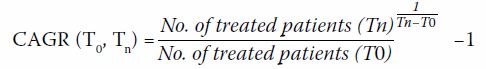Hong
Kong Med J 2019 Jun;25(3):201–8 | Epub 29 May 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Budget impact of introducing tofacitinib to the public
hospital formulary in Hong Kong, 2017-2021
X Li, PhD1; Swathi Pathadka, PharmD1;
Kenneth K Man, BSc, MPH2; Ian CK Wong, PhD1,2;
Esther WY Chan, PhD1
1 Department of Pharmacology and
Pharmacy, The University of Hong Kong, Pokfulam, Hong Kong
2 School of Pharmacy, University College
London, London, United Kingdom
Corresponding author: Dr Esther WY Chan (ewchan@hku.hk)
Abstract
Introduction: As the first
approved oral kinase inhibitor, tofacitinib is effective and
well-tolerated, but more expensive than conventional treatments for
uncontrolled rheumatoid arthritis. Public formulary listing typically
exerts a positive impact on the uptake of new drugs. We aimed to assess
the budgetary impact of introducing tofacitinib into the Hospital
Authority Drug Formulary as a fully subsidised drug in Hong Kong.
Methods: We applied a
population-based budget impact model to trace the number of eligible
patients receiving biologics or tofacitinib treatment, then estimated
the 5-year healthcare expenditure on rheumatoid arthritis treatments,
with or without tofacitinib (2017-2021). We used linear regression to
estimate the number of target patients and compound annual growth rate
to estimate market share. Competing treatments included abatacept,
adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, and
tofacitinib. Retail price was used for drug costs, valued in Hong Kong
dollars (HK$) in 2017 and discounted at 4% per year.
Results: The annual treatment
cost of tofacitinib was HK$74 214 per patient, and the costs of
biologics ranged from HK$64 350 to HK$115 700. Without tofacitinib, the
annual government health expenditures for rheumatoid arthritis treatment
were estimated to increase from HK$147.9 million (2017) to HK$190.6
million (2021). The introduction of tofacitinib to the formulary would
reduce healthcare expenditures by 17.3% to 20.3% per year, with
cumulative savings of HK$192.8 million; this change was estimated to
provide consistent savings (HK$66.4 million to HK$196.8 million) in all
tested scenarios.
Conclusion: Introduction of
tofacitinib to the formulary will provide 5-year savings, given the
current drug price and patient volume.
New knowledge added by this study
- Government healthcare expenditures for treatment of rheumatoid arthritis were estimated to be lowered by approximately 20% upon the introduction of tofacitinib.
- The cost-saving impact of introducing tofacitinib to the Hospital Authority Drug Formulary is determined by interactions between drug prices and market shares of novel disease-modifying anti-rheumatic drugs (DMARDs).
- Based on current drug prices and patient volume, introduction of tofacitinib to the Hospital Authority Drug Formulary will lower healthcare expenditures for at least 5 years.
- Tofacitinib will offer an orally administered option for patients who showed poor response to initial therapy and will intensify market competition for DMARDs.
Introduction
Rheumatoid arthritis (RA) is a chronic connective
tissue autoimmune disorder that leads to considerable functional
disability, reduced quality of life, and loss of earning capacity1 Conventional synthetic disease-modifying anti-rheumatic
drugs (csDMARDs), such as methotrexate, have long been regarded as the
standard of care for RA and are widely used in newly diagnosed patients to
slow disease progression, control disease manifestations, and achieve
remission.2 3 However, csDMARDs exhibit relatively slow onset of
action and require close monitoring due to the potential for adverse
events, especially in patients with chronic co-morbidities.2 4 For patients
who exhibit poor prognostic factors with moderate to high disease activity
after initial therapy with csDMARDs, novel biologic DMARDs (eg,
