Hong
Kong Med J 2019 Jun;25(3):178–82 | Epub 29 May 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Faecal microbiota transplantation for treatment of
recurrent or refractory Clostridioides difficile infection in Hong
Kong
Rashid N Lui, MB, ChB, FHKAM (Medicine)1;
Sunny H Wong, DPhil (Oxon), FHKAM (Medicine)2,3; Louis HS Lau,
MB, ChB, MRCP (UK)1; TT Chan, MB, BS, MRCP (UK)1;
Kitty CY Cheung, BSc, MPH2; Amy YL Li, BSc2; ML
Chin, BSc, MPhil4; Whitney WY Tang, MPhil, MPH2;
Jessica YL Ching, BSN, MPH2; Kelvin LY Lam, MB, BS, FHKAM
(Medicine)1; Paul KS Chan, MD, FRCPath3,4; Justin CY
Wu, MD, FRCP (Edin)2; Joseph JY Sung, MD, PhD2;
Francis KL Chan, MD, FRCP (Edin)2,3; Siew C Ng, PhD, FRCP
(Edin)2,3
1 Division of Gastroenterology and
Hepatology, Department of Medicine and Therapeutics, Prince of Wales
Hospital, Shatin, Hong Kong
2 Institute of Digestive Disease, The
Chinese University of Hong Kong, Shatin, Hong Kong
3 Centre for Gut Microbiota Research,
The Chinese University of Hong Kong, Shatin, Hong Kong
4 Department of Microbiology, The
Chinese University of Hong Kong, Shatin, Hong Kong
Corresponding author: Prof Siew C Ng (siewchienng@cuhk.edu.hk)
Abstract
Introduction: Clostridioides
difficile infection (CDI) is a leading cause of
healthcare-associated infection globally, causing significant morbidity
and mortality. Faecal microbiota transplantation (FMT) has emerged as a
promising option for recurrent and refractory CDI. This study aimed to
assess the safety, efficacy, and feasibility of FMT for CDI in Hong
Kong.
Methods: We conducted a
single-centre, retrospective study for all consecutive cases of
recurrent or refractory CDI who underwent FMT from 2013 to 2018.
Clinical demographics, outcome, and safety parameters were collected.
Results: A total of 24 patients
with recurrent or refractory CDI (median age 70 years, interquartile
range=45.0-78.3 years; 67% male) were included. Over 80% had been
recently hospitalised or were long-term care facility residents. Faecal
microbiota transplantation was delivered by feeding tube in 11 (45.8%),
oesophagogastroduodenoscopy in eight (33.3%), and colonoscopy in six
(25%) of the patients. Resolution of diarrhoea without relapse within 8
weeks was achieved in 21 out of 24 patients (87.5%) after FMT. No deaths
occurred within 30 days. The FMT was well tolerated and no serious
adverse events attributable to FMT were reported.
Conclusion: Our results confirm
that FMT is a safe, efficacious, and feasible intervention for patients
with refractory or recurrent CDI in Hong Kong. Given the increasing
disease burden and the lack of effective alternatives in Hong Kong for
difficult-to-treat cases of CDI, we recommend that a territory-wide FMT
service be established to address increasing demand for this treatment.
New knowledge added by this study
- Faecal microbiota transplantation is safe, efficacious, and feasible in Hong Kong.
- This study raises awareness of this important armamentarium for the treatment of patients with recurrent or refractory Clostridioides difficile infection.
- A concerted effort by policymakers will be necessary if a dedicated centre providing territory-wide faecal microbiota transplantation services is to be established to help these difficult-to-treat patients.
