Hong
Kong Med J 2019 Feb;25(1):30–7 | Epub 18 Jan 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Totally laparoscopic versus open gastrectomy for
advanced gastric cancer: a matched retrospective cohort study
Brian YO Chan, MB, ChB, MRCSEd1; Kelvin
KW Yau, MStats, PhD2; Canon KO Chan, FRCS, FHKAM (Surgery)1
1 Department of Surgery, Queen Elizabeth
Hospital, Jordan, Hong Kong
2 Department of Management Sciences,
City University of Hong Kong, Kowloon Tong, Hong Kong
Corresponding author: Dr Canon KO Chan (chankoc@gmail.com)
 A video clip illustrating totally
laparoscopic subtotal gastrectomy for a patient with gastric cancer is available at www.hkmj.org
A video clip illustrating totally
laparoscopic subtotal gastrectomy for a patient with gastric cancer is available at www.hkmj.orgAbstract
Introduction: Laparoscopic
gastrectomy revolutionised the management of gastric cancer, yet its
oncologic equivalency and safety in treating advanced gastric cancer
(especially that in smaller centres) has remained controversial because
of the extensive lymphadenectomy and learning curve involved. This study
aimed to compare outcomes following laparoscopic versus open gastrectomy
for advanced gastric cancer at a regional institution in Hong Kong.
Methods: Fifty-four patients who
underwent laparoscopic gastrectomy from January 2009 to March 2017 were
compared with 167 patients who underwent open gastrectomy during the
same period. All had clinical T2 to T4 lesions and underwent
curative-intent surgery. The two groups were matched for age, sex,
American Society of Anaesthesiologists class, tumour location,
morphology, and clinical stage. The endpoints were perioperative and
long-term outcomes including survival and recurrence.
Results: All patients had
advanced gastric adenocarcinoma and received D2 lymph node dissection.
No between-group differences were demonstrated in overall complications,
unplanned readmission or reoperation within 30 days, 30-day mortality,
margin clearance, rate of adjuvant therapy, or overall survival. The
laparoscopic approach was associated with less blood loss (150 vs 275
mL, P=0.018), shorter operating time (321 vs 365 min, P=0.003), shorter
postoperative length of stay (9 vs 11 days, P=0.011), fewer minor
complications (13% vs 40%, P<0.001), retrieval of more lymph nodes
(37 vs 26, P<0.001), and less disease recurrence (9% vs 28%,
P=0.005).
Conclusion: Laparoscopic
gastrectomy offers a safe and effective therapeutic option and is
superior in terms of operative morbidity and potentially superior in
terms of oncological outcomes compared with open surgery for advanced,
surgically resectable gastric cancer, even in a small regional surgical
department.
New knowledge added by this study
- This is the first study showcasing the efficacy and safety profile
of laparoscopic gastrectomy for advanced gastric cancer in a small
regional surgical centre in Hong Kong.
- Laparoscopic gastrectomy was superior in terms of operative
morbidity and potentially superior in terms of oncological outcomes.
- Laparoscopic gastrectomy is a viable first-line treatment for
surgically resectable advanced gastric cancer.
- This study could spark a paradigm shift in other local surgical
departments and specialist training centres.
Introduction
With an age-standardised incidence rate of 24.2 per
100 000 population, gastric cancer is a major clinical entity in Eastern
Asia.1 Operative resection remains
the only curative treatment available. Over the years, advances in
minimally invasive surgery have caused a paradigm shift towards
laparoscopic gastrectomy (LG), with high-quality evidence from both the
East and West demonstrating a satisfactory safety profile and enhanced
postoperative recovery related to reduction of surgical trauma.2 3
However, one major concern regarding LG is its
oncologic equivalency compared with the open technique, as LG requires
adequate lymphadenectomy and involves a steep learning curve. Several
overseas studies have shown comparable lymph node harvest and survival
data2 3
4 but are limited by either short
follow-up periods or being published by major centres in Korea or Japan,
where extensive experience is available. Whether or not these results are
reproducible in smaller regional centres is unknown, especially in Hong
Kong, where no comparative studies concerning LG for gastric cancer exist
in the literature. It has been suggested that a case volume of
approximately 50 to 60 LGs is required to achieve proficiency, with
demonstrable decreases in blood loss, conversion rate, and hospital length
of stay (LOS) with increasing experience.5
Furthermore, most of these data were based on operations for early gastric
cancer in patients selected according to strict criteria. In advanced
cases requiring extensive lymphadenectomy, evidence is still emerging, and
the learning curve may be steeper.
