Non-surgical treatment of lung cancer: personalised stereotactic ablative radiotherapy
Hong Kong Med J 2014;20(6):529–36 | Epub 26 Sep 2014
DOI: 10.12809/hkmj144269
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE
Non-surgical treatment of lung cancer: personalised stereotactic ablative radiotherapy
Maverick WK Tsang, FRCR, FHKAM (Radiology)1; Michael KM Kam, FRCR, FHKAM (Radiology)1; SF Leung, MD, FHKAM (Radiology)1; Anthony TC Chan, MD, FRCP2
1 Department of Clinical Oncology, Prince of Wales Hospital, Shatin, Hong Kong
2 State Key Laboratory in Oncology in South China, The Chinese University of Hong Kong, Shatin, Hong Kong
Corresponding author: Dr Maverick WK Tsang (wk_tsang@clo.cuhk.edu.hk)
Abstract
Stereotactic ablative radiotherapy has emerged as a
standard treatment for medically inoperable stage
I non–small-cell lung cancer and selected cases
of lung metastasis. Techniques to freeze or limit
tumour movement during treatment and image-guided
radiation delivery are integral to a successful
stereotactic ablative treatment without overdose
of surrounding normal structures. In this article, the
practice in a local oncology institution will be used
to illustrate the concept of personalised stereotactic
ablative radiotherapy.
Introduction
Lung cancer is the second most common cancer and
number one killer among all cancers in Hong Kong.1
Non–small-cell lung cancer (NSCLC) accounts for
about 80% of all lung cancer cases. Surgery remains
the mainstay of treatment for early-stage NSCLC.
For patients who refuse or are medically unfit for
surgery, stereotactic ablative radiotherapy (SABR)
has emerged as a standard treatment. In the era of
personalised medicine, SABR should be executed
with techniques which are most suitable for the
patient. In this article, the concepts of SABR, tumour
motion control, and image-guided radiation delivery
will be introduced. Then, using the Prince of Wales
Hospital as an example, the approach to selecting the
appropriate techniques for execution of personalised
SABR will be explained.
Stereotactic ablative radiotherapy
Stereotactic ablative radiotherapy, also named as
stereotactic body radiotherapy, is defined as “an
external beam radiotherapy method used to very
precisely deliver a high dose of radiation to an
extracranial target within the body, using either a
single dose or a small number of fractions”.2 The goal
of SABR is to give a very high (ablative) radiation
dose to kill all cancer cells within the target while
avoiding radiation damage to the surrounding
normal tissues. For lung SABR, a radiation dose of 10
to 20 Gray (Gy, a radiation dose unit) per treatment
fraction is delivered for a total of 48 to 60 Gy in five
or less fractions within 2 weeks.
Indications for stereotactic ablative radiotherapy in lung cancer
Stage I non–small-cell lung cancer: medically
inoperable disease or patient refuses surgery
Nowadays, SABR is the gold-standard treatment
for patients with stage I NSCLC who refuse surgical
intervention or are medically unfit to undergo
surgery (mostly due to poor pulmonary function).
Prospective phase I/II studies document local control
rate of over 90% with SABR, with overall survival
approaching that of surgery (Table 13 4 5 6 7 8 9 10). In Hong
Kong, the reported 2-year local control and 2-year
overall survival rates were 89% to 95% and 53% to 87%,
respectively.11 12 13 Generally, SABR is well tolerated,
even by patients with poor pulmonary function.
Guckenberger et al14 demonstrated that SABR had
only very limited acute and chronic pulmonary
toxicity even in patients with poor pulmonary
function and there was no requirement of minimal
pulmonary function for safe practice of SABR.
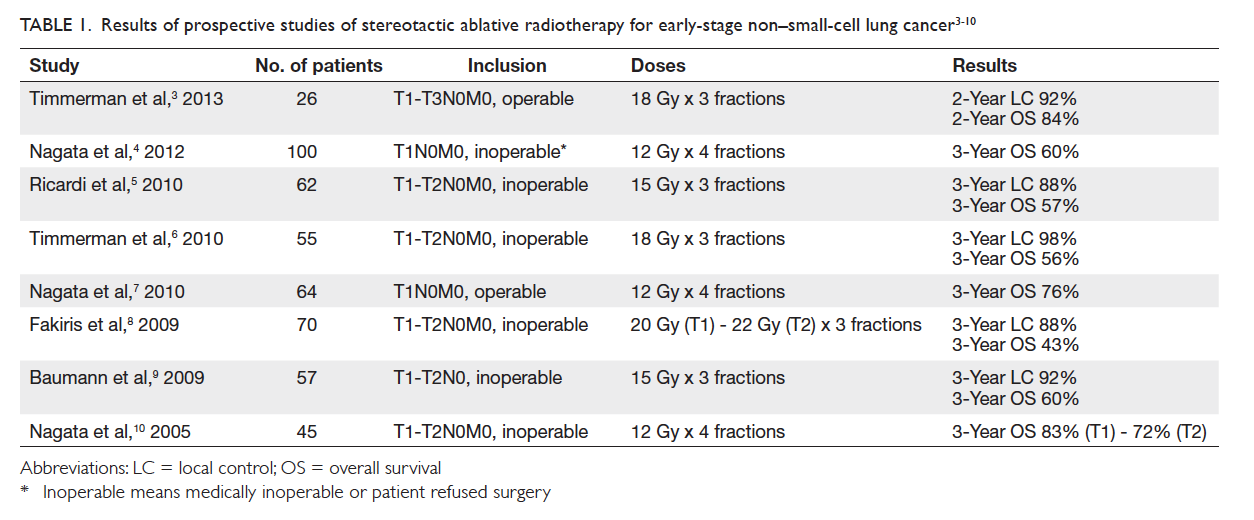
Table 1. Results of prospective studies of stereotactic ablative radiotherapy for early-stage non–small-cell lung cancer3 4 5 6 7 8 9 10
In view of the encouraging data on local control
and mild toxicity of SABR for medically inoperable
cases, the gold-standard role of surgery for operable
stage I NSCLC is now being challenged. At least
two retrospective studies have shown that survival
of patients with operable stage I NSCLC treated
with SABR paralleled that of lobectomy.15 16 Another
two prospective phase 2 trials reported 76% to 84%
2-to-3-year overall survival rates for operable stage I
disease after SABR, which compares favourably to
surgical outcomes.3 7 Unfortunately, all phase 3 trials
comparing SABR with surgery in operable stage I NSCLC were terminated prematurely due to poor
accrual. Thus, the race between SABR and surgery
for the title of standard treatment for operable stage
I NSCLC continues without a foreseeable end.