infliximab, adalimumab, certolizumab pegol, or golimumab) or targeted
synthetic, small-molecule DMARDs (eg, tofacitinib) are recommended for use
in combination with csDMARDs, or as monotherapy.2
3 5
Tofacitinib is a small synthetic molecule Janus
kinase inhibitor which modulates leukocyte recruitment, activation, and
effector cell function at sites of inflammation. It is the first orally
bioavailable therapeutic agent to improve clinical remission in patients
with RA.6 The safety and efficacy
of tofacitinib, relative to those of csDMARDs, have been reported in
recent landmark trials and observational studies. Tofacitinib monotherapy
was superior to methotrexate for reduction of RA symptoms and inhibition
of the progression of structural joint damage in patients who had not
previously received methotrexate or therapeutic doses of methotrexate.7 In patients with inadequate responses to methotrexate,
tofacitinib was non-inferior to adalimumab for symptom control when used
as a combination therapy with methotrexate.8
However, the increased risk of adverse events associated with tofacitinib,
including infections (eg, herpes zoster and tuberculosis) and malignancy,
is a major limitation that requires long-term surveillance and careful
prescribing practices.7 8
Similar to biologic DMARDs, tofacitinib is more
expensive than csDMARDs (eg, methotrexate $1 versus tofacitinib $66 per
tablet in the US9). Several studies have supported the cost-effectiveness
of tofacitinib. When used as an alternative first-line treatment to
csDMARD, tofacitinib was found to be cost-effective in the South Korean
population due to its ability to significantly improve patients’ quality
of life.10 When used in
combination with a csDMARD, a study in the US showed that tofacitinib was
highly cost-effective in patients with severe RA, relative to combinations
of most biologics with csDMARD.11
In a recent modelling study, tofacitinib was also found to be cost-saving
as a second-line therapy following methotrexate failure and as a
third-line therapy following the failure of a biologic therapy.12
Treatment decisions for patients with uncontrolled
RA are increasingly complex because there are no direct head-to-head
comparisons among novel DMARDs from landmark trials, and there are limited
long-term data describing medication safety and compliance. Selection of
biologic DMARDs or tofacitinib is largely dependent on multiple clinical
and socio-economic factors, including disease activity, progression of
structural damage, and co-morbidities within a particular patient, as well
as their preferences for route of administration and dosing frequency, and
the regulatory and cost barriers to drug access.13
Tofacitinib was approved in Hong Kong as a
prescription-only drug in 2014.14
At the time of writing, tofacitinib is listed as a self-financed item
without safety net in the public hospital formulary; thus, the cost of
tofacitinib is entirely out-of-pocket for patients.15 The impact of funding tofacitinib in the public
healthcare system remains unknown. In the present study, we assessed the
budgetary impact of introducing tofacitinib into the Drug Formulary of the
Hospital Authority—the statutory body that manages Hong Kong’s public
hospital services. The objective of this study was to provide guidance for
drug listing decisions from a public institutional perspective.
Methods
Target population
The target population comprised adults with RA who
showed inadequate response to initial csDMARD monotherapy and were
recommended for treatment with novel DMARDs.2
5 Adult patients who had been
treated with novel DMARDs, during the period from 1 January 2009 to 31
December 2015, were identified from the Clinical Data Analysis and
Reporting System (CDARS) of Hong Kong (a territory-wide electronic health
record). Developed by the Hospital Authority, a statutory body that
manages all public hospitals and provides health service to all Hong Kong