At our regional surgical centre in Hong Kong, LG is
currently the first-line modality in the absence of contra-indications. We
aimed to perform a matched retrospective cohort study of laparoscopic
versus open gastrectomy for resectable advanced gastric adenocarcinoma of
all sites, comparing intra- and peri-operative characteristics,
oncological clearance, and long-term outcomes including survival and
recurrence.
Methods
Study design and participants
A prospective gastric cancer database was
maintained at the Department of Surgery, Queen Elizabeth Hospital. From
January 2009 to March 2017, 221 patients who underwent curative
gastrectomy for advanced gastric adenocarcinoma (ie, clinical T2 to T4
lesions of all sites) were identified. Clinical T1 lesions (n=23); cases
with pathologies other than adenocarcinoma, like high-grade dysplasia
(n=1); squamous cell carcinoma (n=2); neuroendocrine tumours (n=4);
gastrointestinal stromal tumours (n=3); and cases involving conversion of
approach (n=6) were excluded. A total of 54 patients operated via a
totally laparoscopic approach were identified and matched with 167
patients who underwent the same operation via an open approach during the
same 8-year period. The case ratio between the laparoscopic and open
groups was 1:3.09. Patients from both groups were matched in terms of age,
sex, American Society of Anesthesiologists (ASA) class, tumour location,
morphology, and clinical stage. Follow-up was performed on all subjects at
the Upper Gastrointestinal Surgical Specialist Outpatient Clinic of our
hospital at 3-month intervals up to 2 years postoperation and every 6
months thereafter.
Operative technique
All 54 LG and 167 open operations were performed by
two experienced upper gastrointestinal surgeons with experience of more
than 100 gastrectomy operations each. The choice of approach was decided
by the attending surgeon. All subjects underwent radical gastrectomy with
D2 lymph node dissection as per the guidelines of the Japanese Gastric
Cancer Association6; that is, in
addition to the perigastric nodes, a second tier of lymph nodes along the
celiac axis branches were removed. Distal subtotal, proximal, or total
gastrectomy was selected depending on tumour location and macroscopic
characteristics. Splenectomy or distal pancreatectomy was performed if
there was direct invasion with the possibility of en bloc complete
resection.
Under general anaesthesia, with the patient in
supine split leg position, LG was performed with the surgeon operating on
either side of the patient and a camera assistant in the middle.
Pneumoperitoneum was created via the open Hasson technique at a pressure
of 12 mm Hg, followed by insertion of a 12-mm infra-umbilical camera port,
then one 12-mm and one 5-mm working port in each upper quadrant of the
abdomen for a total of five ports.
Distal and total gastrectomy accounted for 98% of
all LGs performed. Hence, our discussion of technique shall focus on them.
For total gastrectomy, entry to the lesser sac was obtained via dissection
of the avascular plane between the greater omentum and transverse
mesocolon. The gastrocolic ligament was divided proximally and then
distally towards the pylorus using a laparoscopic energy device. The right
gastroepiploic vessels were doubly clipped and divided at their origin.
Then, dissection of the hepatoduodenal ligament was performed, with
division of the right gastric artery and transection of the duodenum with
a linear stapler. The dissection continued towards the gastroesophageal
junction along the lesser curvature. Along with that dissection,
simultaneous D1 lymphadenectomy of the perigastric nodes was performed.
Then, D2 lymphadenectomy was performed, with removal of the common hepatic
artery (Station 8) nodes. The root of the left gastric artery was doubly
clipped and then divided, followed by dissection of celiac trunk (Station
9) and left gastric artery (Station 7) nodes. The splenic artery lymph
nodes (Station 11) and hilar nodes (Station 10) were excised together with
the surrounding fatty connective tissues. During distal gastrectomy, the
left cardia (Station 2), greater curvature (Station 4sa), splenic hilum
(Station 10), and distal splenic artery (Station 11d) nodes were left
intact.