Oligometastasis involving the lungs with controlled primary tumour
Generally, oligometastasis is defined as one to five
metastatic lesions besides the primary tumour.
Evidence has emerged that patients with limited
metastases can be cured by removal of all metastases.
The reported 10- and 15-year survival rates of
patients undergoing complete lung metastasectomy
were 26% and 22%, respectively. Patients with fewer
metastases and longer disease-free interval fared
even better.17 Prospective studies of limited lung
metastases treated with SABR reported 2-year local
control and 2-year survival rates of 89% to 96% and 39%
to 84%, respectively, which are not inferior to the
surgical results.18 19 20 21
Stereotactic ablative radiotherapy versus conventional radiotherapy
The local control rate of stage I NSCLC after SABR
is ≥90%, in contrast to 50% rate with conventional
radiotherapy.22 23 24 A clinical dose-response curve
of human malignant lung tumours has been
established.25 Thus, a high local tumour control can
be achieved by delivering a high radiation dose. In
lung cancer, however, the intrathoracic normal
structures (normal lung tissues, spinal cord, brachial
plexus, oesophagus, trachea and main bronchi, heart,
great vessels, ribs and skin) close to the tumour may
also receive a high radiation dose which may cause
severe or even fatal treatment complications. It is
this radiation damage of normal structures that
limits the possible radiation dose to the lung tumour
in conventional radiotherapy.
Stereotactic ablative radiotherapy is able to
deliver a very high radiation dose to the target while
sparing the surrounding normal tissues, thanks to
its intrinsic physical advantage. On the other hand,
many normal structures can tolerate a small volume
of high-dose radiation without complications. Thus,
we can deliver high-dose tumour radiation and yet
limit volumes of the surrounding normal tissues
exposed to high-dose radiation by reducing the size
of the radiation field. In SABR, radiation field size
reduction can be achieved through incorporation
of techniques to limit tumour movement during
radiotherapy (tumour motion management) and
image-guided radiation delivery.
Physical property
Compared with conventional radiotherapy, SABR
is able to create a very rapid dose fall-off at the target normal tissue interface so that radiation
can be precisely delivered to the target without
damaging the surrounding normal tissues. This can
be achieved by aligning the treatment field borders
or multi-leaf collimators close to the planning target
volume (PTV) borders (refer to the “Individualised
radiation target volume” section below for definition
of PTV) and by prescribing dose at the part
of the radiation dose depth curve with a steep
slope, ie 60% to 90% isodose line. Such rapid dose
fall-off property of SABR can be further enhanced
by adoption of intensity-modulated radiotherapy
or volumetric-modulated arc therapy techniques
which, in addition, can create a concave radiation
dose distribution to further improve radiation dose
conformity to the target.
Tumour movement restriction during radiotherapy
A lung tumour will move with respiration. In
conventional radiotherapy, the radiation field is
enlarged to encompass the tumour in all respiratory
phases. In SABR, however, tumour movement during
radiotherapy is restricted by various tumour motion
management techniques. As a result, the radiation
field can be smaller, thereby, limiting the amount of
normal tissues exposed to a high radiation dose.
Image-guided radiotherapy
Even with proper tumour motion management,
there will be residual tumour movement both
during the same treatment fraction (intra-fraction)
and between different fractions on different days
(inter-fractions). Therefore, daily pretreatment
verification of tumour position by various image-guided
radiotherapy (IGRT) techniques is essential
to avoid geographical tumour miss. Radiation field
size reduction can only be realised with IGRT.
Techniques to freeze or restrict movement of a lung tumour during stereotactic ablative radiotherapy
Active breathing control/voluntary inspiratory breath-hold
Breath-holding SABR can be achieved actively by
active breathing control (ABC) or voluntarily by
self-initiated breath-hold. The ABC apparatus is a
modified spirometer consisting of two pairs of flow
monitors and scissor valves to control inspiration
and expiration, respectively. The operator activates
ABC at a predefined lung volume by closing both
valves to immobilise the breathing motion for 15
to 20 seconds. At the same time, radiation beam is
switched on. Then, the patient is allowed to breathe
freely until the next ABC activation. The cycle is
repeated until complete delivery of a treatment
fraction, which typically takes 30 minutes. Mostly,
ABC will be activated in inspiration when lungs
expand, resulting in less normal lung irradiation
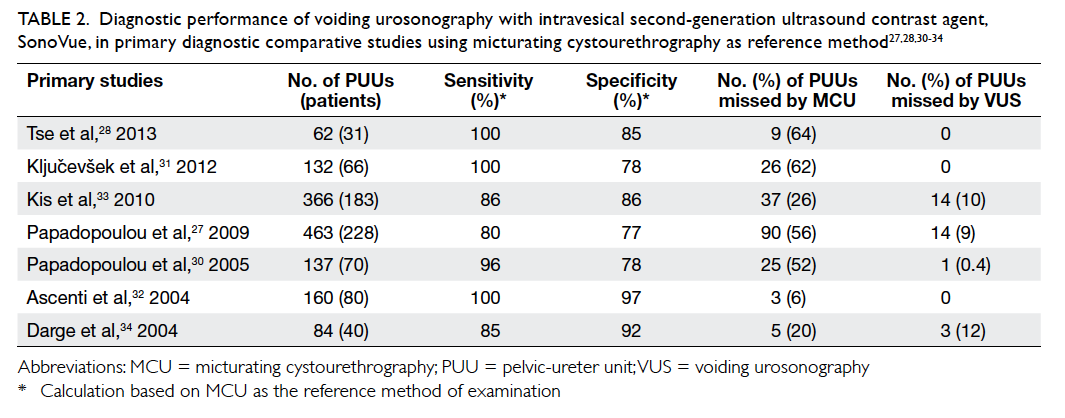
(Fig 1a). A study showed that ABC reduced normal
lung V20 (volume of normal lung tissues receiving a
dose ≥20 Gy) by 34% compared with free breathing.26
There is a good reproducibility of lung tumour
position under ABC both inter-fractionally and
intra-fractionally, with mean tumour displacement
in supero-inferior direction of 1.1 mm and 0.3 mm,
respectively.27 28 Voluntary inspiratory breath-hold
technique is used in case a patient can hold his/her
breath for at least 15 seconds but is unable to hold
the mouthpiece of an ABC apparatus without air
leakage.
