient
cost of CKD was higher than the gross
domestic product per capita.
7 In Australia, it has
been estimated that early diagnosis and prevention
of CKD could save the health system AU$10.2 billion
over 20 years.
9
Although there is regional variation in the
causes of CKD, the risk factors with the highest
population-attributable factors for age-standardised
CKD-related disease-adjusted life years are
hypertension (51.4%), a high fasting plasma glucose
level (30.9%), and a high body mass index (26.5%).
10
These risk factors are also leading risk factors for
mortality worldwide (
Fig 1). Only 40% and 60% of people with hypertension and diabetes, respectively,
are aware of their diagnosis; considerably smaller
proportions of these individuals are receiving
treatment and reaching therapeutic targets.
11 12
Moreover, at least 1 in 5 people with hypertension
and 1 in 3 people with diabetes also have CKD.
13
Most cases of CKD can be prevented through
healthy lifestyles, prevention and management
of risk factors, avoidance of acute kidney injury,
optimisation of maternal and child health, mitigation
of climate change, and efforts to address social and
structural determinants of health.
3 Nevertheless,
the benefits of some of these measures may only
be evident in future generations. Until then, early
diagnosis and risk stratification create opportunities
to introduce therapies that can slow, halt, or even
reverse CKD.
14 It is concerning that CKD awareness
is particularly low among individuals with kidney
dysfunction, such that approximately 80% to 95%
of such patients worldwide are unaware of their
diagnosis (
Fig 2).
15 16 17 18 19 20 Therefore, people are dying
because of missed opportunities to detect CKD early
and deliver optimal care.
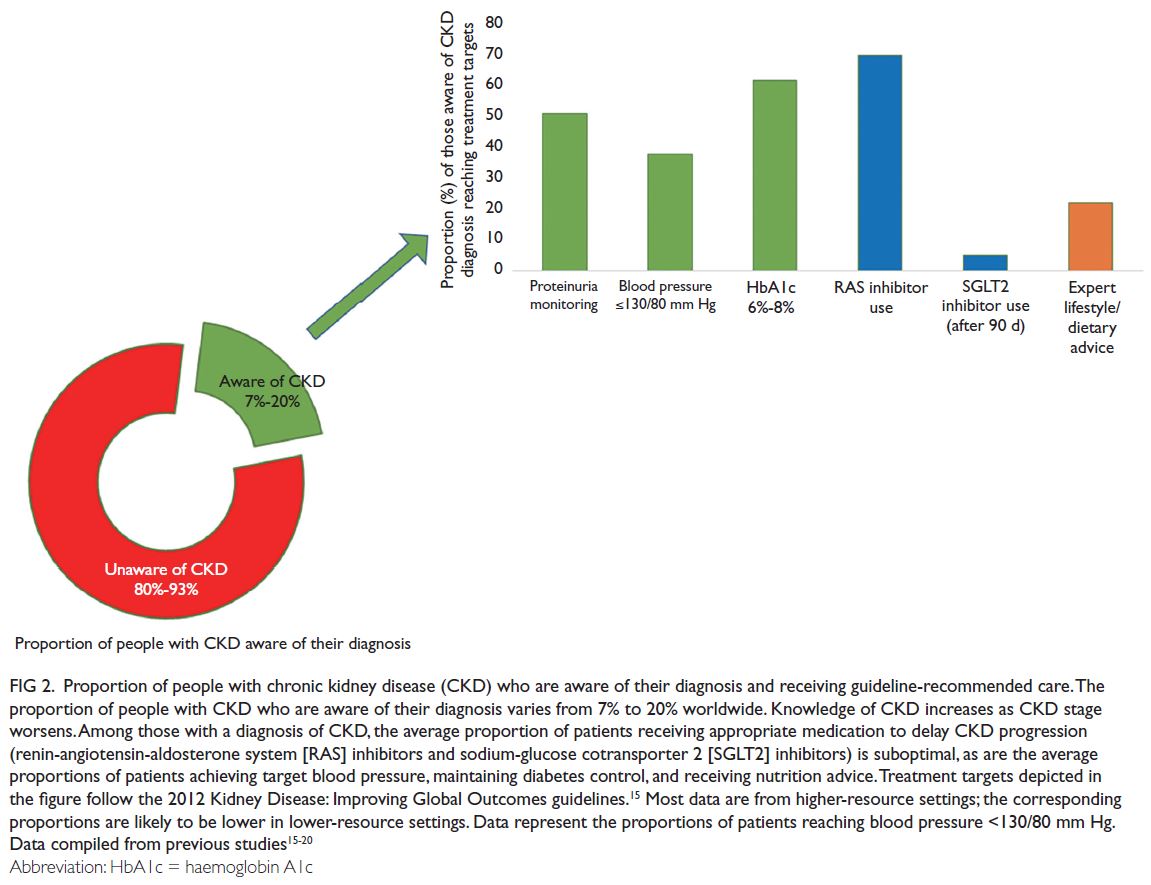 Figure 2.
Figure 2. Proportion of people with chronic kidney disease (CKD) who are aware of their diagnosis and receiving guideline-recommended care. The
proportion of people with CKD who are aware of their diagnosis varies from 7% to 20% worldwide. Knowledge of CKD increases as CKD stage
worsens. Among those with a diagnosis of CKD, the average proportion of patients receiving appropriate medication to delay CKD progression
(renin-angiotensin-aldosterone system [RAS] inhibitors and sodium-glucose cotransporter 2 [SGLT2] inhibitors) is suboptimal, as are the average
proportions of patients achieving target blood pressure, maintaining diabetes control, and receiving nutrition advice. Treatment targets depicted in the figure follow the 2012 Kidney Disease: Improving Global Outcomes guidelines.
15 Most data are from higher-resource settings; the corresponding proportions are likely to be lower in lower-resource settings. Data represent the proportions of patients reaching blood pressure <130/80 mm Hg. Data compiled from previous studies
15 16 17 18 19 20
Furthermore, CKD is a major risk factor
for cardiovascular disease; during kidney disease
progression, cardiovascular death and KF become
competing risks.
21 Indeed, data from the 2019
Global Burden of Disease Study showed that more
deaths were caused by kidney dysfunction–related
cardiovascular disease (1.7 million deaths) than
by CKD itself (1.4 million deaths).
2 Therefore, cardiovascular disease management should also be prioritised for people with CKD.
Gaps between knowledge and implementation in kidney care
Strategies to prevent and treat CKD have been
established on the basis of strong evidence collected
over the past three decades (
Fig 3).
19 22 Clinical
practice guidelines for CKD are clear; however,
adherence to these guidelines is suboptimal (
Fig 2).