After adequate mobilisation, the stomach or distal
oesophagus was divided using a linear stapler with several centimetres of
margin, and the surgical specimen was placed in an endobag for later
retrieval. Following total gastrectomy, oesophagojejunal anastomoses were
fashioned end-to-side using a circular stapler and a transoral anvil
device, whereas distal gastrectomy reconstruction was performed by either
Roux-en-Y gastrojejunostomy or delta-shaped Billroth I anastomosis.
Side-to-side oesophagogastrostomy was utilised in cases of proximal
gastrectomy.
Open gastrectomies followed standard procedures
from the surgical literature and were characterised by a wider range of
reconstructive techniques in our study.
Outcome variables and bias
All clinical data originated from the patients’
electronic and handwritten medical records and were recorded into the
prospective gastric cancer database by one principal investigator. Recall
and observer bias were addressed by this approach. Selection bias was
minimised by matching and controlling for covariates in the outcome
analyses. Our pathological staging followed that of the American Joint
Committee on Cancer (AJCC) for gastric cancer. Complications were graded
from 1 to 5 according to the Clavien-Dindo classification, with 1 to 2
being minor complications and 3 to 5 being major complications. We defined
30-day mortality as any death, inside or outside of the hospital, within
30 days of surgery. Recurrences were documented as either local or
distant, depending on the first recognised disease site. We designated
survival time as the time from the date of the operation until death or
the last available follow-up (if the patient did not experience an event
of interest).
Statistics
All statistical analyses were performed using the
SPSS (Windows version 22.0; IBM Corp, Armonk [NY], United States).
Frequency matching was employed to ensure that the laparoscopic and open
groups had equal distributions of age, sex, ASA class, tumour location,
morphology, and clinical stage. Appropriate univariate analyses like the
Mann-Whitney U test were selected to examine continuous variables,
whereas Chi squared and Fisher’s exact tests were run for dichotomous and
categorical variables, respectively. Operative outcomes like blood loss,
operating time (OT), type of operation, complications, 30-day mortality,
LOS, and oncologic outcomes such as margin clearance, pathological stage,
lymph node yield, adjuvant treatment, survival time, and disease
recurrence were compared. Survival probabilities were estimated using the
Kaplan-Meier method and compared using stratified log-rank tests. All P
values were based on two-tailed statistical analyses with P<0.05 as the
