Hong Kong Med J 2014 Aug;20(4):325–30 | Epub 20 June 2014
DOI: 10.12809/hkmj144243
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
Immunotherapy for peanut allergy
TH Lee, ScD, FRCP1; June Chan,
BSc, MSc1; Vivian WY Lau, BSc, MSc1; WL Lee,
BNurs, MNurs1; PC Lau, BNurs1; MH Lo, BSc,
MSc2
1 Allergy Centre, Hong Kong
Sanatorium and Hospital, 2 Village Road, Happy Valley, Hong
Kong
2 Department of Pathology,
Hong Kong Sanatorium and Hospital, 2 Village Road, Happy Valley,
Hong
Kong
Corresponding author: Dr TH Lee (thlee@hksh.com)
Abstract
Peanut
allergy is one of the commonest food
hypersensitivities causing fatal or near-fatal
reactions. There is, currently, no preventive treatment
and the incidence of severe allergic reactions during
peanut desensitisation has limited its clinical use.
Anti–immunoglobulin E therapy has been shown to
be effective in preventing peanut-induced reactions
but it does not result in long-term tolerance. Two
important advances have recently been reported.
One involves gradual oral introduction of peanut
protein to desensitise, whereas the other approach
uses a combination of anti–immunoglobulin E and
oral peanut immunotherapy. Both approaches could
offer a way to desensitise with a far greater margin of
safety than has, hitherto, been reported. This article
provides an overview of the literature on peanut
immunotherapy and describes the experience in
a small group of children in Hong Kong who were treated
successfully using anti–immunoglobulin E
combined with oral peanut desensitisation.
Introduction
Peanut allergy is the commonest food
hypersensitivity
causing fatal or near-fatal reactions in the western
world.1 There has been a
longstanding but erroneous
belief that peanut allergy is less prevalent in Hong
Kong compared with other countries. Two studies
have estimated the prevalence of allergic reactions
after eating peanuts in children living in Hong Kong
to be 0.6% and 0.3%, respectively,2
3 which is similar
to pooled international data. Strikingly, 700/100 000
of the population in Hong Kong aged 14 years or
younger is estimated to have a risk of anaphylaxis3
and peanut is a leading causative food allergen
alongside shellfish, egg, milk, beef, and tree nuts.2 3
The current medical management of peanut
allergy is to encourage strict avoidance of peanuts
and to use self-administered adrenaline for anaphylaxis
due to inadvertent ingestion. Dietary restrictions
are not only difficult but also stressful for the patient
and families. Reactions from accidental exposure
are common and annual incidence rates range from
3% to 50%.4 Furthermore,
adrenaline is not always
accessible for emergency use. It is, therefore, essential
to discover ways to prevent allergic reactions caused
by peanut exposure. While herbal remedies may
show some promise,5 6 most of the previous studies
have tested the efficacy and safety of desensitisation.
Food desensitisation means an increase
in threshold of food antigen causing allergic
symptoms and depends on the regular (usually
daily) consumption of the food. When dosing is interrupted, any
protective effect may be lost or
attenuated. Mechanisms for desensitisation include
decreased allergen-specific immunoglobulin E
(IgE), increased allergen-specific IgG4, and reduced
responsiveness of mast cells and basophils. In
established oral tolerance, the food can be eaten
without allergic problems even when regular dosing
ceases. Mechanisms responsible for oral tolerance
likely involve recruitment of regulatory T cells with
a shift away from the pro-allergic T helper cell subtype
2 (TH2) phenotype. There is scant information
on long-term outcomes and tolerance following oral
immunotherapy (OIT) in food allergy.
Previous immunotherapy trials
There are no immunotherapy regimens in
routine use for peanut allergy. Most (but not
all) peanut immunotherapy protocols involve an
initial escalation phase (range, 0-7 days) of orally
administered peanut, or a pre-immunotherapy oral
peanut challenge, to determine the starting dose for
OIT. This is followed by administration of further
build-up doses (range, 0-22 months) and then
maintenance doses (range, 1-36 months).
The maximum maintenance doses are
between 300 mg and 4000 mg peanut protein. While
some studies have shown encouraging results,7 8 9 10 11 12
the risk of severe reactions during peanut OIT is of
concern.