15 19 20
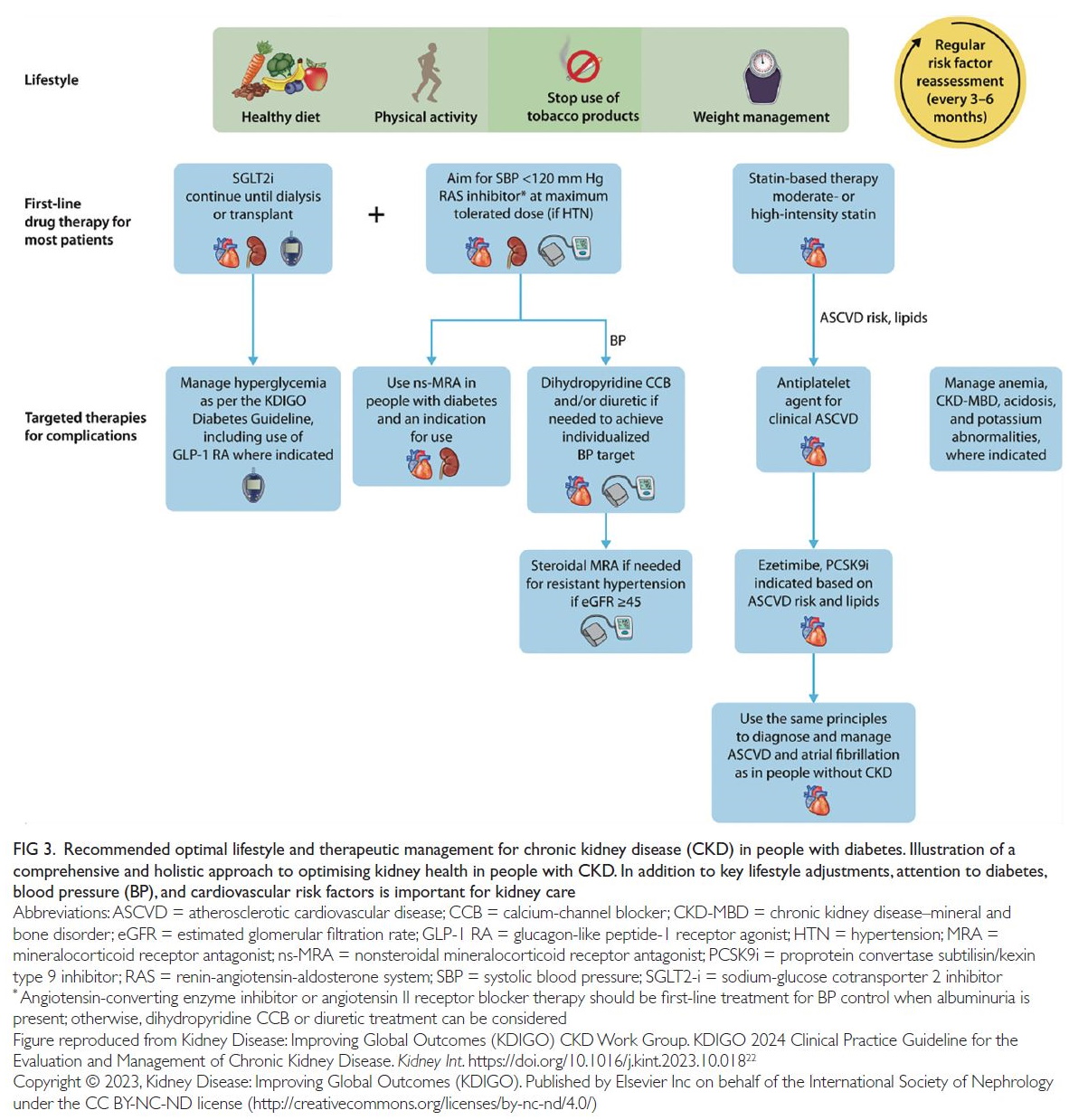 Figure 3.
Figure 3. Recommended optimal lifestyle and therapeutic management for chronic kidney disease (CKD) in people with diabetes. Illustration of a
comprehensive and holistic approach to optimising kidney health in people with CKD. In addition to key lifestyle adjustments, attention to diabetes,
blood pressure (BP), and cardiovascular risk factors is important for kidney care
Regardless of aetiology, management of major
risk factors, particularly diabetes and hypertension,
forms the basis of optimal care for people with
CKD.
19 23 In addition to lifestyle changes and risk
factor control, the earliest pharmacological agents to demonstrate kidney protection were renin-angiotensin-aldosterone system inhibitors in the
form of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers.
14 19
Despite decades of knowledge that these medications
have substantial protective effects on renal and
cardiovascular function in people with CKD, real-world
data from electronic health records show that
their use remains low (
Fig 2). For example, in the
United States, ACEI and angiotensin receptor blocker
utilisation rates ranging from 20% to 40% were
reported ≥15 years after the most recent approval
of these agents for patients with CKD and/or type
2 diabetes.
24 Although more recent data show that
prescribing rates in this population have improved
to 70%, only 40% of such patients continue taking
an ACEI or angiotensin receptor blocker for at least
90 days.
20 These data indicate gaps in prescribing
nephroprotective medication and continuity of care
over time, potentially related to cost, lack of patient
education, polypharmacy, and adverse effects.
25
Although the initial enthusiasm for sodium-glucose
cotransporter 2 (SGLT2) inhibitors focused on their benefits for diabetes and cardiovascular
disease, unprecedented therapeutic benefits have
also been observed regarding CKD. Relative risk
reduction levels with SGLT2 inhibitors approach
40% for substantial decreases in estimated
glomerular filtration rate, KF, and mortality among
populations with several types of CKD, heart failure,
or elevated cardiovascular disease risk.
26 27 These decreases were observed in addition to benefits from
standard-of-care risk factor management and renin-angiotensin-aldosterone system inhibitors. The risks
of heart failure, cardiovascular death, and all-cause
mortality were also reduced in patients with CKD
during SGLT2 inhibitor treatment.
26 The addition of
SGLT2 inhibitors to renin-angiotensin-aldosterone
system inhibitor–based treatment was able to delay the need for renal replacement therapy by several
years, depending on the initial timing of combined
treatment.
28 Moreover, for every 1000 patients with
CKD who received an SGLT2 inhibitor in addition to
standard therapy, 83 deaths, 19 heart failure–related
hospitalisations, 51 instances of dialysis initiation,
and 39 episodes of acute renal function worsening
were prevented.
29
The persistent underuse of these and other
guideline-recommended therapies involving SGLT2
inhibitors is concerning (
Fig 2).
20 24 In the CURE-CKD
(Center for Kidney Disease Research, Education and
Hope–CKD) Registry, only 5% and 6.3% of eligible
patients with CKD and diabetes, respectively,
continued to receive SGLT2 inhibitor and glucagon-like
peptide-1 receptor agonist therapy at 90 days.
