Suggestions to minimise hesitancy and promote vaccination of children in Hong Kong
Hong Kong Med J 2023 Feb;29(1):86 | Epub 6 Jan 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
LETTER TO THE EDITOR
Suggestions to minimise hesitancy and promote
vaccination of children in Hong Kong
Jeffrey Chan1; KS Ng, PhD2; Benny YC Hon, PhD3,4; Simon C Lam, PhD, RN5
1 King George V School, Hong Kong
2 Department of Diagnostic Radiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong
3 Department of Mathematics, City University of Hong Kong, Hong Kong
4 Department of Psychology, The University of Science and Technology of China, Hefei, China
5 School of Nursing, Tung Wah College, Hong Kong
Corresponding author: Prof Simon C Lam (simlc@alumni.cuhk.net)
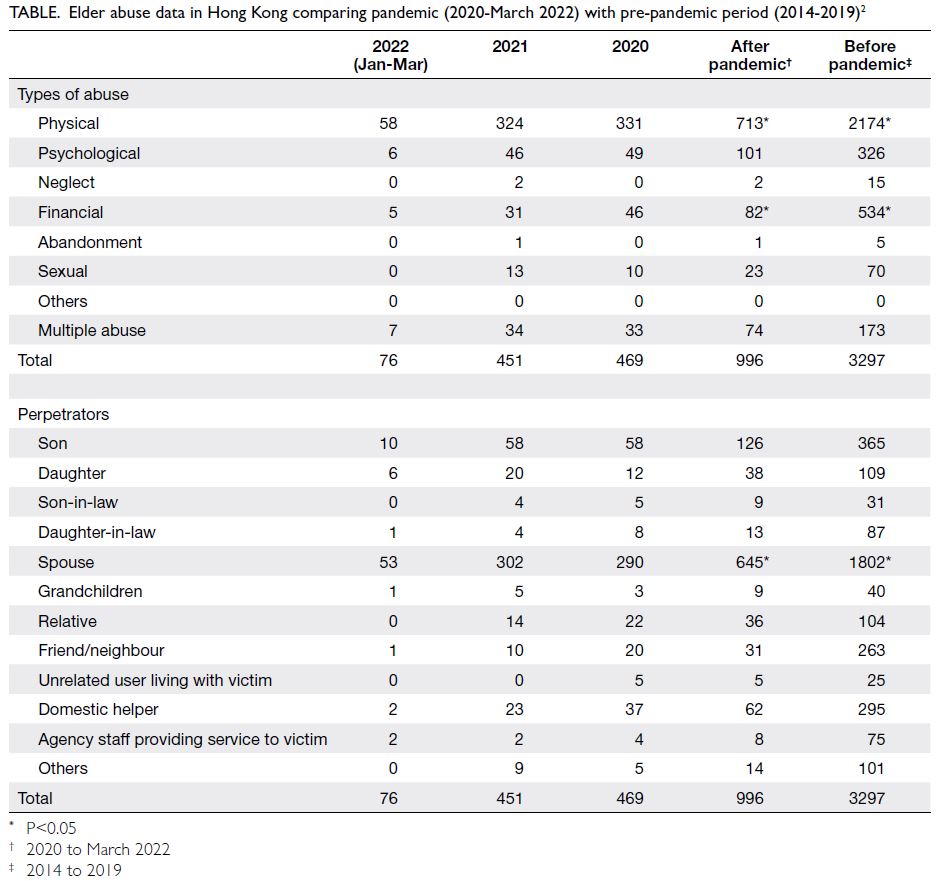
To the Editor—Coronavirus disease 2019 (COVID-19) vaccine hesitancy was high initially1 but persists in
Hong Kong. As of 2 January 2023, 93.0% and 83.3%
of the population had received the second and
third doses of the COVID-19 vaccine, respectively.2
However, vaccination uptake for the third dose was
only 77.06% for age-group of 12-19 years and 30.92%
for those aged 3-11 years.2 The government intends
to lower the vaccine pass age limit to 5 years and allow
schools to hold full-day face-to-face classes only if
90% of students have received three doses of vaccine
before 1 November 2022. According to one study,3
there are four reasons for parental vaccine refusal:
religious beliefs, personal beliefs or philosophical
reasons, safety concerns, and a desire for more
information from healthcare providers. Clearly the
reason for the low vaccination rate in Hong Kong is
mainly due to safety concerns. The Centre for Health
Protection of the Department of Health publishes
public health recommendations for the Hong Kong
Childhood Immunisation Programme. Advised
vaccines from birth onwards include Bacillus
Calmette-Guérin, hepatitis B, and diphtheria,
tetanus, acellular pertussis and inactivated poliovirus
vaccines. Although the side-effects of these vaccines,
such as low fever, redness, and a sore arm, are well
known, parents do not oppose their administration.
We recommend that the Department of Health
collaborate with the Education Bureau to implement
measures that can improve vaccine uptake. These
include: provision of more scientific information,
educational and on-site vaccination interventions
to schools with a low vaccination rate,4 measures to
enhance the biological literacy of school children to
encourage vaccination, rapid response by healthcare
professionals to dispel rumours and conspiracy
theories, education of parents about the benefits of
vaccination, and provision of educational seminars
via Zoom as continued regular education has
been shown to improve the success of vaccination
programmes.5 In addition, the Centre for Health
Protection may consider other options such as
delivering a fractional dose to minimise the side-effects.
Another study6 suggests that using an
intradermal fractional dose (10-20% the amount of
the original dose) vaccination using a microneedle
patch can result in better immunogenicity. Side-effects
are the most common concern among those
who are hesitant or opposed to vaccination. As the
safety profile of fractional doses is comparable to
that of a regular dose,7 fractional dosages may help combat such hesitancy.
Author contributions
All authors contributed to the drafting of the letter and critical revision for important intellectual content. All
authors approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
The authors have declared no conflicts of interest.
Funding/support
The work of this letter was supported by a grant from the Research Grants Council of the Hong Kong Special
Administrative Region (Project No.: CityU 11303521).
References
1. Yu BY, Lam JC, Lam SC, et al. COVID-19 vaccine hesitancy
and resistance in an urban Chinese population of Hong
Kong: a cross-sectional study. Hum Vaccin Immunother
2022;18:2072144. Crossref
2. Hong Kong SAR Government. Hong Kong vaccination
dashboard. Available from: https://www.covidvaccine.gov.hk/en/dashboard. Accessed 2 Jan 2023.
3. McKee C, Bohannon K. Exploring the reasons behind
parental refusal of vaccines. J Pediatr Pharmacol Ther
2016;21:104-9. Crossref
4. Gellert P, Bethke N, Seybold J. School-based educational
and on-site vaccination intervention among adolescents:
study protocol of a cluster randomised controlled trial.
BMJ Open 2019;9:e025113. Crossref
5. Akther T, Nur T. A model of factors influencing COVID-19
vaccine acceptance: a synthesis of the theory of reasoned
action, conspiracy theory belief, awareness, perceived
usefulness, and perceived ease of use. PLoS One
2022;17:e0261869. Crossref
6. Migliore A, Gigliucci G, Di Marzo R, Russo D, Mammucari
M. Intradermal vaccination: a potential tool in the battle
against the COVID-19 pandemic? Risk Manag Healthc
Policy 2021;14:2079-87. Crossref
7. Yang B, Huang X, Gao H, Leung N, Tsang T, Cowling B.
Immunogenicity, efficacy, and safety of SARS-CoV-2
vaccine dose fractionation: a systematic review and meta-analysis.
BMC Med 2022;20:409. Crossref