Clark et al7
reported that four children
underwent successful peanut OIT starting from 5 mg peanut protein
to reach a maintenance
dose of 800 mg peanut protein after 12 biweekly
increments. During the final open challenge, all four
subjects could ingest between 2380 mg and 2760
mg peanut protein reflecting an increase in dose
threshold of at least 48-478 fold. Hofmann et al8
showed that 20 of 28 subjects were able to complete
peanut OIT to reach a daily maintenance dose of 300
mg. Jones et al9 showed
that 27 of 29 subjects with
peanut allergy could be desensitised. Before OIT,
they were developing reactions to eating less than
50 mg peanut protein but after 4 to 22 months of
daily maintenance dosing with 300 mg, they were
able to ingest 3900 mg. Similarly, Blumchen et al10
reported successfully desensitising 14 of 23 subjects
with OIT to reach a maintenance dose of 500 mg
peanut. Anagnostou et al11
reported successful desensitisation in 19 of 22 patients. Thirty weeks into
the maintenance phase of OIT and ingesting 800
mg peanut protein daily, the subjects could eat a
mean dose of peanut that was 1000-fold greater than
baseline. Varshney et al12
published the first double-blind
placebo-controlled study of peanut OIT and
showed that 16 of 19 subjects were able to consume
4000 mg after 12 months of OIT.
In these reports, while allergic symptoms
were uncommon during maintenance dosing (2.1%-3.7% of doses), they were very common during
the initial escalation phase (47%-100% of patients)
and the build-up phase (1.2%-46% of doses).7 8 9 10 11 12
Up to 10.5% of the subjects required adrenaline
treatment on the initial escalation day. The dropout
rate was high (4.5%-10.7%) due to the severity of
allergic complications. These problems have greatly
restricted the use of oral peanut desensitisation.
Use of sublingual immunotherapy (SLIT) may
hold promise but there is limited experience with
this form of desensitisation in peanut allergy. Kim et
al13 successfully desensitised 18 children with peanut
allergy using SLIT over 12 months. As assessed by
double-blind placebo-controlled food challenges, the treatment
group was able to ingest 20 times
more peanut protein compared with the placebo
group (median, 1710 vs 85 mg peanut protein). In
2013, Fleischer et al14
showed that after 44 weeks of
SLIT, 14 out of 20 peanut-allergic subjects showed
increased ability to ingest peanut protein from 3.5 mg
to 496 mg; and after 68 weeks of SLIT, the increase
was twice as high at 996 mg. Allergic symptoms
developing during SLIT were reported with 11.5% of
peanut doses and 8.6% of placebo doses. Of the 4182
active peanut doses, only 0.26% of the doses taken
at home required antihistamine treatment and 0.02%
required use of salbutamol. Thus, with the limited
data available, SLIT appeared to have fewer allergic
side-effects than OIT.
Anti-IgE administration has the potential
to prevent peanut allergy,15
16 as it reduces
free-circulating
IgE levels and inhibits expression of the
high-affinity IgE receptor on mast cells and other
immune cells.17 18 19 20 Leung et al15 showed that 450 mg of
a humanised IgG1 monoclonal antibody against IgE
significantly increased the threshold of sensitivity to
peanut on oral food challenge from approximately
half a peanut to almost nine peanuts. Similarly,
Sampson et al16 have
suggested that the anti-IgE
monoclonal antibody omalizumab (Xolair; Novartis,
Basel, Switzerland), which is approved in Hong Kong
and in many other countries for treating severe
asthma, could increase the tolerability to peanut.
Unfortunately, this latter study was terminated early
because of two severe anaphylactic reactions after
oral peanut challenge during the recruitment phase.
These results are encouraging but Xolair
has to be administered by subcutaneous injection.
As the dose and frequency of administration are
determined by total serum IgE and body weight,
it is suited optimally for only those within 20% of
the ideal body weight. Furthermore, the drug is
expensive and peanut allergy relapses soon after
anti-IgE is discontinued; thus, it cannot induce long-term
tolerance, which may likely require specific
allergen immunotherapy.
Recent developments
There have been some recent advances in
peanut
OIT that look promising. Anagnostou et al21
conducted a randomised controlled cross-over trial
comparing OIT using peanut flour with peanut
avoidance. They reported successful OIT in 62% of
a group of children aged 7 to 16 years with peanut
allergy. There was an initial updosing schedule of
biweekly increments up to a maximum oral intake
of 800 mg peanut protein/day. This was followed by
a maintenance period when the highest dose that
could be safely eaten was taken daily for 26 weeks.
