Hong
Kong Med J 2018 Dec;24(6):554–60 | Epub 3 Dec 2018
DOI: 10.12809/hkmj177087
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Branded versus generic drug use in chronic
disease management in Hong Kong—perspectives of health care professionals and the
general public
Vivian WY Lee, PharmD;
Franco WT Cheng, BPharm, MCP;
Felix YH Fong, BPharm;
Enoch EN Ng, BA;
Laadan LH Lo, BA;
Livia YS Ngai, BPharm;
Amy SM Lam, BPharm
School of Pharmacy, The Chinese University of Hong Kong, Shatin, Hong Kong
Corresponding author: Prof Vivian WY Lee (vivianlee@cuhk.edu.hk)
Abstract
Introduction: The aim of the present study was to
evaluate the understanding of generic substitution
among health care professionals and members of the
general public (“general public”) in Hong Kong.
Methods: This cross-sectional descriptive study was
performed by using a self-completed anonymous
questionnaire from March 2015 to May 2017.
The questionnaire included demographic data,
knowledge of generic drugs, experiences of generic
substitution, and views on policy.
Results: A total of 2106 general public, 73 doctors,
22 nurses, and 50 pharmacists responded the
questionnaire. In all, 41.2% of the general public
was aware that generic drugs have the same active
ingredients. Although a majority of the health care
professionals knew that generic drugs have the
same active ingredients (doctors: 79.5%; nurses:
86.4%; pharmacists: 98.0%), many were unaware
of bioequivalence (doctors: 37.0%; nurses: 18.2%;
pharmacists: 50.0%). “Efficacy” was ranked as the
primary concern among all groups; a substantial
portion of respondents reported experiencing
adverse drug reactions upon generic substitution
(general public: 26.6%; doctors: 23.3%; nurses:
9.1%; pharmacists: 42.0%). At least half of the
general public, nurses, and pharmacists considered
that patients should be given a choice for generic
substitution. However, fewer than one-fifth of
doctors and nurses and approximately one-third of
pharmacists considered that patient consent was
needed prior to generic substitution, compared with
approximately two-thirds of the general public.
Conclusion: The knowledge and perception of
generic substitution remains low, both in the general
public and among health care professionals. This
knowledge deficit could potentially lead to different
perspectives among stakeholders regarding generic
substitution.
New knowledge added by this study
- Knowledge and perception of generic substitution remains low, both in the general public and among health care professionals.
- The general public, nurses, and pharmacists considered that patients should be given a choice for generic substitution.
- Fewer than one-fifth of doctors and nurses and approximately one-third of pharmacists believed that patient consent was needed prior to generic substitution, compared with approximately two-thirds of the general public.
- Disagreements exist among stakeholders, although generic substitution is commonly employed in Hong Kong.
- A number of patients reported adverse drug reactions upon generic substitution, which may be clinically significant. Further investigation is warranted.
Background
Health care expenditures have been escalating
in recent years and have thus become a global
challenge. Generic substitution is an important
approach for lessening health care expenditures.
The use of generic drugs allows patients to use the
same active ingredients, dosage form, strength,
and route of administration, with a similar efficacy
and safety, as well as a lower price than that of the
branded product.1 2 It is estimated that the use of
generic drugs reduces the overall cost of health care
in Europe by €100 billion annually.3
In Hong Kong, drug expenditures comprised
10% of the total expenditures of the Hospital
Authority,4 a statutory body managing all public
hospitals and institutions in Hong Kong, in 2015. In
addition, drug expenditures increased markedly by
15.4% from 2013 to 2015.4 Because the public health
care system is heavily subsidised by the government,
cost containment of drug expenditures is vital in
public hospitals. Therefore, it is not uncommon
to adopt generic substitution in public hospitals,
although it is not legally required in Hong Kong.