threshold for statistical significance. All percentages were rounded off
to nearest integer.
Results
Baseline demographics
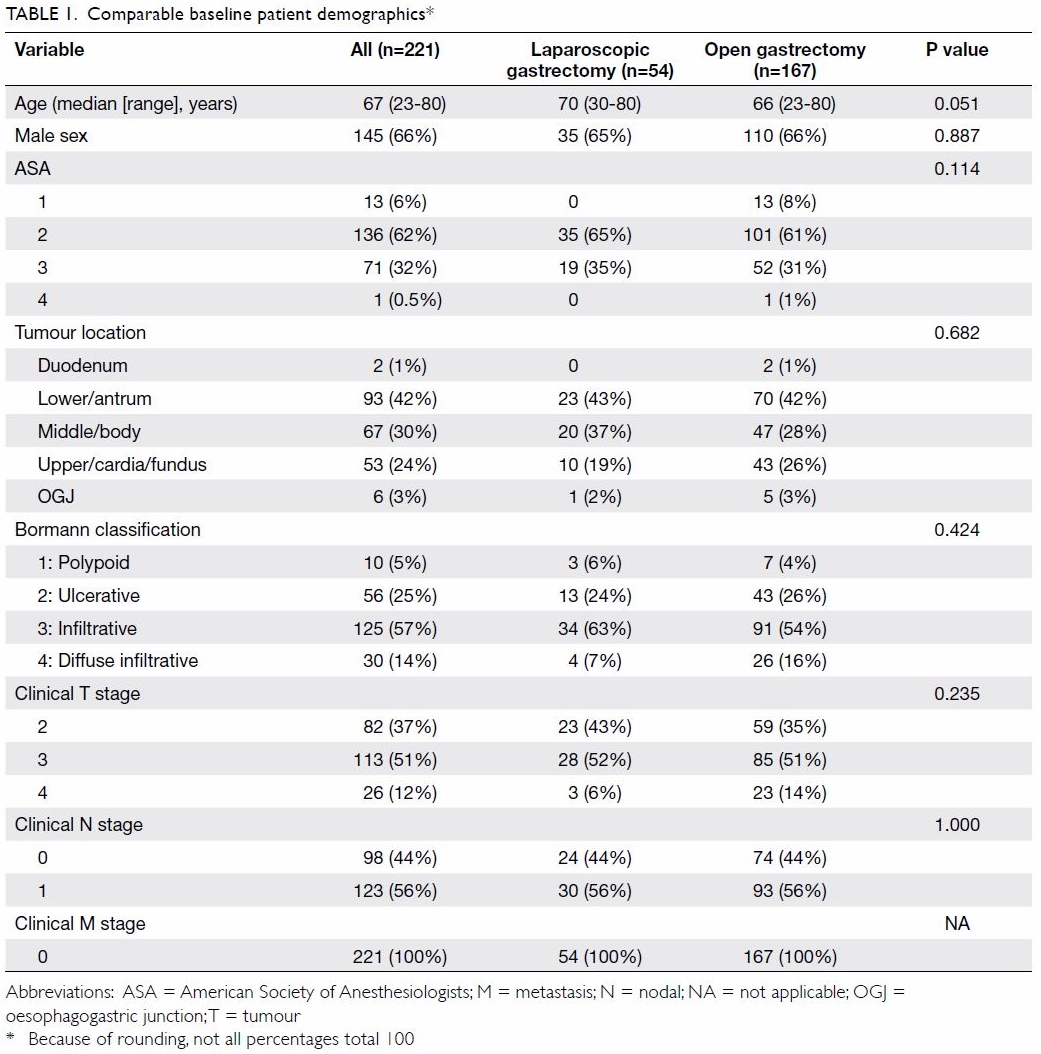
A total of 221 matched patients were evaluated. The
median age at the time of operation was 67 years (range, 23-80 years),
with the majority of patients (145, 66%) being male. Most patients (62%)
were in the ASA 2 category (ie, mild systemic disease without functional
limitation).
In order of descending frequency, 42% of the
tumours were located in the antrum, followed by the gastric body (30%) and
cardia/fundus (24%). All 221 patients had advanced gastric cancer
according to the AJCC clinical staging. Clinical T3 and T2 lesions
accounted for 51% and 37% of cases, respectively, and the remaining 12%
were category T4. Macroscopically, 70% of the tumours were of Bormann
types 3 or 4; only 30% were types 1 or 2 (ie, polypoid or ulcerative with
clear margins). Of all the investigated subjects, 56% had N1 disease on
imaging, while the rest (44%) were negative. No subject had clinically
detectable metastases.
No statistically significant differences were
demonstrated in any of the six matching parameters between the
laparoscopic and open patient groups. The details of the subjects’
demographic variables are charted in Table 1.
Operative outcomes
All 221 patients underwent D2 lymphadenectomy. The
frequency of operation type was comparable between distal and total
gastrectomy (43% and 53%, respectively). Distal pancreatectomy was
performed in six (4%) subjects in the open group only, with no
statistically significant difference between groups (P=0.340). Splenectomy
was performed in 10 (6%) versus 0 subjects in the open and laparoscopic
groups, respectively, and this difference was not statistically
significant (P=0.124). The history of laparotomy was comparable between
groups (7% vs 11% for the laparoscopic and open groups, respectively,
P=0.606).
The laparoscopic group had shorter median OT (321
vs 365 min, P=0.003) and less intra-operative blood loss (150 vs 275 mL,
P=0.018). Operative complications were observed in 41% and 51% of
laparoscopic and open cases, respectively; this trend seemed to favour the
laparoscopic group but failed to reach statistical significance (P=0.210).
Subgroup analyses showed that fewer minor complications were demonstrated
in the laparoscopic group (13% vs 40%, P<0.001). One case of open
distal gastrectomy and laparoscopic total gastrectomy each accounted for
the 30-day mortality among all subjects. Both were older adults in their
70s who developed sudden cardiac arrest and cerebrovascular accident,
respectively, in the days after operation. The median postoperative LOS
was 9 and 11 days, significantly shorter in the laparoscopic group
(P=0.011).