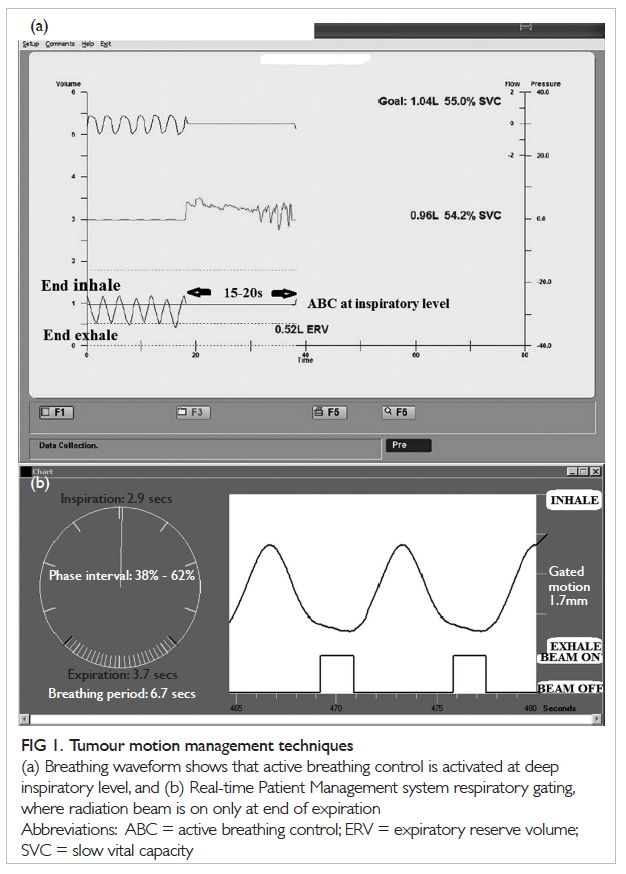
Figure 1. Tumour motion management techniques
(a) Breathing waveform shows that active breathing control is activated at deep inspiratory level, and (b) Real-time Patient Management system respiratory gating, where radiation beam is on only at end of expiration
Respiratory gating
Respiratory gating involves delivery of radiation only
in certain phases of respiration. The gating window
(respiratory phases at which radiation beam will
be turned on) is usually selected at late expiratory
phases as a lung tumour stays for a longer period in
the expiratory phase than in the inspiratory phase,
resulting in a shorter treatment time. In addition,
the tumour position will be more consistent
and reproducible at end of expiration. A four-dimensional
computed tomography (4D-CT; 4D
means 3D + time) for radiotherapy planning purpose
is done with the Real-time Patient Management
(RPM) system (Varian Medical Systems, US), which
consists of an infrared reflective block and an infrared
tracking camera. The reflective block is placed on the
anterior abdominal skin surface midway between the
xiphisternum and umbilicus. The infrared camera
tracks motion of the reflective block. The up-and-down
breathing movement of the abdominal wall
and thus position of the reflective block now reflects
the respiratory phase during which a particular set
of CT images are captured. As a result, positions
of the tumour in various respiratory phases can be
displayed on the 4D-CT images. A radiation field
is designed according to the tumour positions at
selected gating windows. Respiratory gating can
be executed with either the RPM or the ExacTrac
Adaptive Gating systems (Brainlab AG, Germany).
Real-time Patient Management system
During SABR, the infrared reflective block is placed
on the patient’s abdominal wall and serves as an
external indicator to predict the tumour location.
The infrared camera will track movements of the
reflective block to turn the radiation beam on (at
gating window) and off (Fig 1b).