18
Notably, a lack of commercial health insurance and
the receipt of treatment in community-based (versus
academic) institutions were associated with lower
likelihoods of SGLT2 inhibitor, ACEI, or angiotensin
receptor blocker prescriptions among patients with
CKD and diabetes.
20 In low- or middle-income
countries (LMICs), the gap between evidence and
implementation is even wider, considering the
high cost and inconsistent availability of these
medications, although generics are available.
30
Such gaps in delivering optimal care for CKD are
unacceptable.
In addition to SGLT2 inhibitors, nonsteroidal
mineralocorticoid receptor antagonists have been
demonstrated to reduce the risks of CKD progression,
KF, cardiovascular events, and mortality, when used
in addition to standard-of-care treatment involving
renin-angiotensin-aldosterone system inhibitors,
among people with type 2 diabetes.
31 There is a
growing portfolio of promising therapeutic options,
including glucagon-like peptide-1 receptor agonists
(NCT03819153, NCT04865770), aldosterone
synthase inhibitors (NCT05182840), and dual-to-triple
incretins (
online supplementary Table 1).
26 32
Furthermore, there is clear evidence that in patients
with CKD and/or type 2 diabetes, glucagon-like
peptide-1 receptor agonists reduce cardiovascular
events, constitute safe and effective glucose-lowering
therapies, and aid in weight loss.
32
Historically, it has taken an average of 17 years
for new treatments to move from clinical evidence
to routine practice.
33 Considering that millions of
people with CKD die each year, this waiting period
is far too long.
Closing the ‘gap’ between what we know and what we do
Lack of policy and presence of global inequity
Health policy
Since the launch of the World Health Organization Global Action Plan for the prevention and control of non-communicable diseases (NCDs) in 2013, there has
been global progress in the proportion of countries
with a national NCD action plan and dedicated NCD
units.
34 However, CKD is incorporated into NCD
strategies in approximately one-half of countries.
4
Policies are required to integrate kidney care within
essential health packages under universal health
coverage (
Fig 4).
30 Multisectoral policies must also
address social determinants of health, which are
major amplifiers of CKD risk and severity that limit
people’s opportunities to improve their health.
3 A
lack of investment in kidney health promotion, along
with primary and secondary prevention of kidney
diseases, hinders progress.
14
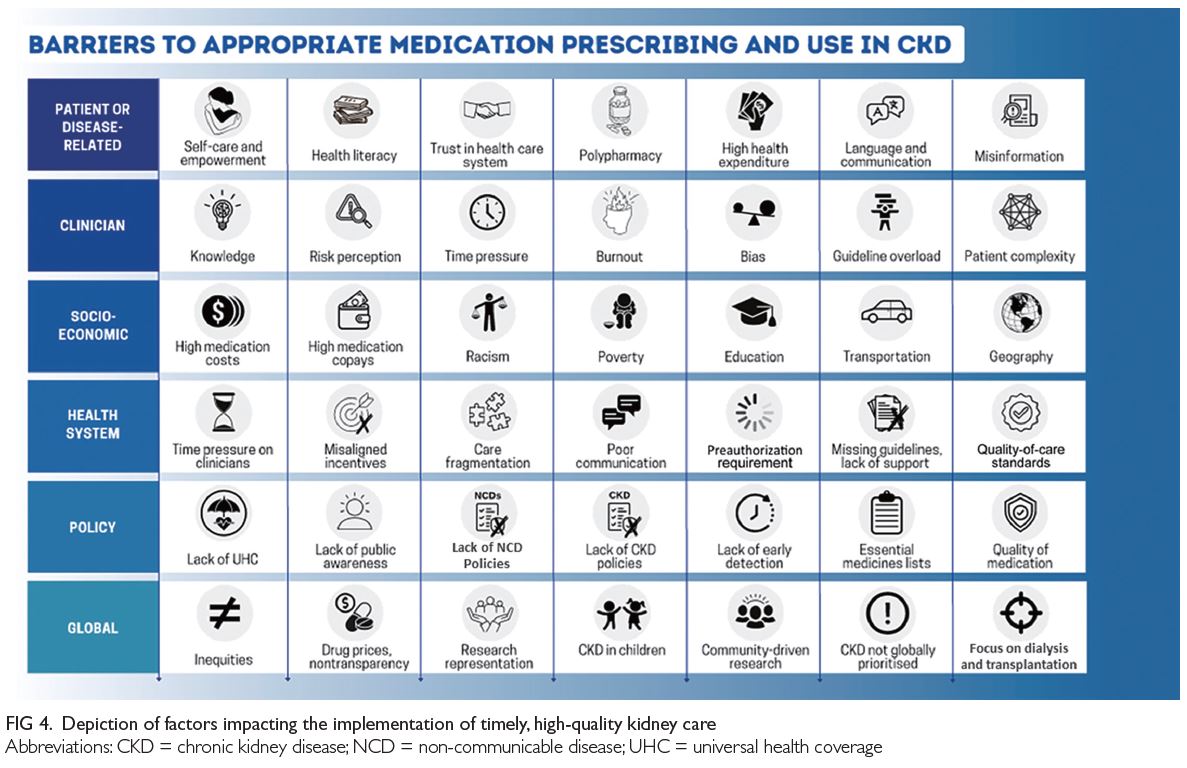 Figure 4.
Figure 4. Depiction of factors impacting the implementation of timely, high-quality kidney care
Health systems
Two major goals of universal health coverage are to
achieve coverage for essential health services and to
reduce financial hardship imposed by healthcare.
However, universal health coverage alone is
insufficient to ensure adequate access to kidney care.
3
Health systems must be strengthened, and quality
of care must be prioritised, because poor quality
care contributes to more deaths than lack of access
in low-resource settings.
35 Quality care requires a
well-trained healthcare workforce, sustainable
availability of accurate diagnostics, reliable
infrastructure, and medication supplies; it should
be monitored through a continuous quality
improvement process (
Fig 4). Medication quality,
especially in LMICs, may be an additional barrier
to successful management of CKD.
36 Regulation
and monitoring of drug manufacturing and quality
standards are important to ensure safe and effective
therapies. Strategies to support regulation and
quality assurance should be developed according
to local circumstances and guidelines, as outlined
elsewhere.
37
The establishment of a credible case for CKD
detection and management based on real-world data
regarding risks, interventions, outcomes, and costs
will help translate theoretical cost-effectiveness
(currently established primarily in high-income
countries with minimal data from other countries)
into economic reality.