By this time, 91% could ingest 800 mg peanut protein
daily versus none in the control group, and 54%
had no reactions to a 1400 mg peanut challenge. Side-effects were
reported in 20% of subjects but
they were mostly mild consisting mainly of gastro-intestinal
symptoms and oral pruritus. The median
peanut threshold dose had increased by 25.5-fold.
In light of the biological activities of
Xolair, it
was logical to combine it with peanut OIT to test
whether the drug can facilitate allergen-specific
desensitisation by reducing incidence of side-effects.
A period of pretreatment with anti-IgE has already
been reported to decrease acute allergic reactions
developing during rush immunotherapy for ragweed-induced
seasonal rhinitis and milk allergy.22
23
Schneider et al24
treated 13 children with a
brief course of Xolair over 20 weeks. At 12 weeks
of Xolair administration, OIT was started. On the
first day of OIT, 11 desensitising doses of peanut
flour were given over 6 hours (rush OIT). This was
followed by a slower escalation phase of peanut
allergen doses at weekly intervals for 7 to 12 weeks
until the subjects were receiving 4000 mg of peanut
flour (equivalent to about 9-10 peanuts) daily at
which time Xolair was discontinued. The children
then continued to ingest 4000 mg peanut flour daily
during maintenance phase. On this regimen, the
subjects were able to ingest 160 to 400 times the dose
that could be eaten before OIT. The rapidity with
which the patients reached 4000 mg was notable
and this was achieved with only about 2% of the
peanut doses associated with mild allergic reactions.
The initial rush desensitisation allowed the patients
to ingest a cumulative dose of 992 mg peanut flour
(about 2 peanuts) after only 24 hours of OIT. This
would have removed the patient very rapidly from
risk of anaphylaxis caused by accidental exposure.
Schneider et al’s report24 is very similar to
our experience in Hong Kong. We have completed
the first phase of a small pilot desensitisation study
in four children with mild-to-moderately severe
peanut allergy in which Xolair and peanut OIT were
combined. The inclusion criteria for the study were
volunteers aged 8 years or older with a history of
peanut allergy manifested by any of the following:
urticaria, angioedema, asthma, gastro-intestinal symptoms, or anaphylaxis
within 60 minutes of ingestion; a serum total IgE
between 30 and 1500 IU/mL; a positive double-blind
placebo-controlled oral peanut challenge; good
general health; within 20% of ideal body weight; a
positive skin prick test (at least 3 x 3 mm wheal greater
than diluent control); a positive serum-specific
IgE to peanut as measured by radioallergosorbent
test (RAST); and no prior exposure to monoclonal
antibodies. Asthma must have been stable with a
forced expiratory volume in 1 second of at least
80% predicted value. Systemic glucocorticoids,
beta blockers, and angiotensin-converting enzyme
inhibitors were prohibited before screening and
throughout the study. Aspirin, antihistamines, and
antidepressants were not permitted for 3 days, 1 week, and 2
weeks, respectively, before skin testing or
oral food challenge. If patients had poorly controlled
asthma and/or atopic dermatitis, or inability to
discontinue antihistamines or other medications for
skin testing and oral challenges, they were excluded.
They were also deemed ineligible if it seemed
unlikely that they would be able to comply with the
study protocol for any reason. The subjects were
recruited from patients attending the Allergy Centre
at the Hong Kong Sanatorium and Hospital. The
study was approved by the Hospital Research Ethics
Committee; both written informed consent from the
children’s parents and the children’s informed verbal
assent were obtained. The inclusion and exclusion
criteria for the previous trials cited in this review are
included in Table 1 7 8 9 10 11 12 13 14 21 24
for comparison.