residents (over 7 million),16
CDARS is recognised as a unique population-based electronic health record
in Hong Kong that enables publication of an increasing number of
high-quality studies.17 18 19 20 21 A
detailed description of CDARS can be found elsewhere.22 23 24 In this study, retrieved data from the electronic
patient records included demographics, date of registered death, date of
hospital admission and discharge, drug dispensing records and diagnoses.
Patient records from CDARS are de-identified and linked with unique
reference keys to protect patient privacy and facilitate data retrieval.
Based on the eligible patients identified from CDARS, the number of
patients on novel DMARDs was calculated annually between the year of 2009
and 2015 and projected for the years from 2017 to 2021 assuming a linear
trend of increasing in patients receiving novel DMARDs (online
supplementary Appendix 1).
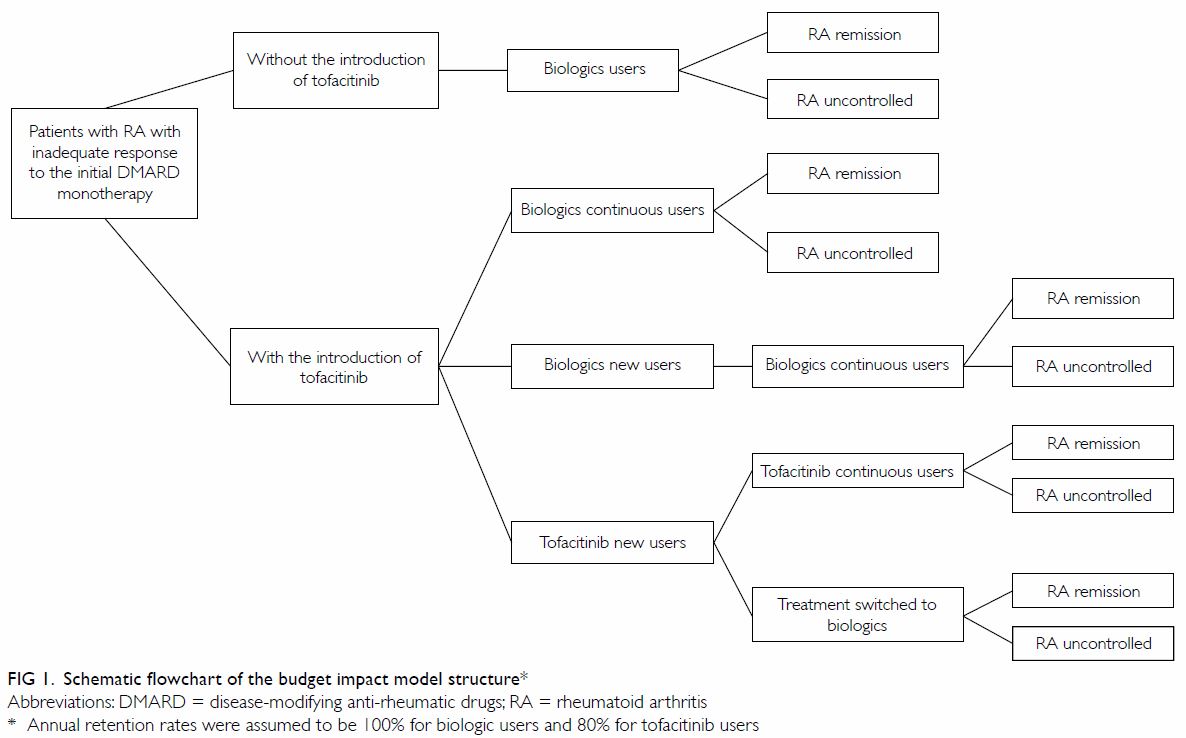
Budget impact model
We developed a population-based budget impact model
to trace the number of patients with RA on novel DMARDs and assess changes
in healthcare expenditures with respect to RA treatments (Fig
1). The model used a cohort of patients diagnosed with RA who showed
inadequate responses to initial DMARD therapy. Without the introduction of
tofacitinib, patients were assumed to use one of the biologic DMARDs
(monotherapy or combination therapy with a csDMARD), according to its
corresponding market share. The introduction of tofacitinib provided an
additional option to patients newly placed on novel DMARDs, with treatment
choices determined by projected market share. We assumed that the annual
retention rate of biologics was 100%, whereas that of tofacitinib was 80%,
in accordance with landmark trial results.7
Dropout patients were assumed to switch to one of the biologics, according
to its corresponding market share in the same year. We also assumed that
the safety and efficacy profiles of biologics and tofacitinib were
equivalent, based on a recent Cochrane review that assessed novel DMARDs
compared to placebo or standard care,25
and based on a head-to-head comparison between tofacitinib and adalimumab.8 The assumptions of the projection
model are summarised in online supplementary Appendix 2.
The analysis was conducted from the public
institutional perspective of Hong Kong, and direct medical costs were
calculated. The numbers of patients on each treatment and overall
medication costs were calculated on a yearly basis, and results were
cumulative over a period of 5 years (2017-2021). Monetary value was
expressed in Hong Kong dollars (HK$) in 2017, discounted at 4% per year.26 27
The study outcome was the difference in healthcare expenditures with
respect to RA treatment, with or without introducing tofacitinib into the
formulary. There was no pre-defined threshold for a favourable budget
impact.
Competing alternatives and market shares
Treatment options were assumed to include biologics
(eg, abatacept, adalimumab, certolizumab pegol, etanercept, golimumab,
infliximab, and tocilizumab) and tofacitinib, using current
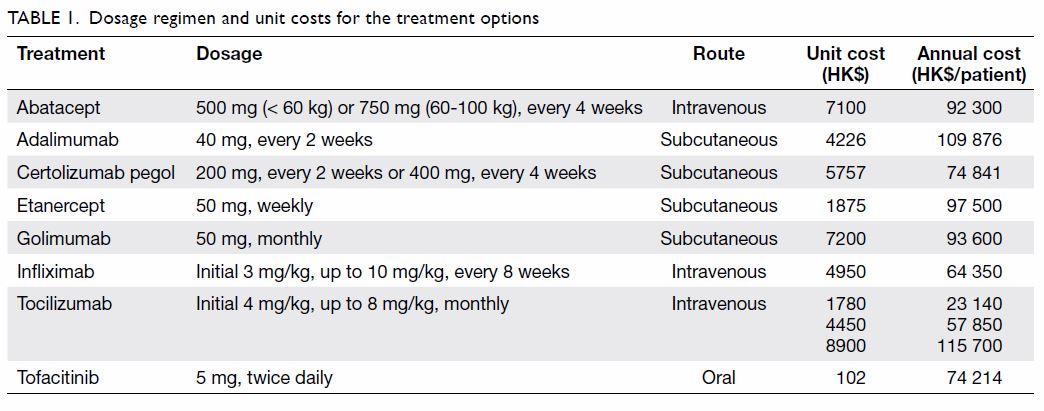
recommendations for patients with inadequate responses to csDMARDs. Table
1 shows dosage regimens, per dose cost in local currency, and annual
medication costs for each treatment. The number of patients on each
treatment was determined by the corresponding market share of the
treatment and the total number of eligible patients in the same year.
Based on the number of biologics that were prescribed from 2013 to 2015
(as recorded in CDARS), the compound annual growth rate (CAGR) was
estimated by following equation28:
We assumed a constant CAGR for each biologic; the
corresponding market share was projected yearly between 2017 and 2021
(online supplementary Appendix 3). The overall market share of novel
DMARDs was assumed to be 100%.