Introduction
Clostridioides difficile infection (CDI) is
a leading cause of healthcare-associated diarrhoea and is associated with
significant morbidity and mortality.1
In the United States, C difficile has become the most lethal acute
enteric pathogen, with the Centers for Disease Control designating it as
an urgent antibiotic resistance threat in 2015, one of only three
pathogens to earn this distinction (http://www.cdc.gov/drugresistance/biggest_threats.html).2 In Hong Kong, an annual increase
of 26% in CDI was noted from 2006 to 2014.3
This is even more alarming for older adults aged ≥65 years, in whom the
estimated crude incidence rate is 133 to 207 cases per 100 000
population.4 It is believed that CDI arises when the normal gut microbiota
is disrupted. This allows for the colonisation of C difficile,
whose production of cytotoxins leads to disease.5
A recent study showed that apart from microbiota dysbiosis, enteric virome
dysbiosis may also play an important role.6
Risk factors include nursing home care, recent hospitalisation,
antibiotics exposure, and proton-pump inhibitor use.4 First-line treatment for non-fulminant C difficile–associated
diarrhoea includes the cessation of unnecessary medications and treatment
with vancomycin or fidaxomicin. For more severe cases, a combination of
vancomycin and intravenous metronidazole with early consideration of
surgery may be required.7 However,
it is well known that a significant proportion of cases relapse or recur
despite first-line therapy.8 For
recurrent cases, taper and pulse regimens of vancomycin can be offered,
but response rates decline further with multiple recurrences.9 Fidaxomicin is another promising treatment option, but
its usage is limited by its price and availability.10 These difficult-to-treat cases are associated with
extended hospital stays and may result in widespread nosocomial outbreaks.
In their seminal paper, van Nood et al11
demonstrated that faecal microbiota transplantation (FMT), in which
infusion of faeces from healthy donors is given to individuals with
disease, was efficacious for recurrent CDI. Since then, this finding has
been confirmed by a systematic review and meta-analysis of >160
clinical studies.12 In view of the
limited options available for treatment of recurrent or refractory cases,
we decided to establish a pilot FMT service to address this treatment gap.
Initially, screening of relatives meeting strict donor criteria and
willing to donate fresh stools was performed; later on, with the
establishment of the Healthy Donor Stool Biobank by the Faculty of
Medicine, The Chinese University of Hong Kong in 2017, pre-screened frozen
stools were made available. The objectives of this study, the first of its
kind in Hong Kong, was to assess the safety, efficacy, and technical and
logistical feasibility of performing FMT.
Methods
We conducted a single-centre, retrospective review
of all consecutive cases of recurrent or refractory CDI with FMT performed
at an academic hospital from 2013 to 2018. Data on patient demographics,
co-morbidities, route of administration of FMT, treatment efficacy,
adverse events, and other relevant clinical data were extracted from the
Clinical Management System of the Hospital Authority, Hong Kong or from
review of case notes under the auspices of the FMT registry. Stringent
donor criteria based on guidance and protocols were applied to fresh and
frozen donor stool to ensure patient safety.13
Screening for viral hepatitis, blood-borne viruses, pathogenic bacteria,
enteric viruses, syphilis, multidrug resistant organisms, Helicobacter
pylori, C difficile, and parasites was performed (online
supplementary Appendix). The FMT product was delivered either via
a feeding tube or through scope channels during
oesophagogastroduodenoscopy (OGD) or colonoscopy. Feeding tube position
was confirmed prior to FMT delivery. For the OGD route, the endoscopist
performed a routine OGD to ensure there were no ulcerations or other
contra-indications for FMT. Afterwards, the endoscopist tried to pass the
scope as distally as possible (at least to the second or third parts of
the duodenum) to minimise reflux of the FMT back into the stomach. During
the procedure carbon dioxide for luminal insufflation was used to minimise
gaseous distention, discomfort, and the urge to retch or vomit. Before FMT
delivery, the patient is sat upright to minimise the risk of aspiration.
For the colonoscopy route, the endoscopist performed a routine colonoscopy
to ensure there are no large ulcers or other contra-indications for FMT.