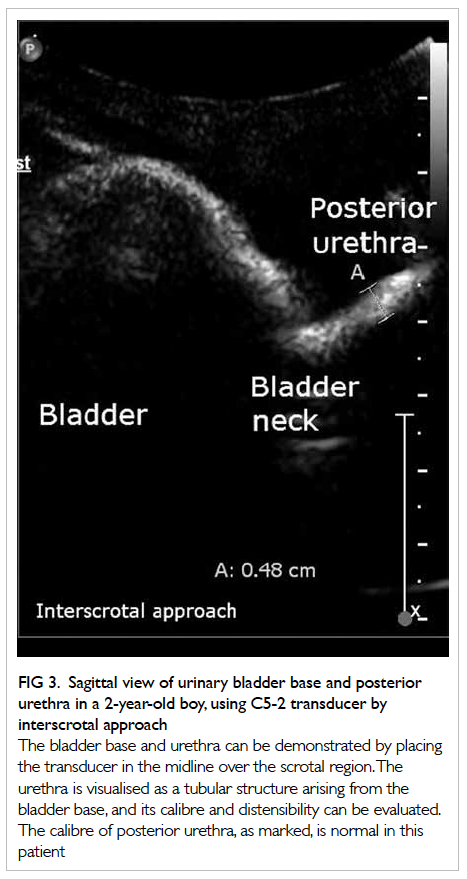
ExacTrac Adaptive Gating system
Similar to the RPM system, ExacTrac has an optical
infrared tracking system comprising several infrared
reflective body markers (usually five to eight) and an
infrared tracking camera mounted on the ceiling of
the radiation treatment room (Fig 2a). The radiation beam will be turned on only at a predefined gating
window. The advantages and disadvantages of
different tumour motion management techniques
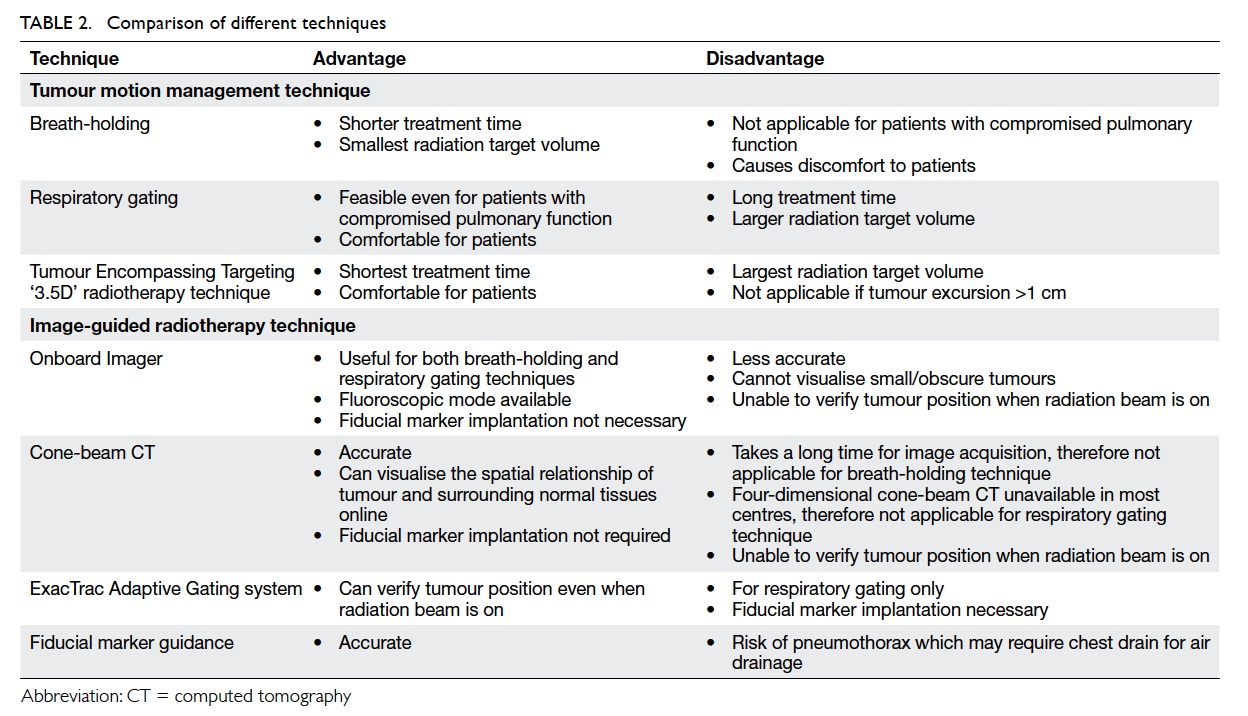
are tabulated in Table 2.
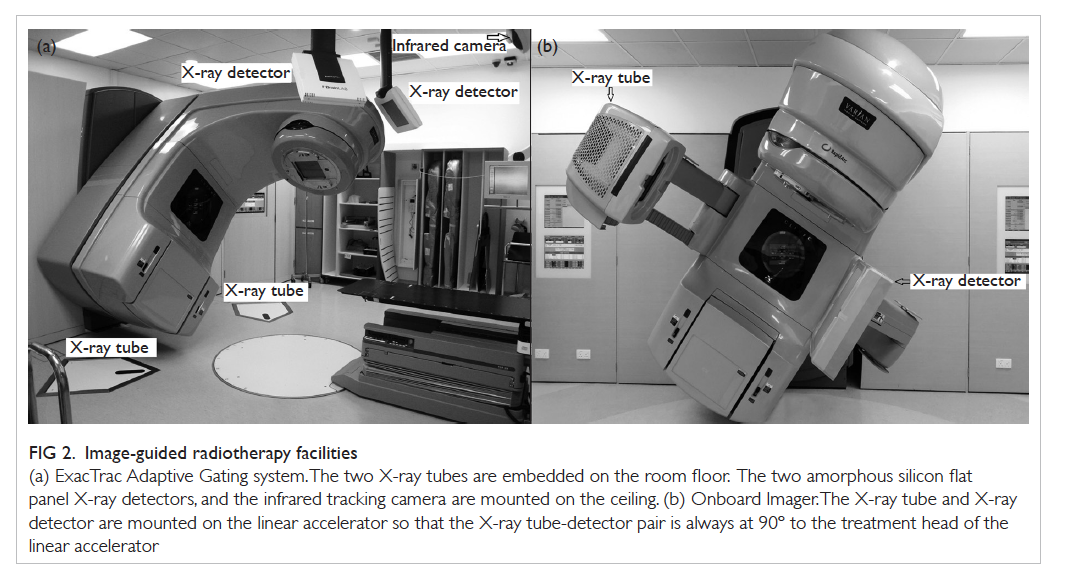
Figure 2. Image-guided radiotherapy facilities
(a) ExacTrac Adaptive Gating system. The two X-ray tubes are embedded on the room floor. The two amorphous silicon flat panel X-ray detectors, and the infrared tracking camera are mounted on the ceiling. (b) Onboard Imager. The X-ray tube and X-ray detector are mounted on the linear accelerator so that the X-ray tube-detector pair is always at 90º to the treatment head of the linear accelerator
Radiation delivery under image guidance
Multiple studies have concluded that neither external
indicators (infrared reflective block in the RPM
system29 30) nor internal indicators (diaphragm,31
bony anatomy such as vertebral bodies32 33) have a
consistent correlation with tumour position over
time. Therefore, direct visualisation of the lung
tumour itself is required for accurate and precise
radiation delivery. Image-guided radiotherapy is
a procedure that uses various imaging techniques
(eg X-ray and CT) to identify a tumour to guide the
radiation beam during SABR treatment. It makes
radiotherapy more accurate and causes less damage
to healthy tissues.