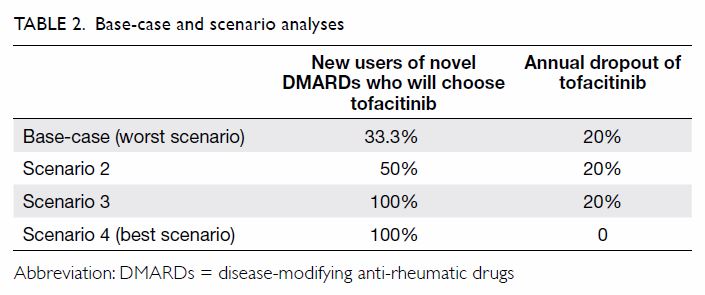
Base-case and scenario analyses
In the base-case analysis, we assumed that
one-third of the eligible patients who were new users of novel DMARDs
would be placed on tofacitinib in the first year. In accordance with the
findings of the landmark trial, 20% of tofacitinib users were expected to
drop out each year, due to adverse events.7
We assumed that the dropout patients would choose one of the biologics and
that the efficacy and safety of tofacitinib and all biologics were
equivalent; hence, the switch would only affect medication costs. Three
scenario analyses were conducted to assess the impact of uncertainties on
the base-case conclusion: specifically, uncertainties were considered in
market share of tofacitinib and dropout of tofacitinib users (Table
2). The base-case scenario was regarded as the worst scenario for
the uptake of tofacitinib (market share of 33.3% among new novel DMARDs
users and 20% annual dropout). In the other three scenarios, the
first-year uptake of tofacitinib increased from 50% to 100%, and the
annual dropout rate ranged from 0% to 20%. Scenario 4 was determined to be
the best scenario, with the highest first-year uptake and 100% retention
over 5 years. We also tested the impact of discounting (0-4%) on base-case
results, as there are no health technology assessment guidelines with
respect to the proper discount rate for Hong Kong.
Statistical Analysis System software (version 9.4,
SAS Inc, Cary [NC], US) was used for data manipulation and analysis.
Microsoft Excel (2003 for Windows; Microsoft Corp, Redmond [WA], US) was
used to establish the budget impact model and generate corresponding
plots.
Ethics
The study was designed and reported in accordance
with the Consolidated Health Economic Evaluation Reporting Standards
Statement.29 The study protocol in
which CDARS was used to estimate the number of eligible patients was
approved by the Hospital Authority Hong Kong West Cluster and The
University of Hong Kong Institutional Review Board (UW14-602). Ethics
approval for the budget impact analysis was waived, as it comprised a
statistical modelling projection without patient contact.
Results
Base-case analysis
Between 2017 and 2021, the estimated number of
eligible patients on novel DMARDs increased from 1466 to 2375. Without
introducing tofacitinib into the formulary, the annual government health
expenditures for RA treatment were projected to increase from HK$147.9
million (2017) to HK$190.6 million (2021) [Fig 2a]. The increased expenditures were driven by
increased patient volume and growth of the biologics market. Addition of
tofacitinib to the formulary would reduce relevant healthcare expenditures
by HK$33.1 million to HK$39.9 million annually (17.3% to 20.3% reduction)
[Fig 2a]. Budgetary savings were expected, regardless
of discount rates (Fig 2). Cumulative savings over the 5-year study
period were projected to be HK$192.8 million (discounted at 4%) and
HK$208.8 million (undiscounted), respectively.
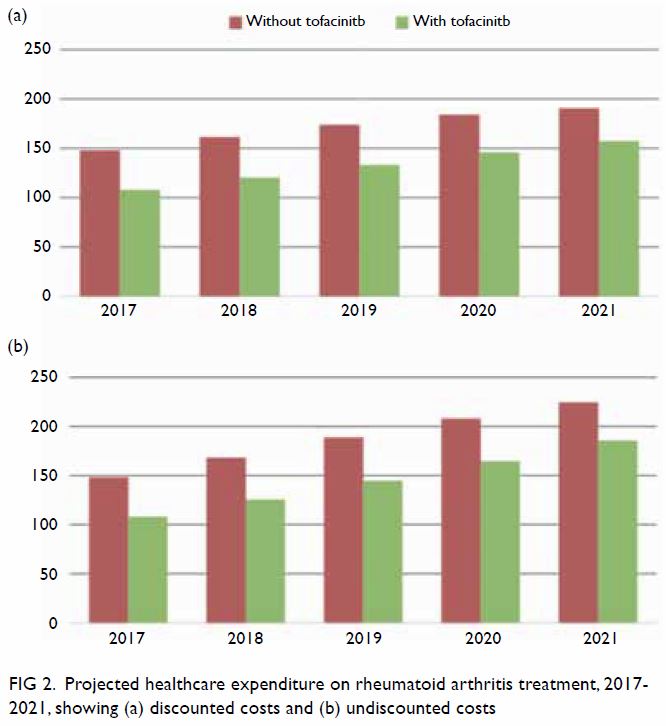
Figure 2. Projected healthcare expenditure on rheumatoid arthritis treatment, 2017-2021, showing (a) discounted costs and (b) undiscounted costs
Scenario analyses
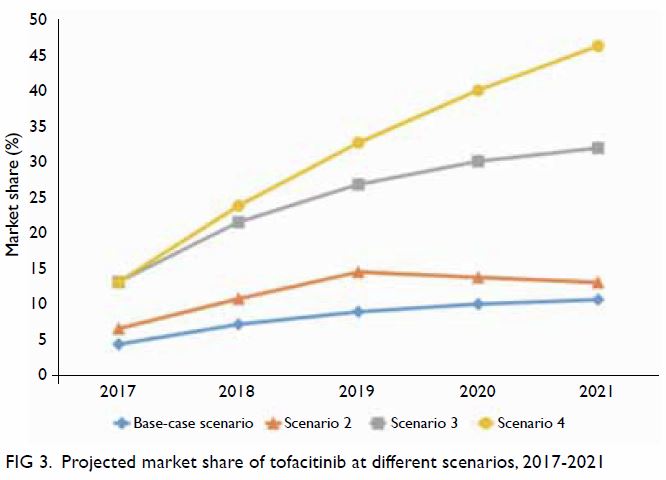
Variations in the market share of tofacitinib were
assessed in different test scenarios (Fig 3). The base-case scenario assumed the most
conservative uptake of tofacitinib, comprising 4.4% to 10.7% of the
overall novel DMARD market. With the assumption that half of the new users
of novel DMARDs would choose tofacitinib, combined with the assumption of
an annual dropout rate of 20% (Scenario 2), the market share of
tofacitinib was expected to increase from 6.6% to 13.1% over the 5-year
study period. With the assumption that all new users of novel DMARDs would
choose tofacitinib (Scenario 3), the market share was expected to increase
from 13.2% to 32% over the 5-year study period. In the best-case scenario
(Scenario 4), 100% uptake was assumed, combined with the assumption of an
annual dropout rate of 0% among new users, the market share of tofacitinib
was expected to increase linearly from 13.2% in 2017 to 46% in 2021.