Pathological characteristics
Tumour location and clinical stage were comparable
between groups, as they were matching variables. All patients had
adenocarcinoma. Margin clearance was satisfactory, ranging from 96% to 98%
in the laparoscopic group and 94% to 96% in the open group, and the P
value showed no significant between-group difference in this metric. Over
half (57%) of the patients were in pathological stage III, with no
significant difference in staging between the groups. Interestingly, the
median number of lymph nodes harvested was higher in the laparoscopic
group at 37 (range, 7-77) compared with 26 (range, 3-95) in the open group
(P<0.001). Adjuvant treatment was prescribed in 41% (22 of 54) of
laparoscopic group patients versus 28% (47 of 167) of open group patients,
but this difference did not reach statistical significance (P=0.093).
Oncological outcomes
The mean postoperative follow-up duration was 33
months (laparoscopic group: 25 months, open group: 35 months). Disease
recurrence was observed in 9% and 28% of laparoscopic and open group
patients, respectively, with a statistically significant between-group
difference (P=0.005). During the entire follow-up period, death occurred
in 19 out of 54 laparoscopic group (35%) and 97 out of 167 open group
(58%) patients. Median disease-free survival (DFS) was 46.9 months and
31.7 months, and median overall survival (OS) was 46.9 months and 34.9
months, for the laparoscopic and open groups, respectively. Using a
60-month cut-off, the estimated 5-year DFS and OS were both 47% for the
laparoscopic group and 39% for the open group (P=0.210 and P=0.233,
respectively). The details of the operative, pathological, and oncological
outcomes are charted in Table 2, and the Kaplan-Meier plots for DFS and OS
are shown in Figures 1 and 2, respectively.
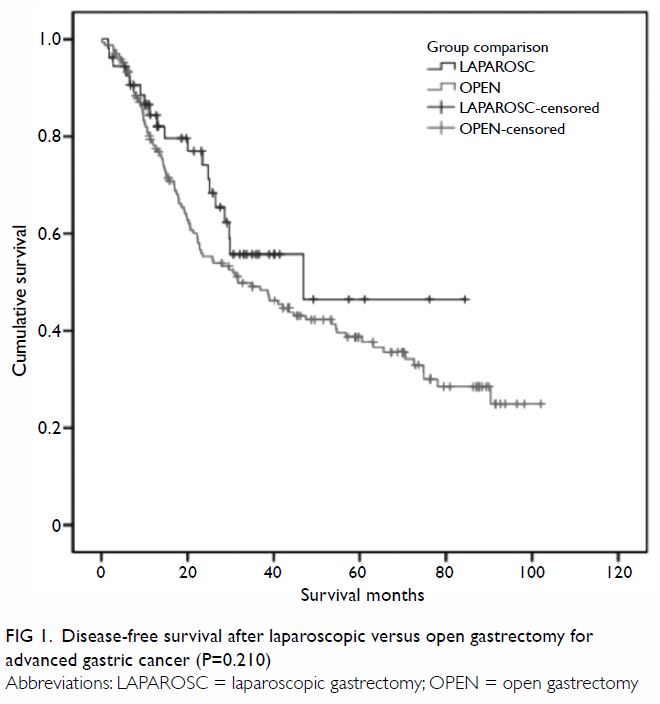
Figure 1. Disease-free survival after laparoscopic versus open gastrectomy for advanced gastric cancer (P=0.210)
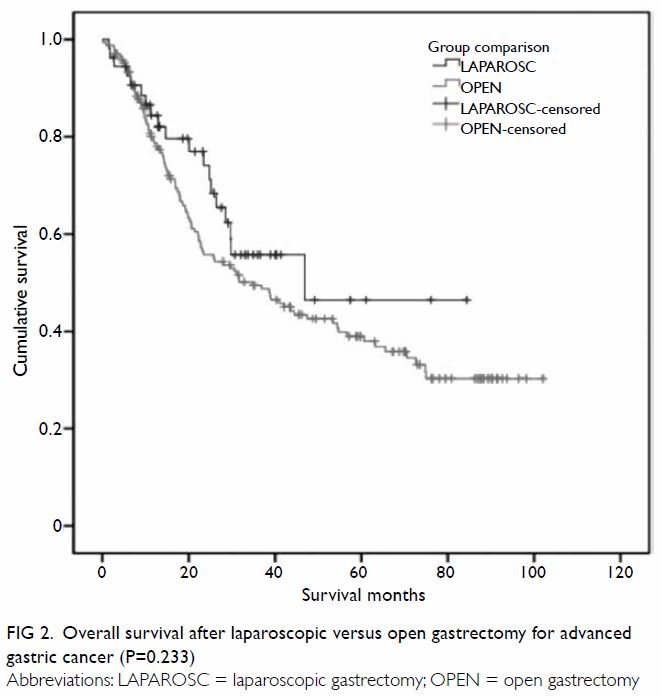
Figure 2. Overall survival after laparoscopic versus open gastrectomy for advanced gastric cancer (P=0.233)
Discussion
Laparoscopic gastrectomy has markedly matured since
its inception by Kitano et al7 in
1994. In early gastric cancer, high-quality evidence including
meta-analyses has demonstrated the equivalence of laparoscopic distal
gastrectomy and open surgery. Early postoperative benefits include less
blood loss, fewer complications, and shorter LOS with comparable
mortality. However, lengthier operations and smaller lymph node yield
remain issues in the laparoscopic approach.8
Technical difficulties in anastomosis and laparoscopic lymph node
dissection have resulted in poorer translation of these results to total
gastrectomies, and such application is often practised only in expert
centres with exceptional case volume.9