30 38 Screening should include
evaluation of risk factors for CKD; identification of
family history; recognition of potential symptoms
(usually advanced, such as fatigue, poor appetite,
oedema, and itching); and measurements of blood
pressure, serum creatinine, urine components (ie,
urinalysis), and urine albumin/protein to creatinine
ratio, as outlined in established guidelines.
19 39 Early
identification of CKD in primary care is expected to
lower costs over time by reducing CKD complications
and KF. Medications required for kidney care are
already included in the World Health Organization
Model List of Essential Medicines (
Table 1). These
medications should be provided at the national level under universal health coverage.
40 Additionally, pharmaceutical companies should provide these medications at affordable prices.
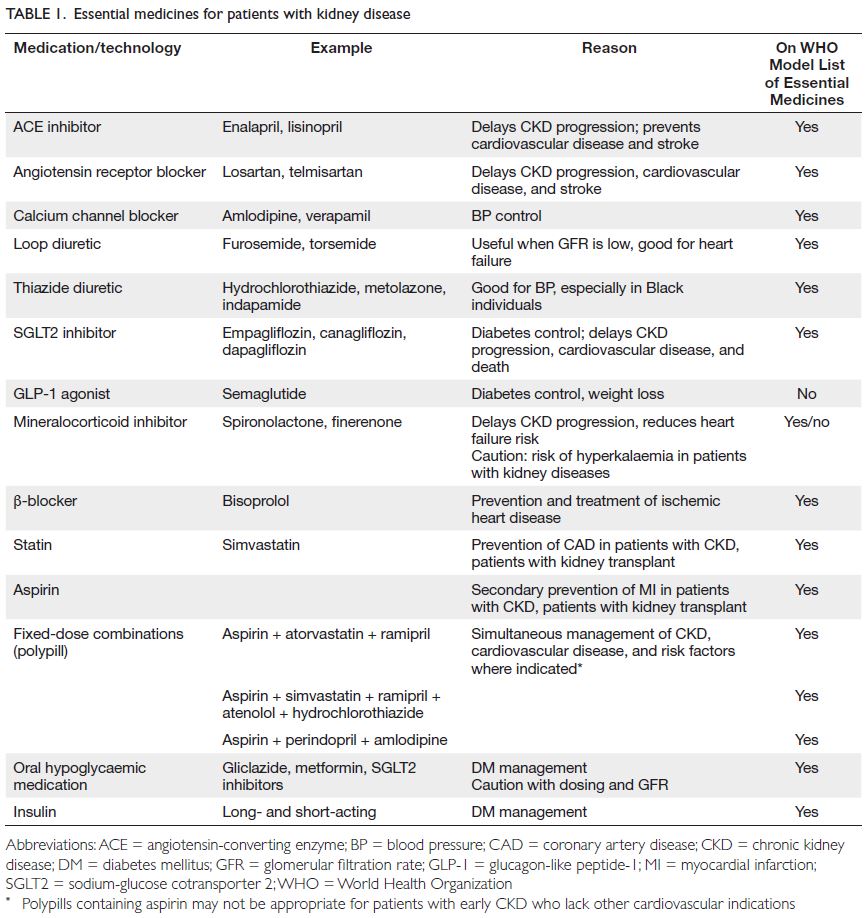 Table 1.
Table 1. Essential medicines for patients with kidney disease
Challenges in primary care and clinical inertia
Healthcare professionals
The shortage of primary care professionals is
exacerbated by inconsistent access to specialists
and allied health professionals in both high-income
countries and LMICs. It is essential to define roles
and responsibilities for kidney care. Solutions
may include multidisciplinary team care (primary
care physicians, pharmacists, specialists, nurses,
therapists, educators, nutritionists, and mental
health professionals), well-established mechanisms
for collaboration among all elements, and rapid
communication technologies (both within health
systems and among health professionals) to support
care and decision-making.
41 42 Brain drain in low-resource
settings is a complex issue that must be
addressed.
The mobilisation of community health workers
yields cost-savings in infectious disease programmes within LMICs; it may facilitate early detection,
diagnosis, and management of NCDs.
43 Protocolised
CKD management, possibly assisted by electronic
decision-support systems, may be appropriate for
interventions at the community level, with the
integration of primary care physicians and aid
from nephrologists and other professionals.
44 45 For
example, in some settings, pharmacists could identify
people with diabetes or hypertension exhibiting CKD
risk, based on their prescriptions, then offer on-site
testing and referral as needed.
46 Pharmacists could
also provide medication reconciliation and advice
regarding safety, effectiveness, and adherence. Social
workers and pharmacists can help patients with
medications to access suitable programmes.
46
Clinical inertia
Clinical ‘inertia’, commonly regarded as a causative
factor in low prescribing rates, has many facets (
Fig 4).
47 Many knowledge gaps regarding CKD exist among primary care physicians.
48 Such gaps can
be remedied with focused public and professional
education. Additional factors include fear of adverse
effects from medication, misaligned incentives within the health system, excessive workload, formulary
restrictions, and clinician burnout.
47 Furthermore,
inconsistent recommendations in guidelines from
different professional organisations may enhance
confusion. A major barrier to optimal care is the
time constraints imposed on individual clinicians. A
typical primary care physician in the United States
would require approximately 26.7 hours per day
to implement guideline-recommended care for a
panel of 2500 patients.
49 Innovation is required to
support guideline implementation, especially for
primary care physicians who must follow multiple
guidelines to meet the diverse needs of their patients.
Electronic health records, reminders, team-based nudges, and decision-support tools offer potentially
valuable assistance for quality kidney care in busy
clinical practices.
50 However, the additional time
and effort involved in negotiating pre-authorisations
or completing medication assistance programme
requests, as well as the need for frequent monitoring
of multiple medications, also hinder appropriate
prescribing.
25 Many primary care physicians only
have a few minutes allocated for each patient because
of institutional pressure or patient volume. The term
‘inertia’ can hardly be applied to clinicians working
at this pace. The number of health professionals
worldwide must increase.
Visits for patients with CKD are complex because multimorbidity is common. Such patients are often managed by multiple specialists, leading
to fragmentation of care, lack of holistic oversight,
and diffusion of responsibility for treatment. Single
and combined outcomes analyses have shown that
multidisciplinary care improves transition to renal
replacement therapy and reduces mortality.
51 Novel
‘combined clinic’ models with on-site collaboration
and joint participation (eg, with nephrologists,
cardiologists, and endocrinologists) may provide
substantial benefits for patients in terms of reduced
fragmentation of care, logistics, and cost savings.
Patient centeredness
Health literacy
Self-management is the most important aspect of
kidney care. A patient’s ability to understand his/her health needs, make healthy choices, feel safe
and respected in the health system, and obtain
psychosocial support are important for promoting
health decision-making (
Fig 4). Good communication
should begin with quality information and
confirmation of ‘understanding’ by the patient (and
family members, as applicable). Electronic apps and
reminders can serve as useful tools that support
patients by improving disease knowledge, promoting
patient empowerment, and improving self-efficacy;
however, a one-size-fits-all approach is unlikely to
be successful.