Afterwards, the endoscopist tried to pass the scope as proximally as
possible, but if the patient’s tolerance was an issue, administering FMT
into the left side of the colon or even the rectum was considered
reasonable, depending on the premorbid state. The majority of FMT
procedures were performed in the Endoscopy Centre with close monitoring,
followed by further observation in the wards for any potential adverse
events. The present report was compiled in accordance with the PROCESS
statement.14
Results
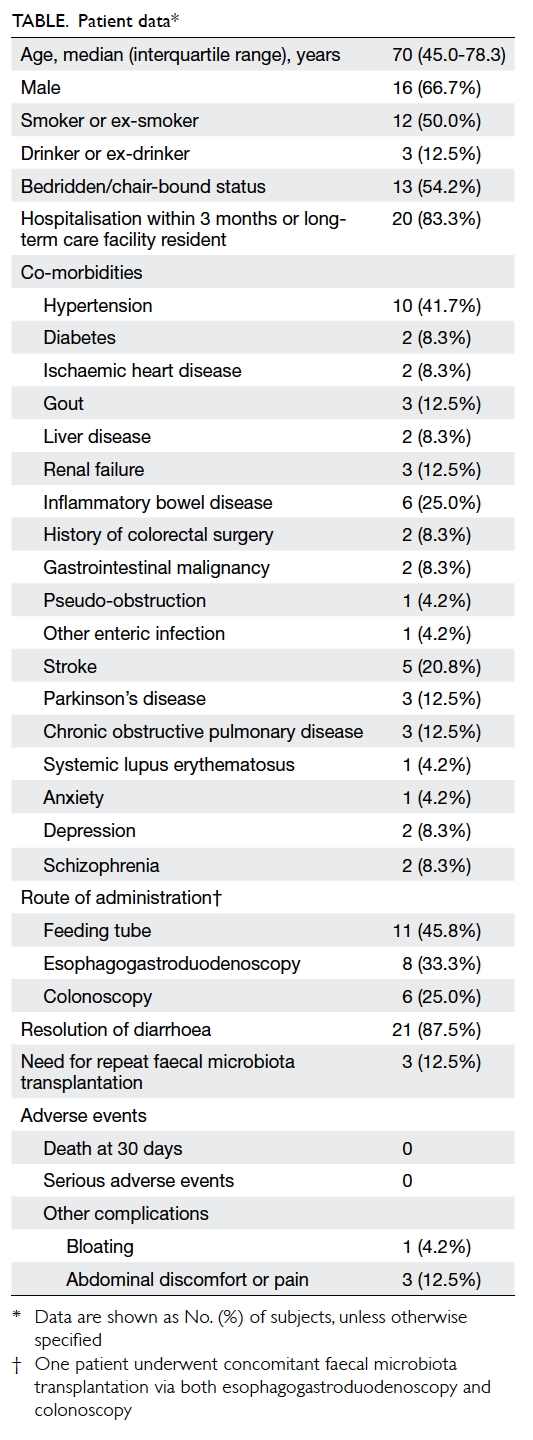
A total of 24 patients with recurrent or refractory
CDI who received FMT were identified. The patients’ baseline
characteristics are shown in the Table. Their median age was 70 years (interquartile
range=45.0-78.3 years). More than two-thirds of the patients were male.
More than half of the patients were in either a bedridden or chair-bound
state, a surrogate of poor functional status. The majority (>80%) of
patients had been hospitalised within the most recent 3 months or were
long-term care facility residents. All patients had at least one
co-morbidity. The most common co-morbidity was hypertension (n=10, 41.7%),
followed by inflammatory bowel disease (IBD) [n=6, 25.0%] and stroke (n=5,
20.8%). The FMT was performed by experienced gastroenterologists in
accordance with published protocols11
and the latest guidelines.15 All
patients were given 50 g of FMT product unless otherwise stated. The FMT
was delivered via feeding tube in 11 (45.8%), OGD in eight (33.3%), and
colonoscopy in six (25.0%) of the patients (one patient had FMT performed
via both OGD and colonoscopy during the same session). Resolution of
diarrhoea without relapse within 8 weeks was achieved in 21 out of 24
patients (87.5%). Three patients (12.5%) did not show significant
improvement in their symptoms after the therapy; therefore, a clinical
decision was made to perform repeat FMT, after which all three patients
showed resolution of diarrhoea. Two of those were confirmed C
difficile–negative on repeat stool testing; the remaining patient
was frail and did not save repeat stools, but no documented recurrence
within 8 weeks was noted. No deaths occurred at 30 days. The procedure was
generally well tolerated, with no serious adverse events attributable to
FMT. The most common complication was abdominal pain (n=3, 12.5%) after
FMT. Bloating was reported in one (4.2%) patient.