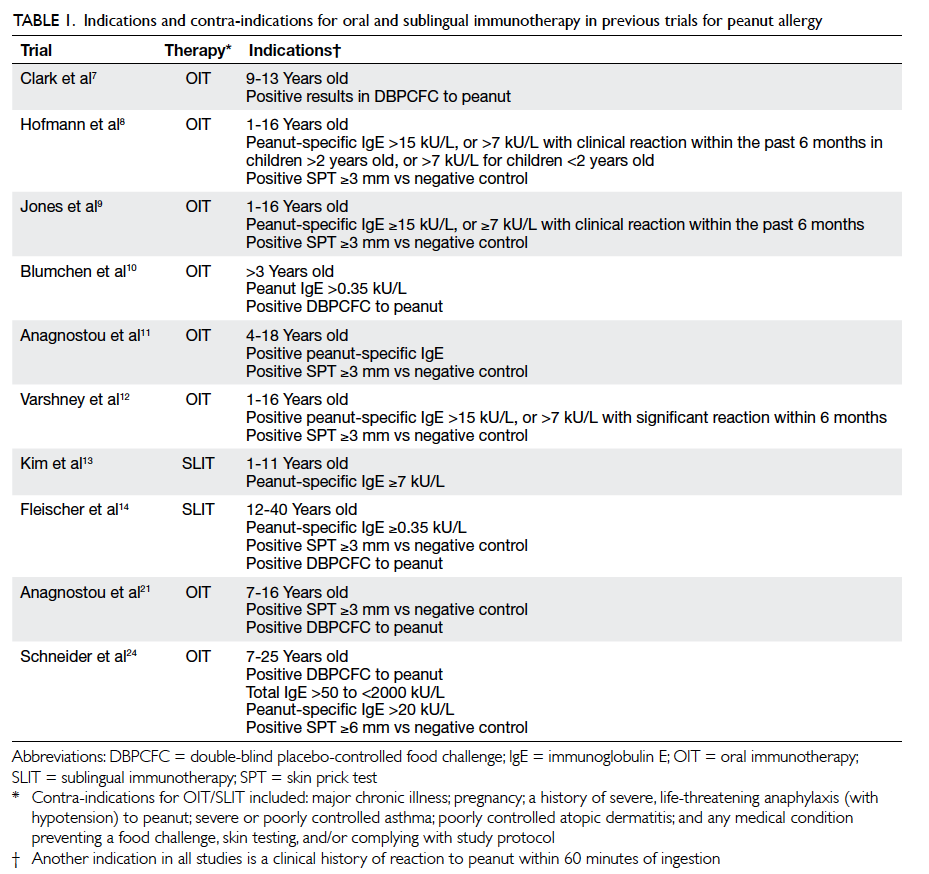
Pretreatment verification of tumour position
by a CT or X-ray imaging device mounted on a linear
accelerator (Linac; a machine for generation and
delivery of radiation beam) should be done daily for
detection of inter-fractional tumour displacement,
with necessary correction if there is significant
tumour displacement from its planned position
(Fig 3). Moreover, interval treatment verification
with X-ray imaging during one treatment fraction is
required for identification of intra-fractional tumour
displacement.
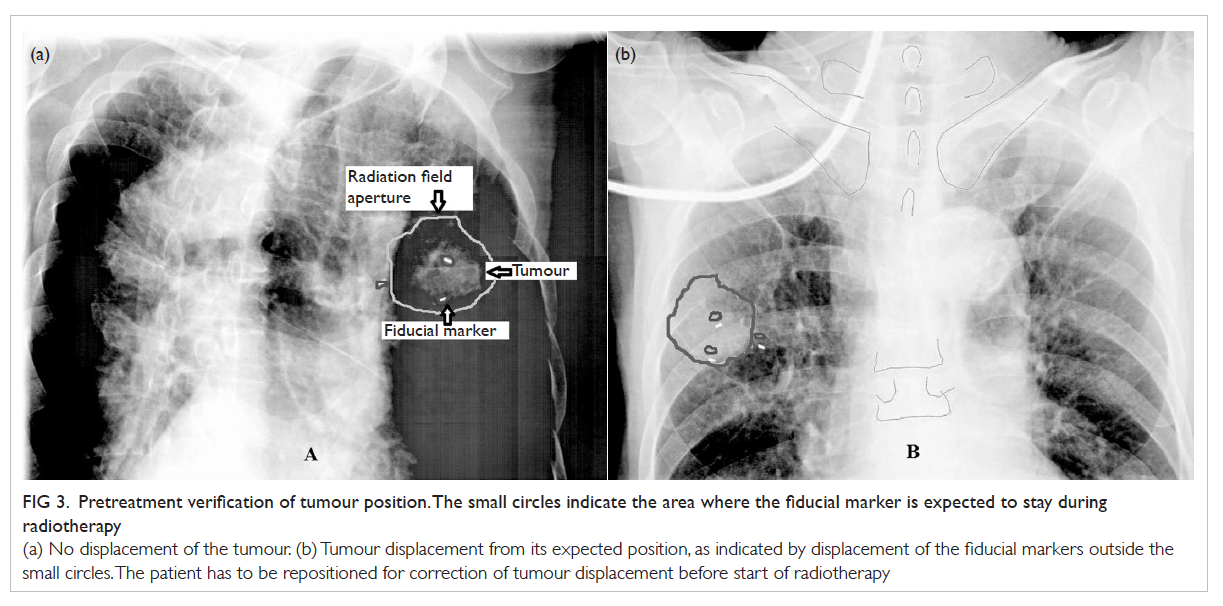
Figure 3. Pretreatment verification of tumour position. The small circles indicate the area where the fiducial marker is expected to stay during radiotherapy
(a) No displacement of the tumour. (b) Tumour displacement from its expected position, as indicated by displacement of the fiducial markers outside the small circles. The patient has to be repositioned for correction of tumour displacement before start of radiotherapy
Onboard Imager
Onboard Imager (OBI; Varian Medical Systems, US)
is a high-resolution X-ray device mounted on the
treatment head of a Linac to display real-time tumour
location (Fig 2b). Radiographic images can be taken at the gating window for online (ie when a patient lies on
treatment table of the Linac) verification of tumour
position in RPM respiratory-gated radiotherapy. In
ABC treatment, the tumour position under ABC can
also be verified online. It should be noted that X-ray
images can be taken only when the radiation beam
is turned off.