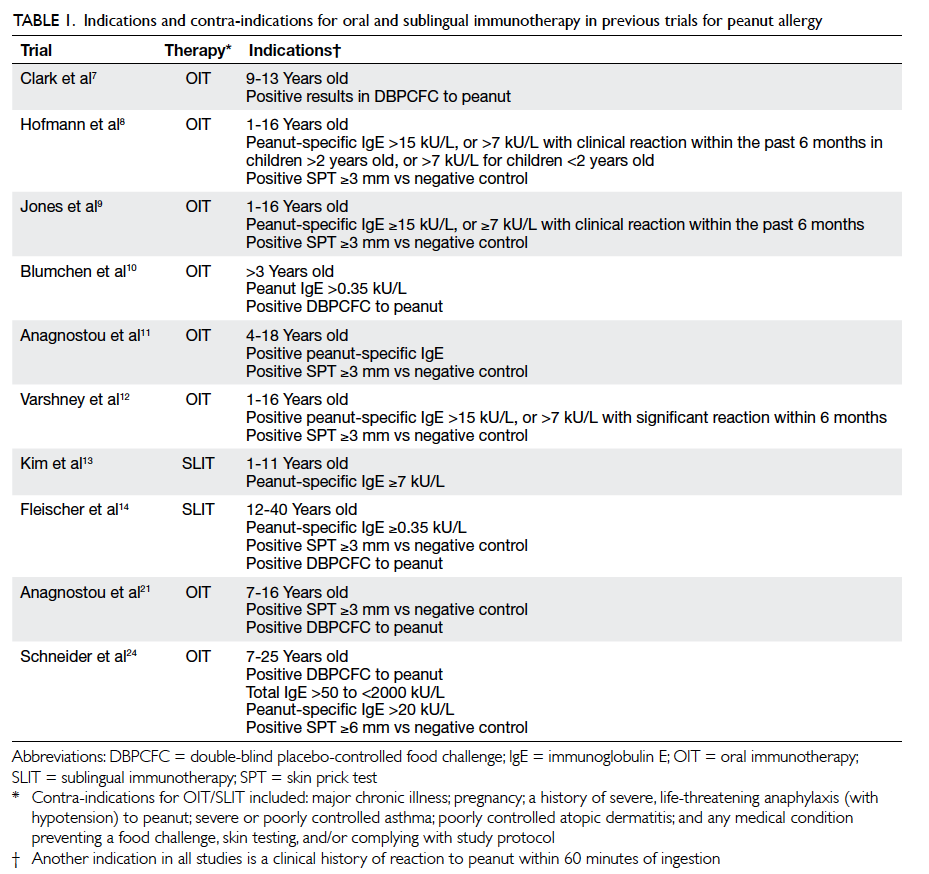
Table 1. Indications and contra-indications for oral and sublingual immunotherapy in previous trials for peanut allergy
The children in our study had a history of
peanut allergy manifested by urticaria, angioedema,
asthma, sore mouth, and anaphylaxis within minutes
of ingestion (Table 2). Their serum total IgE levels
were raised and they had a positive skin prick test
and RAST to peanut. They were also positive for
specific IgE to Ara h 2, a molecular component of
peanut protein which, at high levels, is reported to
identify a subgroup of subjects allergic to peanut
with more severe symptoms, although this issue is
considered debatable.25
Each child had a positive,
double-blinded oral peanut challenge at recruitment
confirming their clinical allergy.
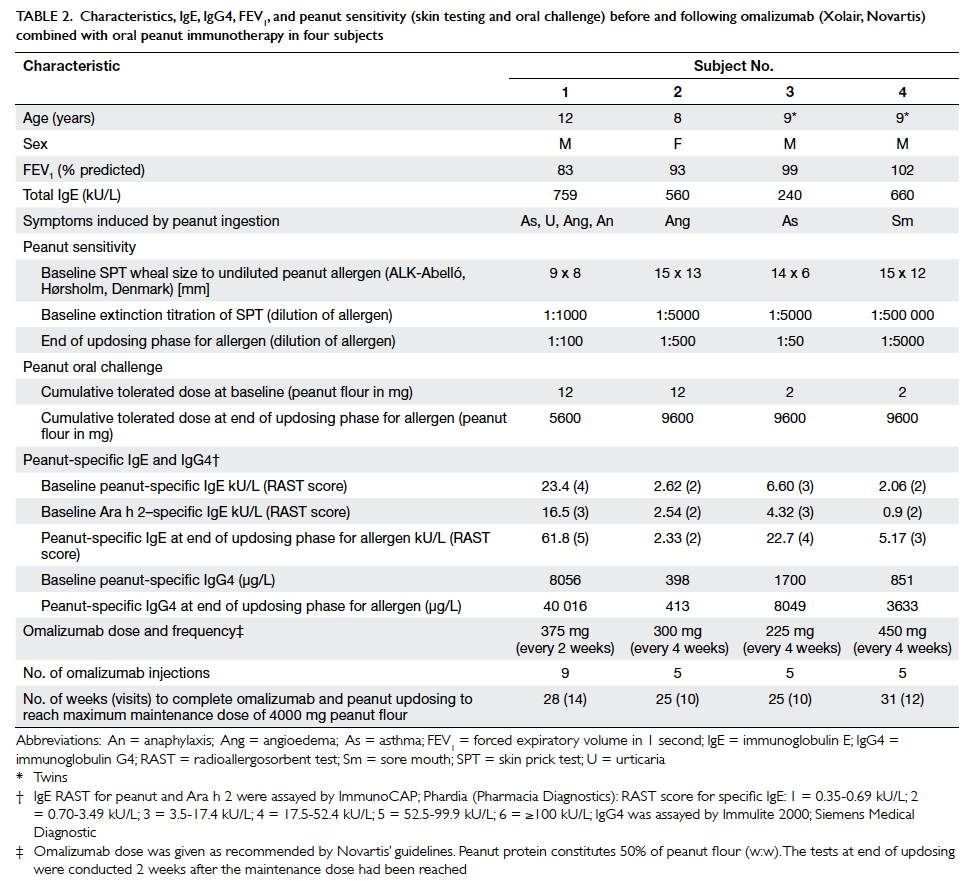
Table 2. Characteristics, IgE, IgG4, FEV1, and peanut sensitivity (skin testing and oral challenge) before and following omalizumab (Xolair, Novartis) combined with oral peanut immunotherapy in four subjects
The study protocol had three stages. In
stage
1, each subject received Xolair for 16 to 18 weeks.
At 12 weeks of Xolair treatment, each subject had a
graded oral peanut challenge to ensure that Xolair
had increased the amount of peanut protein that
could be ingested. If the challenge showed at least
a two-step increase in the threshold dose of peanut
provoking a reaction compared with baseline, OIT was
started. If the increase in threshold was less than
two-dose steps, the peanut challenge was repeated
4 weeks later to ensure that the threshold target
had been met before OIT was initiated; if not, the
subject was withdrawn. In stage 2, OIT had an
escalation phase of peanut oral administration with
updosing at biweekly intervals. In the most sensitive
subjects, the doses could be: 0.5, 1, 2, 5, 12, 25,
50, 100, 200, 400, 800, 1200, 1600, and 2000 mg of
peanut protein, given as defatted peanut flour with
50% peanut protein by weight. However, if subjects
became less sensitised to peanut during Xolair
treatment, as was the case in all our four subjects,
the escalation phase might start in the mid-range of
the dose range indicated above, thus, shortening the
escalation phase considerably. The escalation phase
was followed by maintenance phase when subjects
continued to ingest the top dose of peanut (4000