Discussion
Because it replenishes colonic microbial diversity,
FMT removes ecological niches that would otherwise be occupied by C
difficile.16 A recent study
has shown that the prevalence of CDI in Asia is similar to the high
prevalence in North America and Europe.17
Furthermore, it is well known that the treatment efficacy of antibiotics
declines with multiple recurrences,9
rendering them less effective in difficult-to-treat cases. We established
Hong Kong’s first FMT service to address the treatment gap that currently
exists. Our results suggest that the efficacy of FMT for recurrent or
refractory CDI is comparable to that reported in the literature, with an
excellent safety profile.11 12
According to the Census and Statistics Department,
the population of Hong Kong is projected to increase from 6.8 million in
mid-2003 to 8.38 million in mid-2033 with a continuous ageing trend.18 The proportion of those aged ≥65 years is projected
to rise markedly, from 11.7% in 2003 to 27% in 2033. Life expectancy in
Hong Kong has already increased to age 79.5 years, behind only Japan and
Switzerland.18 Increasing numbers
of elderly people living in residential care homes, together with the
widespread use of antibiotics and proton-pump inhibitors4, mean that the incidence of CDI in elderly people will
likely increase in Hong Kong, as it has in other developed countries.18
The incidence of IBD in Hong Kong has risen by
almost 3-fold in the past two decades.19
Recently in the West, as the incidence and severity of CDI has increased
in the general population, even greater increases have been described in
patients with IBD.2 In the present
study, a significant proportion of patients with CDI also had IBD, and it
is likely that the incidence and severity of CDI in IBD patients in Hong
Kong will increase further in future. Establishment of FMT services has
been advocated for healthcare systems to effectively manage expected
increases in refractory or recurrent CDI.20
A concerted effort by policymakers will be
necessary to establish a dedicated centre to provide territory-wide FMT
services to tackle the growing disease burden. Such a centre would require
a multidisciplinary team involving gastroenterologists, microbiologists,
infectious disease specialists, specialty nurses, and research personnel
together with the cooperation of wards, the healthy donor stool biobank,
and endoscopy centres. Such an FMT centre may receive territory-wide
consultations and referrals. It could also provide training, knowledge
transfer, and accreditation to healthcare professionals. Currently, the
status of FMT as a therapeutic agent is still evolving. The United States
Food and Drug Administration classifies human stool as a biological agent
and asserts that its use in FMT should be regulated, although their draft
guidance mentions that it intends to exercise enforcement discretion for
its use in recurrent CDI that does not respond to standard therapies.21 This highlights the importance of proper oversight
and governance to mitigate any potential risks that may arise. Data on
long-term safety outcomes are also scarce, highlighting the importance and
role of registries to provide more insight, another function a centralised
and specialised FMT centre would be able to undertake.
Our study has several limitations. As a case
series, the data presented are mainly descriptive and uncontrolled with
the possibility of bias. Further, the sample size is relatively small.
Controlled trials with a larger sample size are required to confirm these
findings and to optimise the timing of FMT administration. Despite these
limitations, resolution of diarrhoea was at a similar rate in the present
study to that reported in the literature.11
Furthermore, as the only centre in Hong Kong that has provided FMT, the
results reported here would be highly relevant for both clinicians and
policymakers concerning its safety, efficacy, and feasibility.
Conclusion
Our results show that FMT is safe, efficacious, and
feasible for treating patients with recurrent or refractory CDI in Hong
Kong. Given the lack of effective alternatives for difficult-to-treat
cases of CDI, and demographic trends that will likely lead to increased
incidence of CDI, demand for FMT is expected to rise. A territory-wide FMT
service should be established to address this expected increase in demand.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept or design of the study: All authors.
Acquisition of data: RN Lui, LHS Lau, KCY Cheung.
Analysis or interpretation of data: RN Lui, LHS Lau, KCY Cheung.
Drafting of the manuscript: RN Lui, SH Wong, PKS Chan, SC Ng.
Critical revision for important intellectual content: All authors.
Acquisition of data: RN Lui, LHS Lau, KCY Cheung.
Analysis or interpretation of data: RN Lui, LHS Lau, KCY Cheung.
Drafting of the manuscript: RN Lui, SH Wong, PKS Chan, SC Ng.
Critical revision for important intellectual content: All authors.
Conflicts of interest
All authors have disclosed no conflicts of
interest.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The present study was reviewed and approved by the
Joint Chinese University of Hong Kong/New Territories East Cluster
Clinical Research Ethics Committee (Ref 2017.260).