52 Important barriers include a lack of
patient health information, poor communication,
and mistrust, especially in the marginalised and
minoritised communities where CKD is common.
30
Patients may also be confused by contradictory
recommendations among healthcare professionals,
as well as conflicting messages in mainstream media.
Innovative platforms that improve CKD-related
communication between patients and clinicians
represent a promising approach to promote optimal
prescribing and adherence.
53 54
Patient perspectives are essential when
designing and testing health strategies to overcome
barriers and promote equity. Collaborative care
models must include patients, families, and
community groups, as well as various types
of healthcare professionals, health systems,
government agencies, and payers.
38 Advocacy organisations, local community groups, and peer
navigators with trusted voices and relationships
can serve as conduits for education while providing
input regarding the development of patient tools and
outreach programmes.
55 Most importantly, patients
must be the focus of their own care.
Medication cost and availability
In high-income countries, people without health insurance and people with high copays paradoxically
pay the highest amounts for essential and non-essential nonessential
medications.
38 Across LMICs, kidney
diseases represent the leading cause of catastrophic
health expenditures due to reliance on out-of-pocket
payments.
56 In 18 countries, four cardiovascular
disease medications frequently indicated in CKD
(statins, ACEIs, aspirin, and β-blockers) had greater
availability in private settings than in public settings;
they were mostly unavailable in rural communities,
and they were unaffordable for 25% of people in
upper middle-income countries and 60% of people
in low-income countries.
57 Newer therapies may be
prohibitively expensive worldwide, especially where
generics are not yet available. In the United States,
the retail price for a 1-month supply of an SGLT2
inhibitor or finerenone is approximately US$500
to $700; for glucagon-like peptide-1 receptor
agonists, the retail price is approximately US$800 to
$1200 per month.
38 This reliance on out-of-pocket
payments for vital, life-saving essential medications
is unacceptable (
Fig 4).
Special considerations
Not all kidney diseases are equal. Much of what
has been discussed here applies to the most
common forms of CKD (eg, diabetes-related
and hypertension-related). Some incompletely
understood forms of CKD have different risk
profiles, including environmental exposures, genetic
predisposition, and autoimmune or other systemic
disorders. Highly specialised therapies may be
required. Pharmaceutical companies should be
responsible for ensuring that research studies include
disease-representative participants with appropriate
representation (eg, race, ethnicity, sex, and gender),
that effective drugs are made available after studies,
and that the balance between profits and prices
is fair and transparent. Many novel therapies are
offering new hope for various kidney diseases; once
these therapies are approved, there must be no delay
in extending benefits to all affected patients (
online supplementary Table 1).
An important but often overlooked group
consists of children with kidney diseases. This group
is especially vulnerable in LMICs, where nephrology
services and resources are limited; families must
often decide whether to pay for one child’s treatment
or support the rest of the family.
58 Children with
CKD also have a high risk of cardiovascular disease,
even in high-income settings, and they require
more attention to control risk factors and achieve
treatment targets.
59
Fostering innovation
Implementation science and knowledge translation
Considering that rigorous evidence-based treatments
for CKD have been established, implementation must be optimised.
60 Implementation research aims
to identify effective solutions by understanding
how evidence-based practices, often developed
in high-income countries, can be integrated into
care pathways in lower-resource settings. The
management of CKD is suitable for implementation
research: optimal therapeutic strategies are known,
outcomes are easily measurable, and essential
diagnostics and medications already are in place.
Crucial components of such research are the
identification of local patient preferences and
elucidation of challenges. Ministries of health should
commit to overcoming identified barriers and scaling
up successful and sustainable programmes.
Polypills as an example of simple innovation
Polypills are attractive on multiple levels: fixed doses
of several guideline-recommended medications are
present within a single tablet (
Table 1), the price is
low, the pill burden is reduced, and the regimen is
simple.
61 Polypills can prevent cardiovascular disease
and are cost-effective for patients with CKD.
62 More
studies are needed, but considering the alternatives
of costly renal replacement therapy or premature
death, it is likely that polypills will be a cost-effective
approach for reducing CKD progression.
Harnessing digital technologies
The integration of telehealth and other types of
remotely delivered care can improve efficiency
and reduce costs.
63 Electronic health records and
registries can support monitoring of quality of care
and identify gaps to guide implementation and
improve outcomes within an evolving health system
that is capable of learning. Artificial intelligence
may also be harnessed to stratify risk, personalise
medication prescribing, and facilitate adherence.
64
The use of telenephrology for communication
between primary care physicians and specialists may
also be beneficial for patient care.
65
Patient perspectives
Multiple methods can support the identification
of patient preferences for CKD care, including
interviews, focus groups, surveys, discrete
choice experiments, structured tools, and simple
conversations.
66 67 Many of these methods are
currently in the research phase. Clinical translation
will require contextualisation and assessments of
local and individual acceptability.
The journey of each person living with CKD is
unique; however, common challenges and barriers
exist. As examples of lived experiences, comments
collected from patients about their medications and
care are detailed in
online supplementary Table 2.
These voices must be heard and acknowledged to
close gaps and improve the quality of kidney care
worldwide.
Call to action
The current lack of progress in kidney care has been
tolerated for far too long. New therapeutic advances
offer real hope that many people with CKD can
survive without developing KF. The evidence for
clinical benefit is overwhelming and unequivocal.
These patients cannot wait another 17 years for this
evidence to be translated into clinical practice.
33 It
is time to ensure that all who are eligible for CKD
treatment receive this care in an equitable manner.
Known barriers and global disparities in
access to diagnosis and treatment must be urgently
addressed (
Fig 4). To achieve health equity for
people with kidney diseases and those at risk of
developing kidney diseases, we must raise awareness
among policy makers, patients, and the general
population; harness innovative strategies to support
all cadres of healthcare workers; and balance profits
with reasonable prices (
Table 2). If we narrow the
gap between what we know and what we do, kidney
health will become a reality worldwide.
