Hong
Kong Med J 2019 Jun;25(3):209–15 | Epub 29 May 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Outcomes of transperineal and transrectal
ultrasound-guided prostate biopsy
KL Lo, MB, ChB, FHKAM (Surgery)1; KL
Chui, MB, BS, FHKAM (Surgery)1; CH Leung, MSc2; SF
Ma, MB, ChB1; Kevin Lim, MB, ChB1; Timothy Ng, MB,
ChB1; Julius Wong, MB, ChB1; Joseph KM Li, MB, ChB,
FHKAM (Surgery)1; SK Mak, MB, BS, FHKAM (Surgery)1;
CF Ng, MB, ChB, FHKAM (Surgery)1,2
1 Division of Urology, North District
Hospital, New Territories East Cluster Urology Unit, Prince of Wales
Hospital, Shatin, Hong Kong
2 SH Ho Urology Centre, Department of
Surgery, The Chinese University of Hong Kong, Shatin, Hong Kong
Corresponding author: Prof CF Ng (ngcf@surgery.cuhk.edu.hk)
Abstract
Objective: To compare the
clinical outcomes and pathological findings of transperineal
ultrasound-guided prostate biopsy (TPUSPB) and transrectal
ultrasound-guided prostate biopsy (TRUSPB) in a secondary
referral hospital.
Methods: This was a
retrospective study of 100 TPUSPBs and 100 TRUSPBs performed in our
centre. Pre-biopsy patient parameters (eg, patient age, clinical
staging, serum prostate-specific antigen [PSA] level, prostate size, and
PSA density), as well as pathological results and 30-day complication
and readmission rates, were retrieved from the patients’ medical records
and compared between the two groups.
Results: One hundred TPUSPBs
performed from January 2018 to May 2018 and 100 TRUSPBs performed from
January 2016 to April 2016 were included for analysis. Mean age did not
significantly differ between the groups. The TPUSPB group had a higher
mean PSA level, smaller prostate size, and higher PSA density, compared
with the TRUSPB group. The overall prostate cancer detection rate was
similar between the TPUSPB and TRUSPB groups (35% vs 25%, P=0.123).
There were no significant differences between the groups in prostate
cancer detection rates after stratification according to PSA density and
clinical staging. With respect to complications, no patients developed
fever in the TPUSPB group, while 4% of patients in the TRUSPB group had
fever and required at least 1-week admission for intravenous antibiotic
administration.
Conclusion: For prostate biopsy,
TPUSPB is safer, with no infection complications, and has similar
prostate cancer detection rate compared with TRUSPB.
New knowledge added by this study
- There were no sepsis complications associated with the use of transperineal prostate biopsy (TPUSPB), which avoids penetration of the rectal mucosa and possible transfer of intestinal flora to the blood stream during the procedure.
- In terms of prostate cancer detection, TPUSPB was comparable to transrectal prostate biopsy (TRUSPB). Moreover, TPUSPB may have an advantage over TRUSPB in patients with previous negative biopsy findings, as it does not neglect prostate cancer in the anterior fibromuscular stroma.
- TPUSPB is suitable for use as a routine, 1-day out-patient procedure, which may be helpful for patients who must travel a considerable distance to reach the hospital.
- TPUSPB may be more suitable for patients who cannot undergo general anaesthesia or monitored anaesthesia care.
- TPUSPB might be a good alternative to TRUSPB, particularly for patients with increased risk of sepsis.
Introduction
According to the Hong Kong Cancer Registry,1 prostate cancer is the third most common cancer in men.