mg peanut flour) for 36 months. Stage 3 was started
when OIT ceased after 36 months and subsequent
progress was monitored to assess whether long-term tolerance had
been induced over the next 36 months
(end of stage 3). Our study subjects are in stage 2 of
the pilot study.
The Hong Kong protocol differed from
Schneider et al’s24 in
some respects. We treated
the children with Xolair for 16 to 18 weeks and not
20 weeks. The Xolair treatment only overlapped
the initial few weeks of OIT in the Hong Kong
subjects whereas in Schneider’s protocol, Xolair
was administered during the entire build-up phase
of OIT. The serum elimination half-life of Xolair
averaged about 26 days, so even when the injections
were stopped, the drug effect would likely have
persisted significantly longer. We did not have a
rush OIT phase, preferring to updose more slowly
at biweekly intervals to give a wider margin of safety.
As a consequence, the duration of our escalation
phase was slightly longer (14 weeks) compared with
7 to 12 weeks in the Schneider et al’s study.24 Despite
these differences in protocol design, the results were very
similar between the two studies.
One subject (subject 1) experienced mild
abdominal cramps and mild oral itching when eating
4000 mg peanut flour (2000 mg peanut protein;
equivalent to about 9 peanuts as each peanut
contains about 240 mg peanut protein) as a single
daily dose at home, but was able to ingest the dose
when administered in two 2000 mg doses separated
by at least 30 minutes. Compared with baseline,
when subjects could only eat 2 to 12 mg peanut
flour, at the end of the escalation phase on formal
challenge under supervision, three subjects could
eat a cumulative maximum dose of 9600 mg peanut
flour (about 20 peanuts) [Table 2]. Subject 1 could
eat a cumulative dose of 5600 mg (about 11 peanuts)
but reacted at 9600 mg with mild abdominal cramps
which resolved spontaneously. On the combination
regimen, the children were, therefore, able to eat
between 466- and 4800-fold more peanut
protein than before they were desensitised. Subjects’ threshold
skin prick test reactions to peanut extract
had also increased (10-100 fold) [Table 2]. Thus, at
the end of the escalation phase, all the children could
ingest many more peanuts than would have been
eaten inadvertently, and were protected from severe
allergic reactions after accidental ingestion.