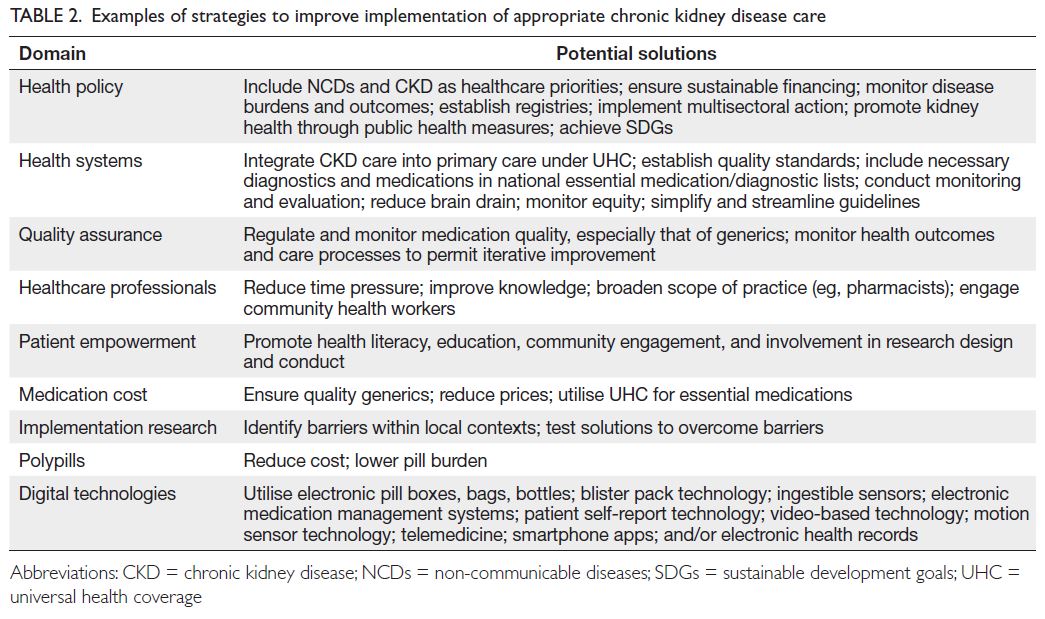 Table 2.
Table 2. Examples of strategies to improve implementation of appropriate chronic kidney disease care
Author contributions
All authors contributed equally to the conception, preparation, and drafting of the manuscript.
Conflicts of interest
VA Luyckx is chair of the Advocacy Working Group of the
International Society of Nephrology and has no financial
disclosures. KR Tuttle has received research grants from the
National Institutes of Health (National Institute of Diabetes
and Digestive and Kidney Diseases, National Heart, Lung, and
Blood Institute, National Center for Advancing Translational
Sciences, National Institute on Minority Health and Health
Disparities, director’s office), the United States Centers for
Disease Control and Prevention, and Travere Therapeutics;
and consultancy fees from AstraZeneca, Bayer, Boehringer
Ingelheim, Eli Lilly, and Novo Nordisk. She is also chair of
the Diabetic Kidney Disease Collaborative for the American
Society of Nephrology. R Correa-Rotter is a member of the
Steering Committee for World Kidney Day, a member of
the Diabetes Committee of the Latin American Society of
Nephrology and Hypertension, and a member of the Latin
American Regional Board of the International Society of
Nephrology. He is also a member of the Steering Committees
for the Dapagliflozin and Prevention of Adverse Outcomes in
Chronic Kidney Disease (DAPA-CKD) trial (AstraZeneca),
the Study Of diabetic Nephropathy with AtRasentan (SONAR)
[AbbVie], A Non-interventional Study Providing Insights Into
the Use of Finerenone in a Routine Clinical Setting (FINE-REAL)
[Bayer], and CKD-ASI (Boehringer Ingelheim). He has
received research grants from AstraZeneca, GlaxoSmithKline,
Roche, Boehringer Ingelheim, and Novo Nordisk, as well
as speaker honoraria from AstraZeneca, Bayer, Boehringer
Ingelheim, and Amgen. All other authors have declared no
competing interests.
Funding/support
This editorial received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration
This article was published in Kidney International (Luyckx VA, Tuttle KR, Abdellatif D, et al. Mind the gap in kidney care: translating what we know into what we do. Kidney Int
2024;105:406-17.
https://doi.org/10.1016/j.kint.2023.12.003), and was reprinted concurrently in several journals. The articles cover identical concepts and wording, but vary in
minor stylistic and spelling changes, detail, and length of
manuscript in keeping with each journal’s style. Any of these
versions may be used in citing this article.
Supplementary material
The supplementary material was provided by the authors
and some information may not have been peer-reviewed.
Accepted supplementary material will be published as
submitted by the authors, without any editing or formatting.
Any opinions or recommendations discussed are solely
those of the author(s) and are not endorsed by the Hong
Kong Academy of Medicine and the Hong Kong Medical
Association. The Hong Kong Academy of Medicine and the
Hong Kong Medical Association disclaim all liability and
responsibility arising from any reliance placed on the content.
References
1. Jager KJ, Kovesdy C, Langham R, Rosenberg M,
Jha V, Zoccali C. A single number for advocacy and
communication-worldwide more than 850 million
individuals have kidney diseases. Kidney Int 2019;96:1048-50.
Crossref2. Institute for Health Metrics and Evaluation (IHME). GBD
compare data visualization. Available from:
http://vizhub.healthdata.org/gbd-compare. Accessed 18 Nov 2023.
3. Luyckx VA, Tonelli M, Stanifer JW. The global burden of
kidney disease and the sustainable development goals. Bull
World Health Organ 2018;96:414-22D.
Crossref4. International Society of Nephrology. ISN Global Kidney Health Atlas, 3rd ed. Available from:
https://www.theisn.org/initiatives/global-kidney-health-atlas/. Accessed 18 Nov 2023.
5. GBD Chronic Kidney Disease Collaboration. Global,
regional, and national burden of chronic kidney disease,
1990-2017: a systematic analysis for the Global Burden of
Disease Study 2017. Lancet 2020;395:709-33.
Crossref6. Vanholder R, Annemans L, Brown E, et al. Reducing the
costs of chronic kidney disease while delivering quality
health care: a call to action. Nat Rev Nephrol 2017;13:393-409.
Crossref7. Nguyen-Thi HY, Le-Phuoc TN, Tri Phat N, et al. The
economic burden of chronic kidney disease in Vietnam.
Health Serv Insights 2021;14:11786329211036011.
Crossref8. United States Renal Data System. Healthcare expenditures
for persons with CKD. Available from:
https://usrds-adr.niddk.nih.gov/2023/chronic-kidney-disease/6-healthcare-expenditures-
for-persons-with-ckd. Accessed 18 Nov 2023.
9. Kidney Health Australia. Changing the chronic kidney
disease landscape: the economic benefits of early detection
and treatment. Available from:
https://kidney.org.au/uploads/resources/Changing-the-CKD-landscape-Economic-benefits-of-early-detection-and-treatment.pdf. Accessed 16 Jan 2024.
10. Ke C, Liang J, Liu M, Liu S, Wang C. Burden of chronic
kidney disease and its risk-attributable burden in 137
low-and middle-income countries, 1990-2019: results from
the Global Burden of Disease Study 2019. BMC Nephrol
2022;23:17.