Cone-beam computed tomography
Cone-beam CT (CBCT) is a 3D mode of OBI, which
is able to acquire and reconstruct 3D volumetric
data in one rotation of treatment head of the Linac
in 1 minute. Because of the long image acquisition
time, CBCT is unsuitable for treatment verification if
breath-holding or respiratory gating techniques are
used. Rather, it is a useful and accurate tool for daily
treatment verification of the Tumour Encompassing
Targeting radiotherapy.
ExacTrac Adaptive Gating
The ExacTrac Adaptive Gating system has two
components: the optical infrared tracking system
for respiratory gating (mentioned above) and the
stereoscopic X-ray imaging system for online
detection and correction of tumour position shift.
The stereoscopic X-ray imaging system consists of
two X-ray tubes embedded in the Linac room floor
and two amorphous silicon flat panel detectors
mounted on the ceiling; the angle between the two
X-ray tube-detector pairs is approximately 90° (Fig 2a). Stereoscopic X-ray can be taken at the gating
window for verification of tumour position. Its
advantage over OBI is that periodic X-ray acquisition
at gating window is possible during both beam-on and
beam-off periods. That means tumour displacement
can be detected anytime during treatment, including
during the beam-on period. Radiation beam can be
turned off automatically if the tumour is displaced
outside its allowed region. Table 2 compares the pros and cons of various IGRT techniques.
Personalised stereotactic ablative radiotherapy adapted to patient’s needs and limitations
Individualised tumour motion management
The criteria for selection of an appropriate tumour
motion management technique include: (1) ability
of the patient to tolerate inspiratory breath-hold for
≥15 seconds, (2) extent of tumour movement at tidal
breathing, and (3) selected IGRT technique (refer
to the ‘Individualised image-guided radiotherapy’
section below for details).
Ideally, all patients should be treated under
breath-holding condition as the lung tumour will
be frozen and a minimal safety margin is required
for creation of a radiation field. In practice, many
lung cancer patients are elderly and smokers with
compromised pulmonary function. These patients
cannot hold the breath long enough for SABR
treatment. For patients unsuitable for breath-holding
techniques, a 4D-CT is done under tidal breathing to
assess the magnitude of tumour movement. If it is
≤1 cm, the Tumour Encompassing Targeting
technique (‘3.5D’ radiotherapy) is used in which the
radiation field will cover all possible tumour positions
at any respiratory phase as shown on 4D-CT. For
tumours with excessive (>1 cm) movement under
tidal breathing, the respiratory gating technique
should be utilised.
A flowchart showing the approach to selecting
appropriate tumour motion management technique
for lung SABR is shown in Figure 4a.
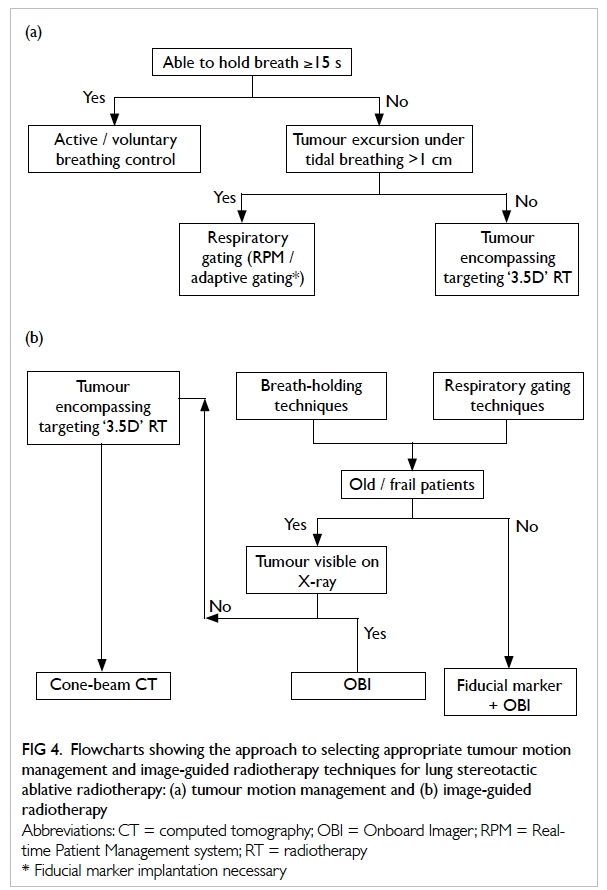
Figure 4. Flowcharts showing the approach to selecting appropriate tumour motion management and image-guided radiotherapy techniques for lung stereotactic ablative radiotherapy: (a) tumour motion management and (b) image-guided radiotherapy
Individualised image-guided radiotherapy
An IGRT technique should be selected to match
requirements of the selected tumour motion
management technique and characteristics of the
tumour (Fig 4b). A fiducial marker (a marker made
of pure gold that can be visualised clearly on X-ray)
can be implanted into or close to the tumour as an
indicator of tumour location under OBI. There are
various commercially available fiducial markers
of different shapes and sizes, such as a cylindrical
marker measuring 0.75 mm in diameter and 10 mm
in length. Fiducial markers are implanted under CT
guidance. In theory, all SABR treatments should be
executed either under fiducial markers guidance or
with CBCT as these are the most accurate methods
for pretreatment tumour position verification. In
fact, CBCT is the IGRT technique of choice for
‘3.5D’ radiotherapy. Nearly all patients cannot hold
the breath long enough for performing CBCT,
which typically takes a minute for 360° acquisition
of a full set of CT images. Furthermore, 4D-CBCT is
unavailable in most oncology centres in Hong Kong.