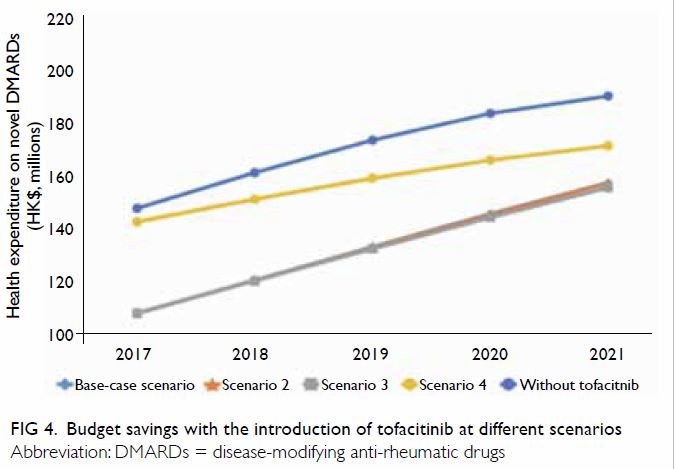
The estimated annual health expenditures for RA
treatments were positively correlated with the uptake of tofacitinib. In
all tested scenarios, the introduction of tofacitinib to the public
hospital formulary provided consistent savings, compared to the current
situation where tofacitinib is self-financed (Fig 4). Similar to the base-case scenario, the
cumulative budget savings over the 5-year study period were estimated to
be HK$193.5 million and HK$196.8 million for Scenarios 2 and 3. In the
best-case scenario with the highest uptake of tofacitinib, the total
savings were reduced to HK$66.4 million (Fig 4).
Discussion
In Hong Kong, patients with uncontrolled RA must
pay HK$20 000 to HK$100 000 per year out-of-pocket to receive novel DMARDs
treatments that facilitate disease remission. In addition to the
progressive loss of working ability associated with RA, the high cost of
therapy poses an additional burden to affected patients, their families,
and society.30 In the present
study, we attempted to provide guidance with respect to introduction of
tofacitinib to the public hospital formulary by analysing the budgetary
impact of this change. Drug listing and subsidy decisions rely on the
principles of efficacy, safety, and cost-effectiveness; thus, they must
consider a variety of factors, including clinical evidence and impact on
healthcare costs.31 Budgetary
impact is a key element of health economic evaluations that must be
determined before formulary approval in many developed countries with
established health technology assessments.32
33 34
Local and international health economists have suggested that systematic
procedures and transparency are needed with respect to formulary
decision-making in Hong Kong.35
Based on the current drug costs of novel DMARDs and the volume of patients
who receive treatment in public hospitals in Hong Kong, our model
projection suggests that the introduction of tofacitinib would provide
savings over the 5-year study period.
In our analysis, governmental healthcare
expenditures for RA treatments were lowered by approximately 20% upon the
introduction of tofacitinib to the public hospital formulary. With the
assumption that none of the novel DMARDs were discontinued or withdrawn
from the market, the annual treatment costs of tofacitinib were lower than
those of all biologics, except infliximab. This may explain the increased
market share of tofacitinib, as well as the reduction in overall RA
treatment costs. In clinical practice, both biologic DMARDs and
tofacitinib are commonly used in combination with a csDMARD.2 3 In our
analysis, we did not consider the cost of csDMARDs, as they are currently
listed as fully subsided drugs in the formulary and are thus expected to
have minimal impact on the cost of RA treatment, regardless of the
addition of tofacitinib to the formulary.