Similar controversies also exist in the field of advanced gastric cancer,
where adequate lymphadenectomy is of the utmost importance. Acceptable
short-term outcomes have been reported only in studies that incorporated
experienced surgeons, with the technique’s long-term safety still unknown.10 11
12
As such, the safety and oncologic efficacy of LG
are influenced to a large extent by regional incidence and the case volume
of individual centres. With an age-standardised incidence rate of 9.1 per
100 000 population in Hong Kong, compared with 41.8 per 100 000 population
in Korea and 24.2 per 100 000 population overall in Eastern Asia, gastric
carcinoma is far from the top in terms of cancer incidence ranking.1 13 While this
low age-standardised incidence rate may be partially explained by the
absence of population-wide screening, this lack of screening also implies
that a higher proportion of patients will present with advanced disease.
These two points, together with the absence of studies evaluating LG in
the local literature, mark the importance of our study in evaluating the
efficacy and safety of such procedures in treatment of advanced gastric
cancer in Hong Kong.
Queen Elizabeth Hospital, the largest acute
hospital in Hong Kong and a tertiary surgical referral centre, has a
significant case volume and a patient pool that is representative of the
local population. Through this study, we aimed to document the local Hong
Kong experience, comparing and contrasting results from Hong Kong with
those from overseas expert centres.
In accordance with other major studies, we
demonstrated that LG was associated with less blood loss, fewer minor
complications, and shorter LOS while achieving similar overall levels of
complications and operative mortality to open surgery. The lesser degrees
of pain, blood loss, ileus, and surgical site infections associated with
laparotomy than open surgery are well-investigated benefits of the
laparoscopic approach, and this explains the scarcity of minor
complications.3 8 Median postoperative LOS was 2 days shorter after LG
than open surgery, a small but statistically significant difference. No
local data on average post-gastrectomy LOS exist, but our results are
comparable with an LOS of 11 days (range, 8-12.5 days) observed in the
United Kingdom.14 The small
difference in LOS between the laparoscopic and open groups may be
partially explained by the fact that, compared with Western counterparts,
local Chinese patients prefer in-patient care over community care despite
being fit for out-patient treatment. Enhanced Recovery After Surgery
(ERAS) protocols have been gradually adapted in local surgical units in
recent years, but no data on their efficacy in gastrectomy patients have
been reported.15 With wider
implementation of ERAS and better patient education, it is expected that
differences in LOS between types of surgery will become even more
apparent.