References
1. Lessa FC, Mu Y, Bamberg WM, et al.
Burden of Clostridium difficile infection in the United States. N
Engl J Med 2015;372:825-34. Crossref
2. Khanna S, Shin A, Kelly CP. Management
of Clostridium difficile infection in inflammatory bowel disease:
expert review from the clinical practice updates committee of the AGA
institute. Clin Gastroenterol Hepatol 2017;15:166-74. Crossref
3. Ho J, Dai RZ, Kwong TN, et al. Disease
burden of Clostridium difficile infections in adults, Hong Kong, China,
2006-2014. Emerg Infect Dis 2017;23:1671-9. Crossref
4. Wong SH, Ip M, Hawkey PM, et al. High
morbidity and mortality of Clostridium difficile infection and its
associations with ribotype 002 in Hong Kong. J Infect 2016;73:115-22. Crossref
5. Mylonakis E, Ryan ET, Calderwood SB. Clostridium
difficile–associated diarrhea: a review. Arch Intern Med
2001;161:525-33. Crossref
6. Zuo T, Wong SH, Lam K, et al.
Bacteriophage transfer during faecal microbiota transplantation in Clostridium
difficile infection is associated with treatment outcome. Gut
2018;67:634-43.
7. McDonald LC, Gerding DN, Johnson S, et
al. Clinical Practice Guidelines for Clostridium difficile
infection in adults and children: 2017 update by the Infectious Diseases
Society of America (IDSA) and Society for Healthcare Epidemiology of
America (SHEA). Clin Infect Dis 2018;66:987-94. Crossref
8. Pépin J, Routhier S, Gagnon S, Brazeau
I. Management and outcomes of a first recurrence of Clostridium
difficile–associated disease in Quebec, Canada. Clin Infect Dis
2006;42:758-64. Crossref
9. Johnson S. Recurrent Clostridium
difficile infection: a review of risk factors, treatments, and
outcomes. J Infect 2009;58:403-10. Crossref
10. Cornely OA, Miller MA, Louie TJ, Crook
DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile
infection: fidaxomicin versus vancomycin. Clin Infect Dis 2012;55 Suppl
2:154-61. Crossref
11. van Nood E, Vrieze A, Nieuwdorp M, et
al. Duodenal infusion of donor feces for recurrent Clostridium
difficile. N Engl J Med 2013;368:407-15. Crossref
12. Lai CY, Sung J, Cheng F, et al.
Systematic review with meta-analysis: review of donor features, procedures
and outcomes in 168 clinical studies of faecal microbiota transplantation.
Aliment Pharmacol Ther 2019;49:354-63. Crossref
13. Woodworth MH, Neish EM, Miller NS, et
al. Laboratory testing of donors and stool samples for fecal microbiota
transplantation for recurrent Clostridium difficile infection. J
Clin Microbiol 2017;55:1002-10. Crossref
14. Agha RA, Borrelli MR, Farwana R, et
al. The PROCESS 2018 statement: updating consensus Preferred Reporting Of
CasE Series in Surgery (PROCESS) guidelines. Int J Surg 2018;60:279-82. Crossref
15. Cammarota G, Ianiro G, Tilg H, et al.
European consensus conference on faecal microbiota transplantation in
clinical practice. Gut 2017;66:569-80. Crossref
16. Seekatz AM, Aas J, Gessert CE, et al.
Recovery of the gut microbiome following fecal microbiota transplantation.
MBio 2014;5:e00893-14. Crossref
17. Borren NZ, Ghadermarzi S, Hutfless S,
Ananthakrishnan AN. The emergence of Clostridium difficile
infection in Asia: a systematic review and meta-analysis of incidence and
impact. PLoS One 2017;12:e0176797. Crossref
18. Census and Statistics Department, Hong
Kong SAR Government. Hong Kong population projection 2004-2033, report of
the task force on population policy. Available from:
https://www.censtatd.gov.hk/media_workers_corner/pc_rm/hong_kong_population_projections_2004_2033__/index.jsp.
Accessed 8 Mar 2019.
19. Lui RN, Ng SC. The same intestinal
inflammatory disease despite different genetic risk factors in the East
and West? Inflamm Intest Dis 2016;1:78-84. Crossref
20. Costello SP, Tucker EC, La Brooy J,
Schoeman MN, Andrews JM. Establishing a fecal microbiota transplant
service for the treatment of Clostridium difficile infection. Clin
Infect Dis 2016;62:908-14. Crossref
21. Guidance for industry: enforcement
policy regarding investigational new drug requirements for use of fecal
microbiota for transplantation to treat Clostridium difficile
infection not responsive to standard therapies. Food and Drug
Administration, US Department of Health and Human Services, US Government;
2016.