As in other cancers, biopsy is needed for histological confirmation of the
diagnosis of prostate cancer before treatment is initiated. With the
increasing age of the population, the incidence rate of this cancer is
expected to increase; the frequency of prostate biopsy will therefore also
increase. Hodge et al2 introduced
the systematic sextant biopsy protocol under transrectal ultrasound
guidance. Transrectal ultrasound-guided prostate biopsy (TRUSPB) has since
become a widely accepted and routinely performed technique to detect
prostate cancer.3 In Hong Kong,
most urologists use TRUSPB to confirm the diagnosis of prostate cancer,
particularly in patients with elevated prostate-specific antigen (PSA) or
abnormal digital rectal examination; TRUSPB is also used in patients
undergoing active surveillance of prostate cancer. However, there are
complications associated with the use of TRUSPB. Most notably, because the
procedure is performed via the rectum, there is a risk of postprocedural
sepsis; the incidence of sepsis ranged was 2% to 4% in contemporary
series,4 5
and sepsis-related mortality has also been reported.6
An increasing number of studies have demonstrated
success in cancer diagnosis with extended biopsy using transperineal
ultrasound-guided prostate biopsy (TPUSPB). In an early report, Kojima et
al7 retrospectively assessed the
usefulness of TPUSPB, which differs from TRUSPB in terms of patient
position, puncture route, puncture site, and ultrasound probe.8 Most importantly, the TPUSPB enables urologists to
thoroughly prepare the perineum with a disinfectant solution to eliminate
the possibility of skin flora contamination of the puncture site.9 In addition, this procedure involves puncture of
perineal skin under the guidance of a side-fire ultrasound probe without
penetration of rectal mucosa, thereby avoiding the possibility that
intestinal flora are transferred to the blood stream. An Australian study
group showed that TPUSPB, in combination with antibiotic prophylaxis,
could almost entirely prevent sepsis complications.10 Some authors have suggested that TPUSPB may be
performed without antibiotic prophylaxis, thus reducing the risk of
generating antibiotic resistance.4
Based on these potential benefits, our centre introduced TPUSPB beginning
in January 2018. Subsequently, we have completely replaced TRUSPB with
TPUSPB. In this study, we aimed to compare the outcomes of our initial
series of patients who underwent TPUSPB with those of our previous cohort
of patients who underwent TRUSPB.
Methods
In this retrospective cohort study, we compared 100
patients who underwent TPUSPB with 100 patients who underwent TRUSPB in
our centre. This study was approved by our institutional ethics committee.
All 100 patients who underwent TPUSPB from January 2018 to May 2018
(TPUSPB group) were included; 100 patients who underwent TRUSPB from
January 2016 to April 2016 were also included. The indications for biopsy
for both groups were serum PSA >4 ng/dL, abnormal digital rectal
examination, and surveillance biopsy for patients under active
surveillance.
The following data were retrieved from hospital
records and compared between the two groups: age, serum PSA level,
prostate size, PSA density, prostate cancer detection rate, and
complications (eg, admission due to acute retention of urine, rectal
bleeding, haematuria, fever, and sepsis). We used the Third International
Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) as an acute
change in total sequential organ failure assessment score ≥2 points due to
the infection11: (1) respiratory
rate ≥22/min, (2) altered mental activity, and (3) systolic blood pressure
≤100 mm Hg.
For both groups, the indications for prostatic
biopsies were serum PSA level >4 ng/dL, abnormal digital rectal
examination, or follow-up biopsy for patients under active surveillance.
All patients underwent pre-procedure blood tests and urine tests to ensure
there was no bleeding tendency or positive urine culture. Patients using
antiplatelet or anticoagulant treatment were required to discontinue drugs
prior to undergoing biopsy. All patients used a sodium phosphate rectal
enema in the morning of the procedure and took oral prophylactic
antibiotics (1 g amoxicillin-clavulanate and 500 mg ciprofloxacin) 2 hours
before the procedure. For TPUSPB, numbing cream (2.5% lidocaine and 2.5%
prilocaine) was applied over the perineal region 1 hour before the
procedure and 1% lidocaine (20 mL) was injected into the perineum as local
anaesthesia (LA) immediately prior to prostate biopsy, at a 45-degree
angle from the midline and approximately 15 mm above the anus on either
side. Details of the two procedures are described below. After either
procedure, all patients were given an additional 1-day course of oral
antibiotics (1 g amoxicillin-clavulanate and 500 mg ciprofloxacin).
When undergoing TRUSPB, patients assumed the left
lateral position, as shown in Figure 1. Prostate size was measured using a
transrectal biplanar ultrasound probe. Subsequently, 10 core biopsies were
taken: five cores were taken from each side of the prostate at the base,
mid, apex, upper lateral, and lower lateral regions. Each procedure was 5
to 10 minutes in duration.