Crossref11. Gregg EW, Buckley J, Ali MK, et al. Improving health outcomes of people with diabetes: target setting for the WHO Global Diabetes Compact. Lancet 2023;401:1302-12.
Crossref12. Geldsetzer P, Manne-Goehler J, Marcus ME, et al. The
state of hypertension care in 44 low-income and middle-income
countries: a cross-sectional study of nationally
representative individual-level data from 1.1 million adults.
Lancet 2019;394:652-62.
Crossref13. Chu L, Bhogal SK, Lin P, et al. AWAREness of diagnosis
and treatment of chronic kidney disease in adults with
type 2 diabetes (AWARE-CKD in T2D). Can J Diabetes
2022;46:464-72.
Crossref14. Levin A, Tonelli M, Bonventre J, et al. Global kidney health
2017 and beyond: a roadmap for closing gaps in care,
research, and policy. Lancet 2017;390:1888-917.
Crossref15. Stengel B, Muenz D, Tu C, et al. Adherence to the Kidney
Disease: Improving Global Outcomes CKD guideline in
nephrology practice across countries. Kidney Int Rep
2020;6:437-48.
Crossref16. Chu CD, Chen MH, McCulloch CE, et al. Patient
awareness of CKD: a systematic review and meta-analysis
of patient-oriented questions and study setting. Kidney
Med 2021;3:576-85.e1.
Crossref17. Ene-Iordache B, Perico N, Bikbov B, et al. Chronic kidney
disease and cardiovascular risk in six regions of the world
(ISN-KDDC): a cross-sectional study. Lancet Glob Health
2016;4:e307-19.
Crossref18. Gummidi B, John O, Ghosh A, et al. A systematic study of
the prevalence and risk factors of CKD in Uddanam, India.
Kidney Int Rep 2020;5:2246-55.
Crossref19. Kidney Disease: Improving Global Outcomes (KDIGO)
Diabetes Work Group. KDIGO 2022 Clinical Practice
Guideline for Diabetes Management in Chronic Kidney
Disease. Kidney Int 2022;102(5S):S1-127.
Crossref20. Nicholas SB, Daratha KB, Alicic RZ, et al. Prescription
of guideline-directed medical therapies in patients with
diabetes and chronic kidney disease from the CURE-CKD
Registry, 2019-2020. Diabetes Obes Metab 2023;25:2970-9.
Crossref21. Grams ME, Yang W, Rebholz CM, et al. Risks of adverse
events in advanced CKD: the Chronic Renal Insufficiency
Cohort (CRIC) study. Am J Kidney Dis 2017;70:337-46.
Crossref22. Kidney Disease: Improving Global Outcomes (KDIGO)
CKD Work Group. KDIGO 2024 Clinical Practice
Guideline for the Evaluation and Management of Chronic
Kidney Disease. Kidney Int 2024;105(4S):S117-314.
Crossref23. Kidney Disease: Improving Global Outcomes (KDIGO)
Blood Pressure Work Group. KDIGO 2021 Clinical
Practice Guideline for the Management of Blood Pressure
in Chronic Kidney Disease. Kidney Int 2021;99(3S):S1-87.
Crossref24. Tuttle KR, Alicic RZ, Duru OK, et al. Clinical characteristics
of and risk factors for chronic kidney disease among adults
and children: an analysis of the CURE-CKD Registry.
JAMA Netw Open 2019;2:e1918169.
Crossref25. Ismail WW, Witry MJ, Urmie JM. The association between
cost sharing, prior authorization, and specialty drug
utilization: a systematic review. J Manag Care Spec Pharm
2023;29:449-63.
Crossref26. Heerspink HJ, Vart P, Jongs N, et al. Estimated lifetime
benefit of novel pharmacological therapies in patients with
type 2 diabetes and chronic kidney disease: a joint analysis
of randomized controlled clinical trials. Diabetes Obes
Metab 2023;25:3327-36.
Crossref27. Nuffield Department of Population Health Renal Studies
Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal
Trialists' Consortium. Impact of diabetes on the effects
of sodium glucose co-transporter-2 inhibitors on kidney
outcomes: collaborative meta-analysis of large placebo-controlled
trials. Lancet 2022;400:1788-801.
Crossref28. Fernández-Fernandez B, Sarafidis P, Soler MJ, Ortiz A.
EMPA-KIDNEY: expanding the range of kidney protection
by SGLT2 inhibitors. Clin Kidney J 2023;16:1187-98.
Crossref29. McEwan P, Boyce R, Sanchez JJ, et al. Extrapolated longer-term
effects of the DAPA-CKD trial: a modelling analysis.
Nephrol Dial Transplant 2023;38:1260-70.
Crossref30. Vanholder R, Annemans L, Braks M, et al. Inequities
in kidney health and kidney care. Nat Rev Nephrol
2023;19:694-708.
Crossref31. Agarwal R, Filippatos G, Pitt B, et al. Cardiovascular and
kidney outcomes with finerenone in patients with type 2
diabetes and chronic kidney disease: the FIDELITY pooled
analysis. Eur Heart J 2022;43:474-84.
Crossref32. Tuttle KR, Bosch-Traberg H, Cherney DZ, et al. Post hoc
analysis of SUSTAIN 6 and PIONEER 6 trials suggests that
people with type 2 diabetes at high cardiovascular risk
treated with semaglutide experience more stable kidney
function compared with placebo. Kidney Int 2023;103:772-81.
Crossref33. Rubin R. It takes an average of 17 years for evidence to
change practice—the burgeoning field of implementation
science seeks to speed things up. JAMA 2023;329:1333-6.
Crossref34. World Health Organization. Mid-point evaluation of the
implementation of the WHO global action plan for the
prevention and control of noncommunicable diseases
2013-2020 (NCD-GAP). Volume 1: Report. 2020. Available
from:
https://cdn.who.int/media/docs/default-source/documents/about-us/evaluation/ncd-gap-final-report.pdf?sfvrsn=55b22b89_5&download=true. Accessed 18 Nov 2023.
35. Kruk ME, Gage AD, Joseph NT, Danaei G, García-Saisó S,
Salomon JA. Mortality due to low-quality health systems in
the universal health coverage era: a systematic analysis of
amenable deaths in 137 countries. Lancet 2018;392:2203-12.
Crossref36. Kingori P, Peeters Grietens K, Abimbola S, Ravinetto R.
Uncertainties about the quality of medical products
globally: lessons from multidisciplinary research. BMJ
Glob Health 2023;6(Suppl 3):e012902.
Crossref37. Pan American Health Organization. Quality control of
medicines. Available from:
https://www.paho.org/en/topics/quality-control-medicines. Accessed 18 Nov 2023.