The clinical improvement was accompanied
by an increase in each subject’s peanut-specific
IgG4, suggesting mechanistic recruitment of the
interleukin-10/Treg pathway and a shift away from
the pro-allergic TH2 phenotype. It was noted that
serum peanut-specific IgE increased in three out
of the four children following Xolair and updosing of allergen, when concentrations
might have been expected to decrease, as in other
forms of allergen-specific desensitisation (Table 2).
Interpretation of IgE measurements following
Xolair administration is difficult because the drug
complexes with free-circulating IgE resulting in an
apparent increase in total IgE levels that may last for
many weeks after treatment.26
Measurement of free-serum
IgE would circumvent this problem but this is
technically difficult to assay and was not performed
in our laboratory. Instead, we used extinction skin
prick tests as a surrogate marker of mast cell-bound
peanut-specific IgE.
The incidence of side-effects during
desensitisation in our limited experience was 0.2%
of total number of peanut doses, which is much
less than the incidence reported previously in the
absence of Xolair cover7 8 9
10 11 12
and even less than the 2%
reported recently.24
Conclusion
The results of recent studies taken
together are
encouraging and strongly suggest that there are
several new strategies, including the use of anti-IgE
with OIT, that could now allow desensitisation to
peanut to be undertaken safely and, in one study,
very rapidly. These approaches may have merit in
the future for treating severe peanut allergy once
protocols have been refined and results validated.
However, these treatment regimens should always
be used by experienced and appropriately trained
clinicians, in an environment where facilities are
available for emergency resuscitation in case a
serious adverse event occurs. Whether the regimens
can induce long-term tolerance will have to await
review of progress when OIT ceases after 3 years.
Acknowledgements
The authors thank The Hong Kong Sanatorium
and
Hospital for support and to the study steering group
(Dr YC Tsao, Dr Walton Li, Prof Raymond Liang,
Prof Kar-nang Lai, Dr Edmond Ma, and Dr Stephen
Till) for advice. We also thank Ms Melissa Tung for
secretarial assistance.
Declaration
No conflicts of interests were declared by
authors.
References
1. Wang J, Sampson HA. Food
anaphylaxis. Clin Exp Allergy 2007;37:651-60. CrossRef
2. Leung TF, Yung E, Wong YS, Lam
CW, Wong GW. Parent-reported adverse food reactions in Hong Kong
Chinese pre-schoolers: epidemiology, clinical spectrum and risk
factors. Pediatr Allergy Immunol 2009;20:339-46. CrossRef
3. Ho MH, Lee SL, Wong WH, Ip P,
Lau YL. Prevalence of self-reported food allergy in Hong Kong
children and teens—a population survey. Asian Pac J Allergy
Immunol 2012;30:275-84.
4. Sicherer SH, Noone SA,
Muñoz-Furlong A. The impact of childhood food allergy on quality
of life. Ann Allergy Asthma Immunol 2001;87:461-4. CrossRef
5. Li XM, Zhang TF, Huang CK, et
al. Food Allergy Herbal Formula-1 (FAHF-1) blocks peanut-induced
anaphylaxis in a murine model. J Allergy Clin Immunol
2001;108:639-46. CrossRef
6. Srivastava KD, Kattan JD, Zou
ZM, et al. The Chinese herbal medicine formula FAHF-2 completely
blocks anaphylactic reactions in a murine model of peanut allergy.
J Allergy Clin Immunol 2005;115:171-8. CrossRef
7. Clark AT, Islam S, King Y,
Deighton J, Anagnostou K, Ewan PW. Successful oral tolerance
induction in severe peanut allergy. Allergy 2009;64:1218-20. CrossRef
8. Hofmann AM, Scurlock AM, Jones
SM, et al. Safety of a peanut oral immunotherapy protocol in
children with peanut allergy. J Allergy Clin Immunol
2009;124:286-91. CrossRef
9. Jones SM, Pons L, Roberts JL, et
al. Clinical efficacy and immune regulation with peanut oral
immunotherapy. J Allergy Clin Immunol 2009;124:292-300, 300.e1-97.