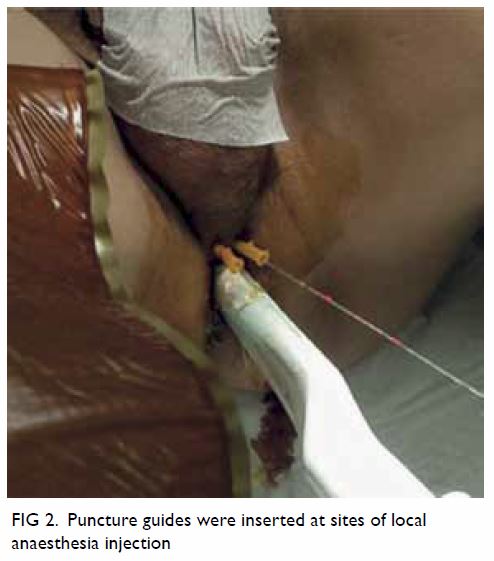
When undergoing TPUSPB, patients assumed the
Lloyd-Davies position prior to injection of lidocaine for LA (described
above). After lidocaine injection, a biplanar ultrasound probe was
inserted through the anus. The prostate size was measured, and 14-gauge
angiocatheters were then inserted at the sites previously used for LA
injection, as shown in Figure 2. Ten core biopsies were obtained in a
manner similar to that of TRUSPB. Because of the different orientation of
the biopsy needle, the apical biopsy was targeted towards the anterior
fibromuscular layer. The biopsy needle was maintained parallel to the
probe to ensure clear visualisation of the targeted area, which was
possible when the whole needle was completely visualised on ultrasound (Fig 3). Each procedure was 10 to 15 minutes in
duration. We also assessed the pain experienced during the TPUSPB at three
time points, namely during probe insertion into the anus, LA injection,
and biopsy procedures, by verbal analogue scale (0-10) during TPUSPB.
Statistical analyses were performed using SPSS
(Windows version 24.0; IBM Corp, Chicago [IL], United States). For
continuous variables, age was compared by independent t test,
while PSA, prostate size, and PSA density were compared by the
Mann-Whitney U test as they did not exhibit normal distributions.
Normality was assessed by normal QQ plots and the Shapiro-Wilk test. When
comparing categorical variables, including cancer detection rates and
complication rates, the Chi squared test was used if the expected count in
each cell was >5; otherwise, Fisher’s exact test was used. In addition,
the Cochran-Mantel-Haenszel test was performed to assess whether there was
an association between the biopsy method and cancer detection rate
according to clinical stage. Differences with a two-sided P value of
<0.05 were considered to be statistically significant.
Results
The patient characteristics, prostate cancer
detection rates, and complications in patients who underwent TPUSPB,
compared with those who underwent TRUSPB, are summarised in Table
1. Age did not significantly differ between the two groups. The
median serum PSA in the TPUSPB group was higher than that in the TRUSPB
group (12.0 ng/dL vs 9.5 ng/dL, P=0.047). Moreover, the median prostate
size in the TPUSPB was smaller than that in the TRUSPB group (46.2 mL vs
56.8 mL, P=0.003). Therefore, the PSA density of TPUSPB group was higher
than that in the TRUSPB group (0.27 vs 0.16, P=0.001).