The introduction of tofacitinib to the formulary
will intensify market competition, which may improve the effectiveness of
disease management36; moreover, this change will provide a more convenient
orally administered option for patients who are reluctant to undergo
subcutaneous or intravenous injections, and who are willing to switch from
biologic DMARDs to an alternative therapy.37
38 Patient preferences regarding
RA treatment may affect compliance, adherence, and quality of life.39 40 The route
of administration significantly influences the decision between
tofacitinib and biologics.41 Among
all factors that impact patients’ therapeutic preferences, the convenience
of oral administration has been shown to exhibit the strongest influence.39 Thus, the oral route of
administration for tofacitinib is likely to provide an advantage over the
parenteral route of administration for biologics.
Given current evidence regarding the
cost-effectiveness of tofacitinib, broader utilisation of tofacitinib can
be expected in the future if safety concerns are appropriately addressed.
The underlying effector mechanism of tofacitinib comprises intracellular
transduction inhibition, which carries the potential for interaction with
the immune system; these factors may contribute to its associations with
serious infections, herpes zoster, tuberculosis, gastrointestinal
disorders, and few malignancies.7 8 However, an integrated safety
summary from Phase I-III trials showed stable adverse events, with an
incidence rate of 0.1-3.9 per 100 patient-years, and no new safety signals
in patients who had used tofacitinib for up to 8.5 years.42 Moreover, a recent systematic review with network
meta-analysis concluded that tofacitinib monotherapy had efficacy
comparable to that of currently available biologics, as well as similar
discontinuation rates due to adverse events.43
Current clinical evidence from trials and real-world observations support
the safety of tofacitinib.
We acknowledge that this study had several
limitations. First, we did not consider the costs of monitoring treatments
or treatment of adverse events; this may have led to underestimation of
overall healthcare expenditures for RA treatments. However, given that
monitoring costs and numbers of adverse events from biologics and
tofacitinib may be similar,43 the
absolute changes in healthcare expenditures are not expected to differ
from those we have described. Second, the expected tofacitinib dropout
rate was established on the basis of landmark trials. Patient compliance
and possible treatment switches in real-life clinical treatment settings
were not analysed in this study. Thus, the results of this study should be
interpreted cautiously with respect to the safety, efficacy, and adherence
of tofacitinib and biologics. Third, we assumed constant costs for all
treatments over the study period; in practice, these may be affected by
the dynamic state of the market and a variety of possible interactions
between costs and market share. Finally, structural and parametric
uncertainties from the model were not tested comprehensively. Although we
do not expect deviation from the base-case conclusion, future studies
should assess model uncertainties while considering current clinical
evidence with respect to the effectiveness and safety of novel DMARDs.
Conclusion
The introduction of tofacitinib to the Hospital
Authority Formulary in Hong Kong for the treatment of patients with
uncontrolled RA is expected to lower healthcare expenditures over the
5-year study period. The conclusion is robust in all scenario analyses
with respect to uncertainties in drug costs, as well as in tofacitinib
uptake and compliance.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Conception and design: X Li, EWY Chan.
Acquisition of data: X Li, KK Man.
Analysis or interpretation of data: X Li, KK Man, S Pathadka, EWY Chan.
Drafting of the manuscript: X Li.
Critical revision for important intellectual content: All authors.
Study supervision: ICK Wong, EWY Chan.
Acquisition of data: X Li, KK Man.
Analysis or interpretation of data: X Li, KK Man, S Pathadka, EWY Chan.
Drafting of the manuscript: X Li.
Critical revision for important intellectual content: All authors.
Study supervision: ICK Wong, EWY Chan.
Acknowledgements
We acknowledge Mr Joseph E Blais and Dr In Hye Suh
from the Department of Pharmacology and Pharmacy, The University of Hong
Kong, for their critical review, thoughtful comments, and proofreading of
the manuscript.
Conflicts of interest
EWY Chan has received funding from the Early Career
Scheme and the General Research Fund from the Hong Kong Research Grants
Council; the Health and Medical Research Fund, Food and Health Bureau,
Hong Kong SAR Government; the Beat Drugs Fund from the Narcotics Division,
Security Bureau; and the Young Scientist Fund, National Science Foundation
Science Foundation of China, all unrelated to the current work. EWY Chan
has also received research grants from Bayer, Bristol-Myers Squibb,
Janssen Pharmaceutica, Pfizer, and Takeda, and honorarium from the Hong
Kong Hospital Authority, all unrelated to the current work. ICK Wong
received grants from the Hong Kong Research Grants Council, Innovative
Medicines Initiative, Shire, Janssen Pharmaceutica, Eli Lilly, Pfizer,
Bayer, and the European Union FP7 programme, all unrelated to the current
work. ICK Wong was a member of the National Institute for Health and
Clinical Excellence ADHD Guideline Group and the British Association for
Psychopharmacology ADHD guideline group and acted as an advisor to Shire.
X Li received a research grant from the Health and Medical Research Fund,
Food and Health Bureau, Hong Kong SAR Government and consulting fees from
Pfizer, unrelated to this work. KK Man received the CW Maplethorpe
Fellowship and personal fees from IQVIA Holdings, Inc. (previously known
as QuintilesIMS Holdings, Inc.), unrelated to this work. The other
author(s) declare no conflicts of interest.