About half (53%) of the operations performed in
this study were total gastrectomies, and all patients had advanced gastric
cancer; both of these factors have been associated with longer OT in the
literature.16 The OT inherent to
the laparoscopic approach has been reported as longer in many studies, but
the median OT of LG was 44 minutes shorter than that of open surgery in
our series. This may be partly explained by the more complex procedures
expected in patients chosen for open gastrectomies. For example, en bloc
splenectomy and distal pancreatectomy were only performed in the open
group, despite the between-group differences in frequency not reaching
statistical significance. Further, the overall histories of laparotomy,
tumour location, and clinical and pathological staging were comparable
between the two groups. Another explanation for the shorter OT observed in
LG in our study is the maturation of our surgeons’ laparoscopic technique.
The higher ratio of total gastrectomies (50%-54%) compared with literature
values was caused by pathological characteristics and surgeon preference.
The 42% of cases with distally located tumours accounted for a compatible
43% of cases in which distal gastrectomies were performed. In contrast,
for the remaining tumours in the gastric cardia or body, because 70% of
tumours were Bormann types 3 and 4, total gastrectomy was the curative
operation of choice.
The median number of lymph nodes harvested was
significantly higher in the LG group (37 compared with 26 in the open
group). Both groups had more lymph nodes harvested than the 15 required
for proper staging. Laparoscopic D2 lymphadenectomy is a technically
challenging procedure, especially at Stations 4, 6, 9, and 11 and in
spleen-preserving lymphadenectomy at the splenic hilum. However, advances
in optics have offered unparalleled amplified clarity for identification
of anatomical structures. The latest laparoscopic energy devices have also
enabled pinpoint precision while performing dissection and sealing in
extensive lymphadenectomies.17
With time and experience, there are indications that our centre’s surgeons
have overcome the learning curve involved.
The importance of adjuvant chemotherapy in curing
advanced gastric cancer cannot be undermined, as many cases have occult
micrometastases. Yet, it has been reported that only 48% to 67% of
patients indicated for adjuvant chemotherapy had it successfully
administered, with postoperative morbidity being a significant factor
behind this deficiency.3 The
advantages of fewer minor complications, shorter LOS, and overall better
general condition of patients may potentially benefit those who undergo LG
and are eligible for adjuvant therapy. Such eligibility was shown in 41%
of patients who underwent LG versus 28% in the open group, but the
difference barely fell short of reaching statistical significance
(P=0.093). Higher rates of receiving adjuvant treatment may translate into
the significantly lower disease recurrence of 9% in the LG group compared
with 28% in the open group (P=0.005). No differences in 5-year DFS nor OS
were demonstrated between the groups. Further large-scale, multicentre
randomised controlled trials like the Korean Laparo-endoscopic
Gastrointestinal Surgery Study (KLASS-02; registered at
www.clinicaltrials.gov as NCT01456598), the Japanese Laparoscopic Gastric
Surgery Study Group (JLSSG 0901; registered at www.umin.ac.jp/ctr/ as
UMIN000003420), and the Chinese Laparoscopic Gastrointestinal Surgery
Study (CLASS-01; registered at www.clinicaltrials.gov as NCT01609309) are
needed to elucidate the short- and long-term results of LG for advanced
gastric cancer.
The limitations of our study include its
retrospective and single-centre nature and its limited number of
participants and follow-up period. Anticipated en bloc distal
pancreatectomy and splenectomy were handled exclusively via the open
approach in this series. With increasing experience, it may be possible to
perform these adjunct procedures laparoscopically, yielding more
homogenous groups for comparison. Efforts have been made to minimise
recall and observer bias and to reduce selection bias through matching.
In summary, LG was associated with shorter OT, less
blood loss, fewer minor complications, shorter LOS, higher lymph node
yield, and, importantly, lower rates of disease recurrence. Overall
complications, 30-day mortality, margin clearance, pathological stage,
percentage receiving adjuvant therapy, and survival time were comparable
between groups. Despite this study’s retrospective cohort nature, which
limits its generalisability, because of the characteristics of our patient
base and the level of our hospital, we believe that our results are
representative of the latest Hong Kong experience.
Conclusion
Laparoscopic gastrectomy is effective and safe as a
curative treatment for patients with advanced gastric adenocarcinoma in
Hong Kong. Apart from its overall equivalent operative and oncological
outcomes, it benefited patients by being associated with less morbidity,
shorter LOS, and higher lymph node clearance than open surgery. This
represents the first local study of its type and illustrates the maturity
of LG as a first-line treatment in our surgical department.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept and design of study: All authors.