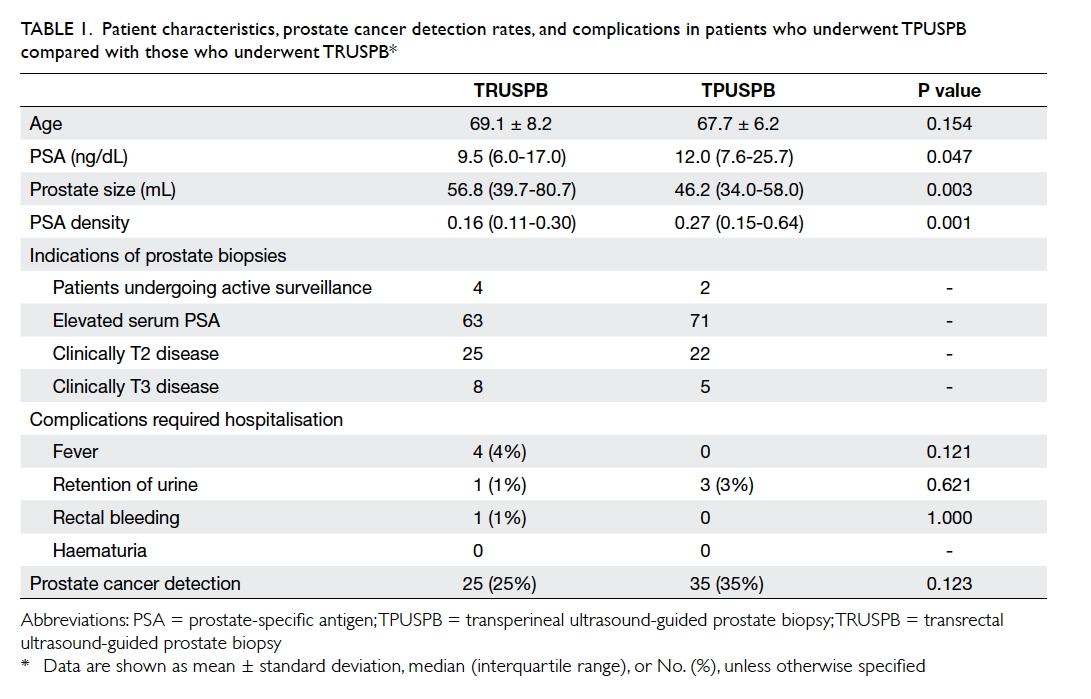
Table 1. Patient characteristics, prostate cancer detection rates, and complications in patients who underwent TPUSPB compared with those who underwent TRUSPB
Stratified prostate cancer detection rates in
patients who underwent TPUSPB, compared with those who underwent TRUSPB,
are listed in Table 2. There was no statistically significant
difference in overall prostate cancer detection rate between the two
groups. In subgroup analysis stratified by serum PSA level, the TPUSPB
group had a higher prostate cancer detection rate than the TRUSPB group
among patients with 20 to 100 ng/mL PSA (50% vs 15%, P=0.036). However,
there were no statistically significant differences in prostate cancer
detection rates among other subgroups according to PSA levels. There were
also no statistically significant differences in prostate cancer detection
rates between the two groups upon stratification according to PSA density
or clinical staging.
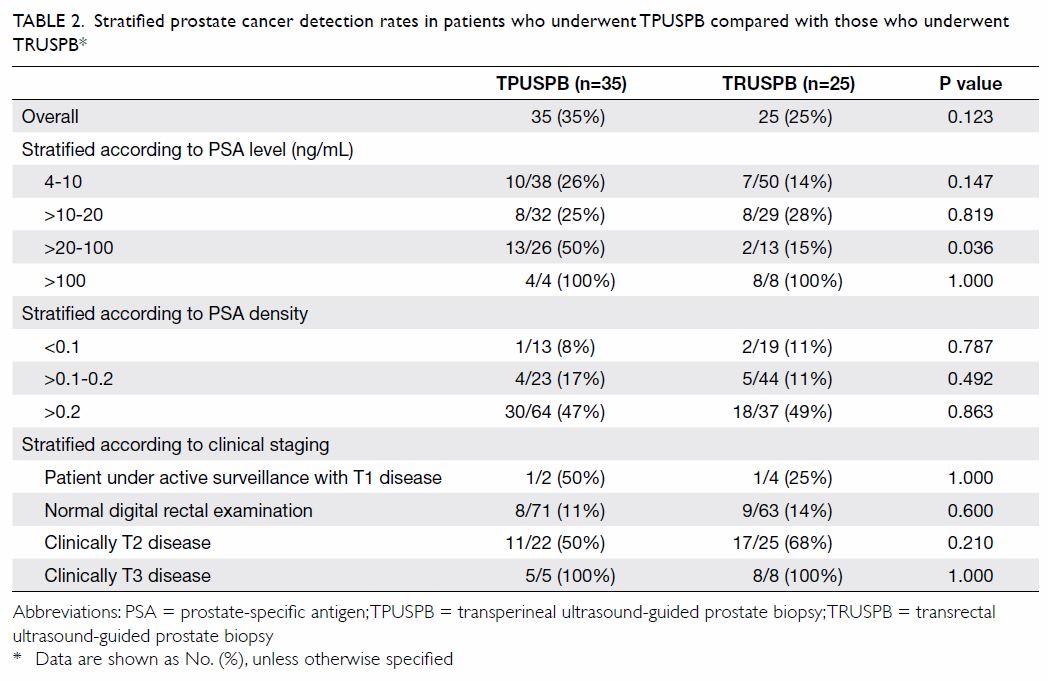
Table 2. Stratified prostate cancer detection rates in patients who underwent TPUSPB compared with those who underwent TRUSPB*
In analysis of 35 patients with positive cores in
the TPUSPB group, 16 (45.7%) patients had at least one positive core in
the anterior fibromuscular stroma. Among these 16 patients, 14 were
diagnosed with high-risk prostate cancers, with multiple positive cores in
each patient. The relatively high proportion of high-risk prostate cancer