38. Tuttle KR, Wong L, St Peter W, et al. Moving from evidence
to implementation of breakthrough therapies for diabetic
kidney disease. Clin J Am Soc Nephrol 2022;17:1092-103.
Crossref39. Kalyesubula R, Conroy AL, Calice-Silva V, et al. Screening
for kidney disease in low- and middle-income countries.
Semin Nephrol 2022;42:151315.
Crossref40. Francis A, Abdul Hafidz MI, Ekrikpo UE, et al. Barriers to
accessing essential medicines for kidney disease in low- and
lower middle-income countries. Kidney Int 2022;102:969-73.
Crossref41. Rangaswami J, Tuttle K, Vaduganathan M. Cardio-renal-metabolic care models: toward achieving effective
interdisciplinary care. Circ Cardiovasc Qual Outcomes
2020;13:e007264.
Crossref42. Neumiller JJ, Alicic RZ, Tuttle KR. Overcoming barriers to implementing new therapies for diabetic kidney disease: lessons learned. Adv Chronic Kidney Dis 2021;28:318-27.
Crossref43. Mishra SR, Neupane D, Preen D, Kallestrup P, Perry HB.
Mitigation of non-communicable diseases in developing
countries with community health workers. Global Health
2015;11:43.
Crossref44. Joshi R, John O, Jha V. The potential impact of public health
interventions in preventing kidney disease. Semin Nephrol
2017;37:234-44.
Crossref45. Patel A, Praveen D, Maharani A, et al. Association of
multifaceted mobile technology-enabled primary care
intervention with cardiovascular disease risk management
in rural Indonesia. JAMA Cardiol 2019;4:978-86.
Crossref46. Ardavani A, Curtis F, Khunti K, Wilkinson TJ. The effect
of pharmacist-led interventions on the management and
outcomes in chronic kidney disease (CKD): a systematic
review and meta-analysis protocol. Health Sci Rep
2023;6:e1064.
Crossref47. Sherrod CF, Farr SL, Sauer AJ. Overcoming treatment
inertia for patients with heart failure: how do we build
systems that move us from rest to motion? Eur Heart J
2023;44:1970-2.
Crossref48. Ramakrishnan C, Tan NC, Yoon S, et al. Healthcare
professionals’ perspectives on facilitators of and barriers to
CKD management in primary care: a qualitative study in
Singapore clinics. BMC Health Serv Res 2022;22:560.
Crossref49. Porter J, Boyd C, Skandari MR, Laiteerapong N. Revisiting
the time needed to provide adult primary care. J Gen
Intern Med 2023;38:147-55.
Crossref50. Peralta CA, Livaudais-Toman J, Stebbins M, et al.
Electronic decision support for management of CKD in
primary care: a pragmatic randomized trial. Am J Kidney
Dis 2020;76:636-44.
Crossref51. Rios P, Sola L, Ferreiro A, et al. Adherence to
multidisciplinary care in a prospective chronic kidney
disease cohort is associated with better outcomes. PLoS
One 2022;17:e0266617.
Crossref52. Stevenson JK, Campbell ZC, Webster AC, et al. eHealth
interventions for people with chronic kidney disease.
Cochrane Database Syst Rev 2019;8:CD012379.
Crossref53. Tuot DS, Crowley ST, Katz LA, et al. Usability testing of the
kidney score platform to enhance communication about
kidney disease in primary care settings: qualitative think-aloud
study. JMIR Form Res 2022;6:e40001.
Crossref54. Verberne WR, Stiggelbout AM, Bos WJ, van Delden JJ.
Asking the right questions: towards a person-centered
conception of shared decision-making regarding treatment
of advanced chronic kidney disease in older patients. BMC
Med Ethics 2022;23:47.
Crossref55. Taha A, Iman Y, Hingwala J, et al. Patient navigators for CKD and kidney failure: a systematic review. Kidney Med
2022;4:100540.
Crossref56. Essue BM, Laba TL, Knaul F, et al. Economic burden
of chronic ill health and injuries for households in low- and
middle-income countries. In: Jamison DT, Gelband
H, Horton S, et al, editors. Disease Control Priorities:
Improving Health and Reducing Poverty. 3rd ed. The
International Bank for Reconstruction and Development/The World Bank; 2017: 121-43.
Crossref57. Khatib R, McKee M, Shannon H, et al. Availability and
affordability of cardiovascular disease medicines and their
effect on use in high-income, middle-income, and low-income
countries: an analysis of the PURE study data.
Lancet 2016;387:61-9.
Crossref58. Kamath N, Iyengar AA. Chronic kidney disease (CKD):
an observational study of etiology, severity and burden of
comorbidities. Indian J Pediatr 2017;84:822-5.
Crossref59. Cirillo L, Ravaglia F, Errichiello C, Anders HJ, Romagnani P,
Becherucci F. Expectations in children with glomerular
diseases from SGLT2 inhibitors. Pediatr Nephrol
2022;37:2997-3008.
Crossref60. Donohue JF, Elborn JS, Lansberg P, et al. Bridging the
“know-do” gaps in five non-communicable diseases using
a common framework driven by implementation science. J
Healthc Leadersh 2023;15:103-19.
Crossref61. Population Health Research Institute. Polypills added
to WHO essential medicines list. 2023. Available from:
https://www.phri.ca/eml/". Accessed 18 Nov 2023.
62. Sepanlou SG, Mann JF, Joseph P, et al. Fixed-dose
combination therapy for prevention of cardiovascular
diseases in CKD: an individual participant data meta-analysis.
Clin J Am Soc Nephrol 2023;18:1408-15.
Crossref63. Dev V, Mittal A, Joshi V, et al. Cost analysis of telemedicine
use in paediatric nephrology–the LMIC perspective.
Pediatr Nephrol 2024;39:193-201.
Crossref64. Musacchio N, Zilich R, Ponzani P, et al. Transparent
machine learning suggests a key driver in the decision to
start insulin therapy in individuals with type 2 diabetes. J
Diabetes 2023;15:224-36.
Crossref65. Zuniga C, Riquelme C, Muller H, Vergara G, Astorga C,
Espinoza M. Using telenephrology to improve access to
nephrologist and global kidney management of CKD
primary care patients. Kidney Int Rep 2020;5:920-3.
Crossref66. van der Horst DE, Hofstra N, van Uden-Kraan CF, et al.
Shared decision making in health care visits for CKD:
patients' decisional role preferences and experiences. Am
J Kidney Dis 2023;82:677-86.
Crossref67. Hole B, Scanlon M, Tomson C. Shared decision making: a
personal view from two kidney doctors and a patient. Clin
Kidney J 2023;16(Suppl 1):i12-9.
Crossref 