Declaration
Preliminary results from this study were presented
(poster, title: Budget impact analysis of introducing tofacitinib for the
treatment of patients with rheumatoid arthritis in Hong Kong) at ISPOR 7th
Asia-Pacific Conference (Tokyo, Japan, 8-11 September 2018). The
conference abstract was published in Value in Health (Volume 21, S80, DOI:
https://doi.org/10.1016/j.jval.2018.07.599).
Funding/support
This work was supported by Pfizer Corporation Hong
Kong Limited (grant No. RC170156). The funder had no role in the study
design, data collection and analysis, preparation of the manuscript, or
decision to publish.
References
1. World Health Organization. Chronic
rheumatic conditions. Available from:
http://www.who.int/chp/topics/rheumatic/en/. Accessed 23 Jun 2017.
2. Singh JA, Saag KG, Bridges SL, et al.
2015 American College of Rheumatology Guideline for the Treatment of
Rheumatoid Arthritis. Arthritis Rheumatol 2016;68:1-26. Crossref
3. Smolen JS, Landewé R, Bijlsma J, et al.
EULAR recommendations for the management of rheumatoid arthritis with
synthetic and biological disease-modifying antirheumatic drugs: 2016
update. Ann Rheum Dis 2017;76:960-77. Crossref
4. Curtis JR, Singh JA. Use of biologics in
rheumatoid arthritis: current and emerging paradigms of care. Clin Ther
2011;33:679-707. Crossref
5. Mok CC, Tam LS, Chan TH, Lee GK, Li EK;
Hong Kong Society of Rheumatology. Management of rheumatoid arthritis:
consensus recommendations from the Hong Kong Society of Rheumatology. Clin
Rheumatol 2011;30:303-12. Crossref
6. Ghoreschi K, Jesson MI, Li X, et al.
Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J Immunol 2011;186:4234-43. Crossref
7. Lee EB, Fleischmann R, Hall S, et al.
Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med
2014;370:2377-86. Crossref
8. Fleischmann R, Mysler E, Hall S, et al.
Efficacy and safety of tofacitinib monotherapy, tofacitinib with
methotrexate, and adalimumab with methotrexate in patients with rheumatoid
arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head,
randomised controlled trial. Lancet 2017;390:457-68. Crossref
9. GoodPx. Tofacitinib. Available from:
https://www.goodrx.com/tofacitinib. Accessed 14 Nov 2018.
10. Lee MY, Park SK, Park SY, et al.
Cost-effectiveness of tofacitinib in the treatment of moderate to severe
rheumatoid arthritis in South Korea. Clin Ther 2015;37:1662-76.e2. Crossref
11. Claxton L, Jenks M, Taylor M, et al.
An economic evaluation of tofacitinib treatment in rheumatoid arthritis:
modeling the cost of treatment strategies in the United States. J Manag
Care Spec Pharm 2016;22:1088-102. Crossref
12. Claxton L, Taylor M, Gerber RA, et al.
Modelling the cost-effectiveness of tofacitinib for the treatment of
rheumatoid arthritis in the United States. Curr Med Res Opin
2018;34:1991-2000. Crossref
13. Mehta N, Schneider LK, McCardell E.
Rheumatoid arthritis: selecting monotherapy versus combination therapy. J
Clin Rheumatol 2017 Jan 18. Epub ahead of print. Crossref
14. Drug Office, Department of Health,
Hong Kong SAR Government. Detail Information: XELJANZ TABLETS 5MG.
Available from:
https://www.drugoffice.gov.hk/eps/drug/productDetail/en/consumer/76216.
Accessed 19 Jun 2017.
15. Hong Kong Hospital Authority Drug
Formulary. Available from:
http://www.ha.org.hk/hadf/en-us/Updated-HA-Drug-Formulary/Drug-Formulary.
Accessed 23 Oct 2017.
16. Lai EC, Man KK, Chaiyakunapruk N, et
al. Brief report: databases in the Asia-Pacific region: the potential for
a distributed network approach. Epidemiology 2015;26:815-20. Crossref
17. Raman SR, Man KK, Bahmanyar S, et al.
Trends in attention-deficit hyperactivity disorder medication use: a
retrospective observational study using population-based databases. Lancet
Psychiatry 2018;5:824-35. Crossref
18. Wong AY, Root A, Douglas IJ, et al.
Cardiovascular outcomes associated with use of clarithromycin: population
based study. BMJ 2016;352:h6926. Crossref
19. Cheung KS, Chan EW, Wong AY, Chen L,
Wong IC, Leung WK. Long-term proton pump inhibitors and risk of gastric
cancer development after treatment for Helicobacter pylori: a
population-based study. Gut 2018;67:28-35. Crossref
20. Lau WC, Chan EW, Cheung CL, et al.
Association between dabigatran vs warfarin and risk of osteoporotic
fractures among patients with nonvalvular atrial fibrillation. JAMA
2017;317:1151-8. Crossref
21. Law SW, Lau WC, Wong IC, et al.
Sex-based differences in outcomes of oral anticoagulation in patients with
atrial fibrillation. J Am Coll Cardiol 2018;72:271-82. Crossref
22. Man KK, Chan EW, Ip P, et al. Prenatal
antidepressant use and risk of attention-deficit/hyperactivity disorder in
offspring: population based cohort study. BMJ 2017;357:j2350. Crossref
23. Chan EW, Lau WC, Leung WK, et al.
Prevention of dabigatran-related gastrointestinal bleeding with
gastroprotective agents: a population-based study. Gastroenterology
2015;149:586-95.e3. Crossref
24. Man KK, Coghill D, Chan EW, et al.
Association of risk of suicide attempts with methylphenidate treatment.