Acquisition of data: BYO Chan, CKO Chan.
Analysis and interpretation of data: All authors.
Drafting of manuscript: BYO Chan.
Critical revision for important intellectual content: All authors.
Acquisition of data: BYO Chan, CKO Chan.
Analysis and interpretation of data: All authors.
Drafting of manuscript: BYO Chan.
Critical revision for important intellectual content: All authors.
Conflicts of interest
The authors have no conflicts of interest to
disclose.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Hospital Authority
Kowloon Central Cluster/Kowloon East Cluster Research Ethics Committee
(Ref No. KC/KE-18-0100/ER-1).
References
1. International Agency for Research on
Cancer, World Health Organization. GLOBOCAN 2012 v1.1, cancer incidence
and mortality worldwide: IARC CancerBase No. 11. 2014. Available from:
http://globocan.iarc.fr. Accessed 1 Jun 2018.
2. Wang JF, Zhang SZ, Zhang NY, et al.
Laparoscopic gastrectomy versus open gastrectomy for elderly patients with
gastric cancer: a systematic review and meta-analysis. World J Surg Oncol
2016;14:90. Crossref
3. Kelly KJ, Selby L, Chou JF, et al.
Laparoscopic versus open gastrectomy for gastric adenocarcinoma in the
west: a case-control study. Ann Surg Oncol 2015;22:3590-6. Crossref
4. Kim HH, Han SU, Kim MC, et al. Long-term
results of laparoscopic gastrectomy for gastric cancer: a large-scale
case-control and case-matched Korean multicenter study. J Clin Oncol
2014;32:627-33. Crossref
5. Gholami S, Cassidy MR, Strong VE.
Minimally invasive surgical approaches to gastric resection. Surg Clin
North Am 2017;97:249-64. Crossref
6. Japanese Gastric Cancer Association.
Japanese classification of gastric carcinoma: 3rd English edition. Gastric
Cancer 2011;14:101-12. Crossref
7. Kitano S, Iso Y, Moriyama M, Sugimachi
K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc
1994;4:146-8.
8. Viñeula EF, Gonen M, Brennan MF, Coit
DG, Strong VE. Laparoscopic versus open distal gastrectomy for gastric
cancer: a meta-analysis of randomized controlled trials and high-quality
nonrandomized studies. Ann Surg 2012;255:446-56. Crossref
9. Son T, Hyung WJ. Laparoscopic gastric
cancer surgery: current evidence and future perspectives. World J
Gastroenterol 2016;22:727-35. Crossref
10. Wei HB, Wei B, Qi CL, et al.
Laparoscopic versus open gastrectomy with D2 lymph node dissection for
gastric cancer: a meta-analysis. Surg Laparosc Endosc Percutan Tech
2011;21:383-90. Crossref
11. Uyama I, Suda K, Satoh S. Laparoscopic
surgery for advanced gastric cancer: current status and future
perspectives. J Gastric Cancer 2013;13:19-25. Crossref
12. Shinohara T, Satoh S, Kanaya S, et al.
Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a
retrospective cohort study. Surg Endosc 2013;27:286-94. Crossref
13. Hospital Authority. Hong Kong Cancer
Registry. Available from: www3.ha.org.hk/cancereg. Accessed 1 Jun 2018.
14. Tang J, Humes DJ, Gemmil E, Welch NT,
Parsons SL, Catton JA. Reduction in length of stay for patients undergoing
oesophageal and gastric resections with implementation of enhanced
recovery packages. Ann R Coll Surg Engl 2013;95:323-8. Crossref
15. Mortensen K, Nilsson M, Slim K, et al.
Consensus guidelines for enhanced recovery after gastrectomy: Enhanced
Recovery After Surgery (ERAS®) Society recommendations. Br J Surg
2014;101:1209-29. Crossref
16. Nozoe T, Kouno M, Iguchi T, Maeda T,
Ezaki T. Effect of prolongation of operative time on the outcome of
patients with gastric carcinoma. Oncol Lett 2012;4:119-22. Crossref
17. Rosati R, Parise P, Giannone
Codiglione F. Technical pro & cons of the laparoscopic
lymphadenectomy. Transl Gastroenterol Hepatol 2016;1:93. Crossref