As a result, CBCT pretreatment verification is
impractical for both breath-holding and respiratory gating
techniques. On the other hand, fiducial
marker implantation under CT guidance will result
in significant pneumothorax
necessitating insertion of a chest drain. Thus, it
is not recommended in old and/or frail patients.
Provided that the tumour can be visualised on X-ray,
OBI alone can be used for pretreatment verification
in such patients. Unfortunately, a lung tumour may
not be visible on X-ray because of its small size
or close proximity to the ribs, mediastinum, or
heart. If a fiducial marker is not implanted and the
tumour is invisible on OBI, neither breath-holding
nor respiratory-gated SABR treatment is realistic.
Instead, the ‘3.5D’ radiotherapy technique should be
utilised with CBCT pretreatment verification.
Individualised radiation target volume
The International Commission on Radiation Units and Measurements
Report 62 clearly defines various target volumes
for radiation.34 Gross tumour volume (GTV) is the
tumour mass shown on clinical examination or by
imaging. Clinical target volume (CTV) encompasses
the subclinical microscopic disease around GTV.
In SABR, the tissue immediately around GTV will
receive a dose high enough to eradicate all possible
microscopic disease. Therefore, CTV margin is not
required in most of the cases, ie GTV = CTV. To
compensate for possible inter-fractional and intra-fractional
tumour movement, a further margin
(internal margin [IM]) is added to CTV to create
the internal target volume (ITV). As 4D-CT scan for
radiotherapy planning only delineates the snapshot
tumour movement at the time of scanning, an IM is
still required to allow for residual tumour movement.
A final setup margin (SM) for all uncertainties
in patient-radiation beam positioning during
radiotherapy treatment is added to ITV to become
the final PTV. The required IM and SM
depend on the selected tumour motion management
and IGRT techniques. As the breathing pattern
of patients may change significantly both within
one treatment and between different treatment
sessions, a larger IM is indicated for respiratory
gating techniques. Compared with fiducial markers
and CBCT, OBI is less accurate for determination
of tumour position during treatment, thereby,
requiring a larger SM (Table 3).
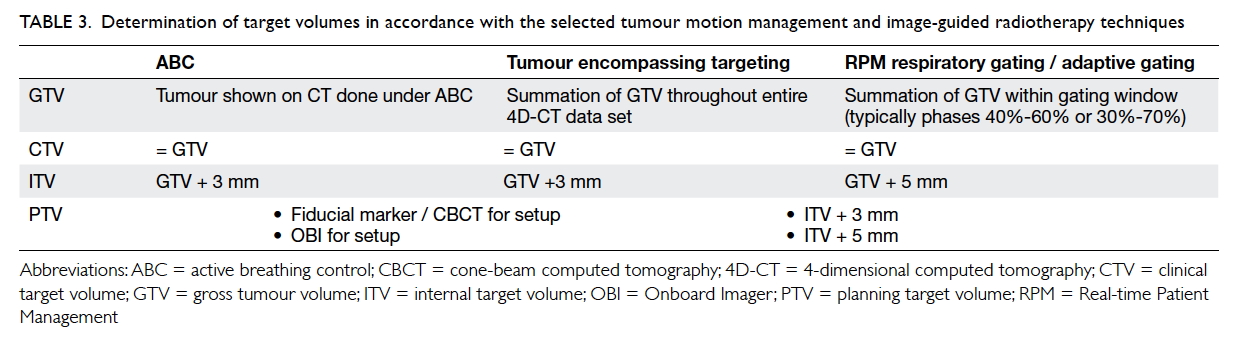
Table 3. Determination of target volumes in accordance with the selected tumour motion management and image-guided radiotherapy techniques
Conclusion
Stereotactic ablative radiotherapy is the standard
treatment for medically inoperable stage I NSCLC.
Phase 3 trials are eagerly awaited to settle the debate
on superiority of SABR or surgery in the treatment
of operable stage I disease. Various tumour motion
management and IGRT techniques are available for
effective execution of SABR. Personalised SABR
should be offered to suit each patient’s needs and
limitations.
References
1. Hong Kong Cancer Registry. Available from: http://www3.ha.org.hk/cancereg/. Accessed Jun 2014.
2. Practice guideline for the performance of stereotactic
body radiation therapy. Reston VA: American College of
Radiology (ACR); 2009: 8.
3. Timmerman RD, Paulus R, Pass HI, et al. RTOG 0618:
Stereotactic body radiation therapy (SBRT) to treat
operable early-stage lung cancer patients [abstract]. J Clin
Oncol 2013;31(Suppl):abstract 7523.
4. Nagata Y, Hiraoka M, Shibata T, et al. Stereotactic body
radiation therapy for T1N0M0 non–small cell lung cancer:
first report for inoperable population of a phase II trial by
Japan Clinical Oncology Group (JCOG 0403). Int J Radiat
Oncol Biol Phys 2012;84(Suppl):S46. CrossRef
5. Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body
radiation therapy for early stage non–small cell lung cancer:
results of a prospective trial. Lung Cancer 2010;68:72-7. CrossRef
6. Timmerman R, Paulus R, Galvin J, et al. Stereotactic body
radiation therapy for inoperable early stage lung cancer.
JAMA 2010;303:1070-6. CrossRef
7. Nagata Y, Hiraoka M, Shibata T, et al. A phase II trial of
stereotactic body radiation therapy for operable T1N0M0
non–small cell lung cancer: Japan Clinical Oncology
Group (JCOG0403). Int J Radiat Oncol Biol Phys
2010;78(Suppl):S27-8. CrossRef
8. Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic
body radiation therapy for early-stage non–small-cell lung
carcinoma: four-year results of a prospective phase II
study. Int J Radiat Oncol Biol Phys 2009;75:677-82. CrossRef
9. Baumann P, Nyman J, Hoyer M, et al. Outcome in a
prospective phase II trial of medically inoperable stage
I non–small-cell lung cancer patients treated with
stereotactic body radiotherapy. J Clin Oncol 2009;27:3290-6. CrossRef
10. Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of
a phase I/II study of 48 Gy of stereotactic body radiotherapy
in 4 fractions for primary lung cancer using a stereotactic
body frame. Int J Radiat Oncol Biol Phys 2005;63:1427-31. CrossRef
11. Chan OS, Yeung RM, Hung AW, et al. Stereotactic ablative
radiotherapy for medically inoperable early stage lung
cancer: early outcomes. Hong Kong Med J 2012;8:412-8.