in the TPUSPB cohort might explain the relatively high number of positive
cores in the anterior fibromuscular stroma.
In the TPUSPB group, 19 patients had previous
negative findings in TRUSPB; three of these 19 (15.7%) were diagnosed with
prostate cancer based on the findings of TPUSPB. Two of the three tumours
were detected in the anterior fibromuscular layer, and the remaining
tumour was found in the apical zone. In the TRUSPB group, 36 patients had
previous negative findings in TRUSPB; six (16.7%) of these were diagnosed
with prostate cancer based on the findings of the current TRUSPB.
Concerning about the pain experienced during
TPUSPB, the pain scores reported by patients during probe insertion into
the anus, LA injection, and biopsy procedures were 1-2, 1-2, and 2-4,
respectively.
With respect to complications, we initially planned
to use the Sepsis-3 described above, but no patients in this study
developed clinical signs of infection that met the criteria for sepsis.
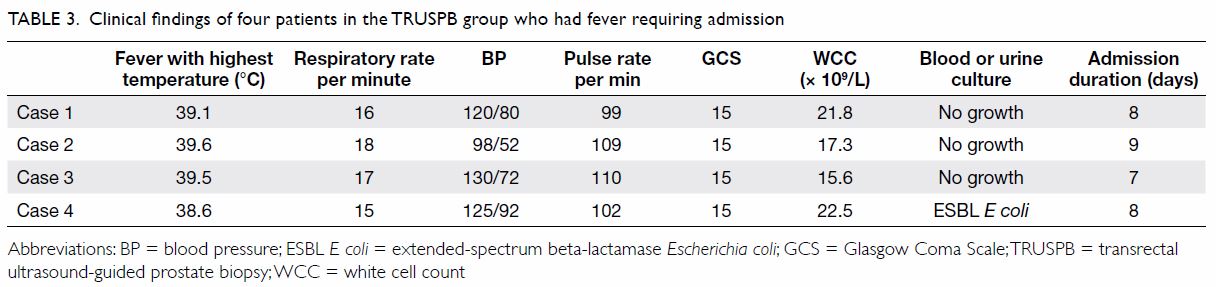
However, four (4%) patients in the TRUSPB group developed fever requiring
hospital admission compared with none in the TPUSPB group (P=0.121) [Table
3]. At least 1 week of intravenous antibiotic treatment was
prescribed for all four of those patients with fever. Three patients in
the TPUSPB group and one in the TRUSPB group developed acute retention of
urine (P=0.621). No patients in the TPUSPB group and one in the TRUSPB
group had rectal bleeding (P=1.000). There were no admissions due to
haematuria in either group.