10. Blumchen K, Ulbricht H, Staden
U, et al. Oral peanut immunotherapy in children with peanut
anaphylaxis. J Allergy Clin Immunol 2010;126:83-91. CrossRef
11. Anagnostou K, Clark A, King Y,
Islam S, Deighton J, Ewan P. Efficacy and safety of high-dose
peanut oral immunotherapy with factors predicting outcome. Clin
Exp Allergy 2011;41:1273-81. CrossRef
12. Varshney P, Jones SM, Scurlock
AM, et al. A randomized controlled study of peanut oral
immunotherapy: clinical desensitization and modulation of the
allergic response. J Allergy Clin Immunol 2011;127:654-60. CrossRef
13. Kim EH, Bird JA, Kulis M, et
al. Sublingual immunotherapy for peanut allergy: clinical and
immunologic evidence of desensitization. J Allergy Clin Immunol
2011;127:640-6. CrossRef
14. Fleischer DM, Burks AW,
Vickery BP, et al. Sublingual immunotherapy for peanut allergy: a
randomized, double-blind, placebo-controlled multicenter trial. J
Allergy Clin Immunol 2013;131:119-27.e1-7.
15. Leung DY, Sampson HA,
Yunginger JW, et al. Effect of anti-IgE therapy in patients with
peanut allergy. N Engl J Med 2003;348:986-93. CrossRef
16. Sampson HA, Leung DY, Burks
AW, et al. A phase II, randomized, double-blind, parallel-group,
placebo-controlled oral food challenge trial of Xolair
(omalizumab) in peanut allergy. J Allergy Clin Immunol
2011;127:1309-10. CrossRef
17. Djukanović R, Wilson SJ, Kraft
M, et al. Effects of treatment with anti-immunoglobulin E antibody
omalizumab on airway inflammation in allergic asthma. Am J Respir
Crit Care Med 2004;170:583-93. CrossRef
18. Milgrom H, Fick RB Jr, Su JQ,
et al. Treatment of allergic asthma with monoclonal anti-IgE
antibody. rhuMAb-E25 Study Group. N Engl J Med 1999;341:1966-73. CrossRef
19. MacGlashan DW Jr, Bochner BS,
Adelman DC, et al. Down-regulation of Fc(epsilon)RI expression on
human basophils during in vivo treatment of atopic patients with
anti-IgE antibody. J Immunol 1997;158:1438-45.
20. Prussin C, Griffith DT, Boesel
KM, Lin H, Foster B, Casale TB. Omalizumab treatment downregulates
dendritic cell FcepsilonRI expression. J Allergy Clin Immunol
2003;112:1147-54. CrossRef
21. Anagnostou K, Islam S, King Y,
et al. Assessing the efficacy of oral immunotherapy for the
desensitisation of peanut allergy in children (STOP II): a phase 2
randomised controlled trial. Lancet 2014;383:1297-304. CrossRef
22. Klunker S, Saggar LR,
Seyfert-Margolis V, et al. Combination treatment with omalizumab
and rush immunotherapy for ragweed-induced allergic rhinitis:
inhibition of IgE-facilitated allergen binding. J Allergy Clin
Immunol 2007;120:688-95. CrossRef
23. Nadeau KC, Schneider LC, Hoyte
L, Borras I, Umetsu DT. Rapid oral desensitization in combination
with omalizumab therapy in patients with cow’s milk allergy. J
Allergy Clin Immunol 2011;127:1622-4. CrossRef
24. Schneider LC, Rachid R,
LeBovidge J, Blood E, Mittal M, Umetsu DT. A pilot study of
omalizumab to facilitate rapid oral desensitization in high risk
peanut-allergic patients. J Allergy Clin Immunol 2013;132:1368-74. CrossRef
25. Lopes de Oliveira LC, Aderhold
M, Brill M, et al. The value of specific IgE to peanut and its
component Ara h 2 in the diagnosis of peanut allergy. J Allergy
Clin Immunol Pract 2013;1:394-8. CrossRef
26. Hamilton RG, Marcotte GV,
Saini SS. Immunological methods for quantifying free and total
serum IgE levels in allergy patients receiving omalizumab (Xolair)
therapy. J Immunol Methods 2005;303:81-91. CrossRef