12. Tsang WK. Stereotactic and respiratory-gated
radiotherapy: local experience. Proceedings of the 4th Asia
Pacific Perspectives in Lung Cancer 2010; 2010 Sep 3-4.
Hong Kong.
13. Ng AW, Tung SY, Wong VY. Hypofractionated stereotactic
radiotherapy for medically inoperable stage I non–small
cell lung cancer—report on clinical outcome and dose to
critical organs. Radiother Oncol 2008;87:24-8. CrossRef
14. Guckenberger M, Kestin LL, Hope AJ, et al. Is there a
lower limit of pretreatment pulmonary function for safe
and effective stereotactic body radiotherapy for early-stage
non–small cell lung cancer? J Thorac Oncol 2012;7:542-51. CrossRef
15. Shirvani SM, Jiang J, Chang JY, et al. Comparative
effectiveness of 5 treatment strategies for early-stage non–small
cell lung cancer in the elderly. Int J Radiat Oncol Biol
Phys 2012;84:1060-70. CrossRef
16. Onishi H, Shirato H, Nagata Y, et al. Stereotactic body
radiotherapy (SBRT) for operable stage I non–small-cell
lung cancer: can SBRT be comparable to surgery? Int J
Radiat Oncol Biol Phys 2011;81:1352-8. CrossRef
17. Long-term results of lung metastasectomy: prognostic
analyses based on 5206 cases. The International Registry of
Lung Metastases. J Thorac Cardiovasc Surg 1997;113:37-49. CrossRef
18. Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic
body radiation therapy for lung metastases. Lung Cancer
2012;75:77-81. CrossRef
19. Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional
phase I/II trial of stereotactic body radiation
therapy for lung metastases. J Clin Oncol 2009;27:1579-84. CrossRef
20. Norihisa Y, Nagata Y, Takayama K, et al. Stereotactic body
radiotherapy for oligometastatic lung tumors. Int J Radiat
Oncol Biol Phys 2008;72:398-403. CrossRef
21. Yoon SM, Choi EK, Lee SW, et al. Clinical results of
stereotactic body frame based fractionated radiation
therapy for primary or metastatic thoracic tumors. Acta
Oncol 2006;45:1108-14. CrossRef
22. Haffty BG, Goldberg NB, Gerstley J, Fischer DB, Peschel
RE. Results of radical radiation therapy in clinical stage I,
technically operable non–small cell lung cancer. Int J Radiat
Oncol Biol Phys 1988;15:69-73. CrossRef
23. Kaskowitz L, Graham MV, Emami B, Halverson KJ, Rush
C. Radiation therapy alone for stage I non–small cell lung
cancer. Int J Radiat Oncol Biol Phys 1993;27:517-23. CrossRef
24. Krol AD, Aussems P, Noordijk EM, Hermans J, Leer JW.
Local irradiation alone for peripheral stage I lung cancer:
could we omit the elective regional nodal irradiation? Int J
Radiat Oncol Biol Phys 1996;34:297-302. CrossRef
25. Fletcher GH. Clinical dose-response curves of human
malignant epithelial tumours. Br J Radiol 1973;46:1-12. CrossRef
26. Barnes EA, Murray BR, Robinson DM, Underwood LJ,
Hanson J, Roa WH. Dosimetric evaluation of lung tumor
immobilization using breath hold at deep inspiration. Int J
Radiat Oncol Biol Phys 2001;50:1091-8. CrossRef
27. Kashani R, Balter JM, Hayman JA, Henning GT, van Herk
M. Short-term and long-term reproducibility of lung
tumor position using active breathing control (ABC). Int J
Radiat Oncol Biol Phys 2006;65:1553-9. CrossRef
28. Cheung PC, Sixel KE, Tirona R, Ung YC. Reproducibility
of lung tumor position and reduction of lung mass within
the planning target volume using active breathing control
(ABC). Int J Radiat Oncol Biol Phys 2003;57:1437-42. CrossRef
29. Koch N, Liu HH, Starkschall G, et al. Evaluation of internal
lung motion for respiratory-gated radiotherapy using MRI:
Part I—correlating internal lung motion with skin fiducial
motion. Int J Radiat Oncol Biol Phys 2004;60:1459-72. CrossRef
30. Berbeco RI, Nishioka S, Shirato H, Chen GT, Jiang SB.
Residual motion of lung tumours in gated radiotherapy
with external respiratory surrogates. Phys Med Biol
2005;50:3655-67. CrossRef
31. Wang J, Liang J, Hugo G, et al. Distance between thoracic
tumor position and diaphragm position during the course
of radiotherapy: does it remain constant? Int J Radiat
Oncol Biol Phys 2005;63:S524. CrossRef
32. Guckenberger M, Meyer J, Wilbert J, et al. Cone-beam
CT based image-guidance for extracranial stereotactic
radiotherapy of intrapulmonary tumors. Acta Oncol
2006;45:897-906. CrossRef
33. Purdie TG, Bissonnette JP, Franks K, et al. Cone-beam
computed tomography for on-line image guidance of lung
stereotactic radiotherapy: localization, verification, and
interfraction tumor position. Int J Radiat Oncol Biol Phys
2007;68:243-52. CrossRef
34. Prescribing, recording and reporting photon beam therapy (Report 62 by International Commission on Radiation Units and Measurements). Available from: http://www.icru.org/home/reports/prescribing-recording-and-reporting-photon-beam-therapy-report-62. Accessed Sep 2014.