Discussion
Since Hodge et al2
introduced the systematic sextant biopsy protocol, TRUSPB has become the
main approach to detect prostate cancer worldwide. In recent years, many
urologists have described increased risks of infection and sepsis
associated with TRUSPB.12
Fluoroquinolone was previously thought to provide effective antibiotic
prophylaxis, thus preventing infections associated with TRUSPB; however,
fluoroquinolone-resistant bacteria and extended-spectrum
beta-lactamase–producing bacteria are present within the intestinal flora
of 40.4% and 41.0% of Chinese patients, respectively.13 Accordingly, the rate of post-TRUSPB sepsis is rising
both in Hong Kong6 and worldwide.14 To reduce the rates of
infection, many strategies have been attempted, including augmented
prophylactic antibiotic protocols. However, no strategies have prevented
the development of sepsis due to the transfer of faecal bacteria into the
blood stream through TRUSPB puncture sites.10
Transperineal ultrasound-guided prostate biopsy has been suggested as a
potentially safer alternative. Notably, the indications, workups,
medications, and numbers of cores are identical between TRUSPB and TPUSPB.
However, the techniques differ with respect to multiple aspects, including
patients’ position and puncture route,15
as described in the Methods section of this paper.
From the pain scores recorded during TPUSPB, most
patients tolerated the procedure well. Because the entire procedure was
performed under LA, all patients could be discharged on the same day
without the need for general anaesthesia or monitored anaesthesia care. In
Asian nations, many patients must travel a considerable distance to reach
the hospital; therefore, 1-day out-patient procedures are preferable for
patients and their relatives. Moreover, some patients are high-risk or
unfit for general anaesthesia or monitored anaesthesia care; procedures
performed under LA are therefore much safer and more practical for them.
Transrectal ultrasound-guided prostate biopsy
neglects prostate cancer located in the anterior fibromuscular stroma,
whereas the TPUSPB does not.16 In
our study, a significant proportion of positive prostatic cores were found
in the anterior fibromuscular stroma among patients in the TPUSPB group.
Moreover, some patients with prior negative findings in TRUSPB were
diagnosed with prostate cancer in the anterior fibromuscular stroma based
on the results of TPUSPB. Therefore, TPUSPB may have an advantage over
TRUSPB in patients with previous negative biopsy findings.
This study had some limitations. First, a
consistent number of cores was biopsied in all patients in the TPUSPB
group, irrespective of prostate size. To improve the rate of prostate
cancer detection, some experts have advocated for the use of different
numbers of prostate biopsies, based on prostate size16—more biopsies should be taken in patients with larger
prostates. Second, TPUSPB required more time than TRUSPB. However, as each
step is standardised, the duration of the procedure may decrease. Third,
there was no documentation of pain scores in the TRUSPB group; thus, a
comparison could not be performed. Future studies should address these
limitations. In particular, a larger sample size is needed to confirm
whether TPUSPB is superior with respect to the rate of prostate cancer
detection.
Conclusion
In summary, TPUSPB avoids penetration of the rectal
mucosa and possible transfer of intestinal flora to the blood stream
during the procedure. This contributed to the lack of infections in the
present study. With respect to prostate cancer detection rate, TPUSPB is
at least comparable to TRUSPB. Therefore, an increasing number of
urologists may adopt this technique in the future.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept or design of study: KL Lo, KL Chui, CF Ng.
Acquisition of data: KL Lo, K Lim, JKM Li, J Wong, SK Mak.
Analysis or interpretation of data: KL Lo, CF Ng, SCH Leung, SF Ma.
Drafting of the manuscript: KL Lo, CF Ng.
Critical revision for important intellectual content: All authors.
Acquisition of data: KL Lo, K Lim, JKM Li, J Wong, SK Mak.
Analysis or interpretation of data: KL Lo, CF Ng, SCH Leung, SF Ma.
Drafting of the manuscript: KL Lo, CF Ng.
Critical revision for important intellectual content: All authors.
Conflicts of interest
As an editor of the journal, CF Ng was not involved
in the peer review process. Other authors have disclosed no conflicts of
interest.