JAMA Psychiatry 2017;74:1048-55. Crossref
25. Singh JA, Hossain A, Tanjong Ghogomu
E, et al. Biologics or tofacitinib for rheumatoid arthritis in incomplete
responders to methotrexate or other traditional disease-modifying
anti-rheumatic drugs: a systematic review and network meta-analysis.
Cochrane Database Syst Rev 2016;(5):CD012183. Crossref
26. National Institute for Health and Care
Excellence. Guide to the methods of technology appraisal 2013. Apr 2013.
Available from: https://www.nice.org.uk/article/pmg9/chapter/Foreword.
Accessed 7 Jul 2015.
27. Sanders GD, Neumann PJ, Basu A, et al.
Recommendations for conduct, methodological practices, and reporting of
cost-effectiveness analyses: Second panel on cost-effectiveness in health
and medicine. JAMA 2016;316:1093-103. Crossref
28. Mai Q, Aboagye-Sarfo P, Sanfilippo FM,
Preen DB, Fatovich DM. Predicting the number of emergency department
presentations in Western Australia: a population-based time series
analysis. Emerg Med Australas 2015;27:16-21. Crossref
29. Husereau D, Drummond M, Petrou S, et
al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)
statement. Value Health 2013;16:e1-5. Crossref
30. Fazal SA, Khan M, Nishi SE, et al. A
clinical update and global economic burden of rheumatoid arthritis. Endocr
Metab Immune Disord Drug Targets 2018;18:98-109. Crossref
31. Hospital Authority, Hong Kong SAR
Government. Drug review and selection mechanism. Available from:
http://ha.org.hk/visitor/ha_visitor_text_index.asp?Content_ID=208041&Lang=ENG&Dimension=100&Parent_ID=206049&Ver=TEXT.
Accessed 29 Mar 2017.
32. National Institute for Health and Care
Excellence. Budget impact test. Available from:
https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-technology-appraisal-guidance/budget-impact-test.
Accessed 19 Jun 2017.
33. Scott AM. Health technology assessment
in Australia: a role for clinical registries? Aust Health Rev
2017;41:19-25. Crossref
34. Canadian Agency for Drugs and
Technologies in Health. About the health technology assessment service.
Available from:
https://www.cadth.ca/about-cadth/what-we-do/products-services/hta.
Accessed 19 Jun 2017.
35. Wong CK, Wu O, Cheung BM. Towards a
transparent, credible, evidence-based decision-making process of new drug
listing on the Hong Kong Hospital Authority Drug Formulary: challenges and
suggestions. Appl Health Econ Health Policy 2018;16:5-14. Crossref
36. Goddard M. Competition in healthcare:
good, bad or ugly? Int J Health Policy Manag 2015;4:567-9. Crossref
37. Genovese MC, van Vollenhoven RF,
Wilkinson B, et al. Switching from adalimumab to tofacitinib in the
treatment of patients with rheumatoid arthritis. Arthritis Res Ther
2016;18:145. Crossref
38. Vieira MC, Zwillich SH, Jansen JP,
Smiechowski B, Spurden D, Wallenstein GV. Tofacitinib versus biologic
treatments in patients with active rheumatoid arthritis who have had an
inadequate response to tumor necrosis factor inhibitors: results from a
network meta-analysis. Clin Ther 2016;38:2628-41.e5. Crossref
39. Alten R, Krüger K, Rellecke J, et al.
Examining patient preferences in the treatment of rheumatoid arthritis
using a discrete-choice approach. Patient Prefer Adherence
2016;10:2217-28. Crossref
40. Li Z, An Y, Su H, et al. Tofacitinib
with conventional synthetic disease-modifying antirheumatic drugs in
Chinese patients with rheumatoid arthritis: Patient-reported outcomes from
a Phase 3 randomized controlled trial. Int J Rheum Dis 2018;21:402-14. Crossref
41. Augustovski F, Beratarrechea A,
Irazola V, et al. Patient preferences for biologic agents in rheumatoid
arthritis: a discrete-choice experiment. Value Health 2013;16:385-93. Crossref
42. Cohen SB, Tanaka Y, Mariette X, et al.
Long-term safety of tofacitinib for the treatment of rheumatoid arthritis
up to 8.5 years: integrated analysis of data from the global clinical
trials. Ann Rheum Dis 2017;76:1253-62. Crossref
43. Bergrath E, Gerber RA, Gruben D, Lukic
T, Makin C, Wallenstein G. Tofacitinib versus biologic treatments in
moderate-to-severe rheumatoid arthritis patients who have had an
inadequate response to nonbiologic DMARDs: systematic literature review
and network meta-analysis. Int J Rheumatol 2017;2017:8417249. Crossref