Acknowledgement
We would like to thank for the support of the
nursing staff in the Integrated Ambulatory Care Centre, North District
Hospital for their support to the procedures.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Joint Chinese
University of Hong Kong–New Territories East Cluster Clinical Research
Ethics Committee (Ref CREC 2018.323).
References
1. Hong Kong Cancer Registry, Hospital
Authority, Hong Kong SAR Government. Prostate cancer in 2016. Available
from: http://www3.ha.org.hk/cancereg/pdf/top10/rank_2016.pdf. Accessed 17
May 2019.
2. Hodge KK, McNeal JE, Terris MK, Stamey
TA. Random systematic versus directed ultrasound guided transrectal core
biopsies of the prostate. J Urol 1989;142:71-4. Crossref
3. Heidenreich A, Bastian PJ, Bellmunt J,
et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis,
and local treatment with curative intent-update 2013. Eur Urol
2014;65:124-37. Crossref
4. Toner L, Bolton DM, Lawrentschuk N.
Prevention of sepsis prior to prostate biopsy. Investig Clin Urol
2016;57:94-9. Crossref
5. Chan ES, Lo KL, Ng CF, Hou SM, Yip SK.
Randomized controlled trial of antibiotic prophylaxis regimens for
transrectal ultrasound-guided prostate biopsy. Chin Med J (Engl)
2012;125:2432-5.
6. Ng CF, Chan SY. Re: the incidence of
fluoroquinolone resistant infections after prostate biopsy—are
fluoroquinolones still effective prophylaxis? J Urol 2008;180:1570-1. Crossref
7. Kojima M, Hayakawa T, Saito T, Mitsuya
H, Hayase Y. Transperineal 12-core systematic biopsy in the detection of
prostate cancer. Int J Urol 2001;8:301-7. Crossref
8. Xue J, Qin Z, Cai H, et al. Comparison
between transrectal and transperineal prostate biopsy for detection of
prostate cancer: a meta-analysis and trial sequential analysis. Oncotarget
2017;8:23322-36. Crossref
9. Chang DT, Challacombe B, Lawrentschuk N.
Transperineal biopsy of the prostate—is this the future? Nat Rev Urol
2013;10:690-702. Crossref
10. Grummet JP, Weerakoon M, Huang S, et
al. Sepsis and ‘superbugs’: should we favour the transperineal over the
transrectal approach for prostate biopsy? BJU Int 2014;114:384-8. Crossref
11. Singer M, Deutschman CS, Seymour CW,
et al. The Third International Consensus Definitions for Sepsis and Septic
Shock (Sepsis-3). JAMA 2016;315:801-10. Crossref
12. Steensels D, Slabbaert K, De Wever L,
Vermeersch P, Van Poppel H, Verhaegen J. Fluoroquinolone-resistant E.
coli in intestinal flora of patients undergoing transrectal
ultrasound-guided prostate biopsy—should we reassess our practices for
antibiotic prophylaxis? Clin Microbiol Infect 2012;18:575-81. Crossref
13. Tsu JH, Ma WK, Chan WK, et al.
Prevalence and predictive factors of harboring fluoroquinolone-resistant
and extended-spectrum β-lactamase-producing rectal flora in Hong Kong
Chinese men undergoing transrectal ultrasound-guided prostate biopsy.
Urology 2015;85:15-21. Crossref
14. Williamson DA, Barrett LK, Rogers BA,
Freeman JT, Hadway P, Paterson DL. Infectious complications following
transrectal ultrasound-guided prostate biopsy: new challenges in the era
of multidrug-resistant Escherichia coli. Clin Infect Dis. 2013;57:267-74.
Crossref
15. Emiliozzi P, Corsetti A, Tassi B,
Federico G, Martini M, Pansadoro V. Best approach for prostate cancer
detection: a prospective study on transperineal versus transrectal
six-core prostate biopsy. Urology 2003;61:961-6. Crossref
16. Ong WL, Weerakoon M, Huang S, et al.
Transperineal biopsy prostate cancer detection in first biopsy and repeat
biopsy after negative transrectal ultrasound-guided biopsy: the Victorian
Transperineal Biopsy Collaboration experience. BJU Int 2015;116:568-76. Crossref