Generic substitution, either voluntary or
mandatory, has been introduced in many countries
around the world.5 6 However, patients and health
care professionals remain sceptical regarding
the use of generic drugs,6 7 8 9 10 11 12 despite the lack of
evidence for significant clinical risks.13 14 15 In Hong
Kong, medicines are typically supplied in clinics
or hospitals, because prescribing and dispensing
are not separated. As a result, the prescribing and
dispensing of generic drugs relies on the attending
physicians and internal policies established by public
hospitals. There are few choices for members of
the general public (“general public”). Furthermore,
only stability data are required for the registration
of pharmaceutical products in Hong Kong,16 while
pharmacokinetic studies are generally required in
many other countries, such as the United States
and New Zealand.17 18 In 2009, a review committee
recommended the inclusion of bioavailability and
bioequivalence (BABE) studies as requirements
for registration of generic drugs, as well as phased
implementation of these new requirements. As of
2016, Phase 2 requirements for BABE studies were
implemented; BABE studies are now mandatory for
29 antiepileptic drugs and 38 drugs with narrow
therapeutic ranges.19 However, the suitability for
most generic substitutions, especially in terms
of the bioequivalence of the products, remains
questionable. Owing to the lack of pharmacokinetic
data, uncertainty may have greater impact in cases
of generic-to-generic substitution. Generic-to-generic
substitution due to manufacturer-related
interruptions in drug distribution is common in
Hong Kong.4
The present study aimed to evaluate the
understanding of generic substitution among health
care professionals and the general public, and to
identify their experiences in terms of undesirable
clinical outcomes after substitution.
Methods
A cross-sectional descriptive study was conducted
from March 2015 to May 2017 by using a selfcompleted
anonymous questionnaire in Hong Kong.
No consent was obtained because the questionnaire
was conducted in an anonymous manner. The private
information collected was considered non-sensitive
and participants were informed that precautions
would be taken to preserve the confidentiality of
the research data. The background and aims of the
questionnaire were disclosed to participants before
they completed the questionnaire.
Two sets of questionnaires were developed
for the general public and health care professionals,
respectively. Both questionnaires cover four areas,
including the demographic data of the interviewees
(age, gender, monthly income, level of education,
chronic diseases and insurance), knowledge
and perception of generic drugs, experiences of
generic substitution, and policy views for generic
substitution. To ascertain the overall reliability of the
results of the questionnaire, it was further modified
in accordance with feedback from a pilot study that
involved 50 physicians and 500 patients during
January to February 2015.
Five statements, regarding active ingredient,
dosage form, efficacy, similarity to branded drugs,
price and quality, were used to assess knowledge
regarding generic drugs in the general public; four
separate statements, regarding active ingredient,
strength, dosage form, and excipient, were used to
assess knowledge among health care professionals.
Health care professionals were further asked about
the legal requirements for registration of generic
drugs in Hong Kong, the definition of bioequivalence,
and their perceptions of generic drugs. A 5-point
Likert scale was used to assess perceptions of generic
drugs with regard to price, side-effect profile, quality,
efficacy, and preferences for using branded drugs.
In the third part of the questionnaires,
experiences of using generic drugs were assessed.
These included adverse drug reactions encountered
after switching from branded to generic products, as
well as factors that affected the use of generic drugs
compared with branded drugs. The questionnaire for
the general public further assessed previous usage
of generic drugs, previous experience of switching
from branded to generic, and the respondents’
willingness-to-pay for branded products.
Respondents’ preferences regarding branded or
generic products, with respect to changes in the
price of branded products, were documented; the
hypothetical price decreased from 200% of the
generic price to equivalent to the price of generic
products, in intervals of 20% reductions. Only
respondents with chronic illnesses were included
in this portion of the analysis, as this population is
more sensitive to changes in drug price.
The last portion of the questionnaire assessed
views regarding the need to obtain patients’ consent
for substitution of branded products with generic
products. The general public was further queried
whether notification and explanation were required
upon generic substitution.
The questionnaire for the general public was
distributed during an outreach programme and
public lecture series organised by the School of
Pharmacy, The Chinese University of Hong Kong.
Physicians from public sectors were recruited from
the Prince of Wales Hospital; private physicians
were randomly identified and recruited from the
list of the Hong Kong Doctor Association, accessed
via http://www.hkdoctors.org. Questionnaires were
distributed via email to pharmacists and nurses who
had participated in outreach programmes organised
by the School of Pharmacy, The Chinese University
of Hong Kong since 2010.
Discrete data were presented as frequency
percentages; mean 5-point Likert scale ratings
were used. Cumulative frequency was employed to
determine the rank order of factors that affected
the use of generic drugs. All data were analysed
using Excel 2017 (Microsoft Corp., Redmond [WA],
United States).
Results
A total of 5748 individuals were invited to complete
the questionnaire, and 2251 complete responses were
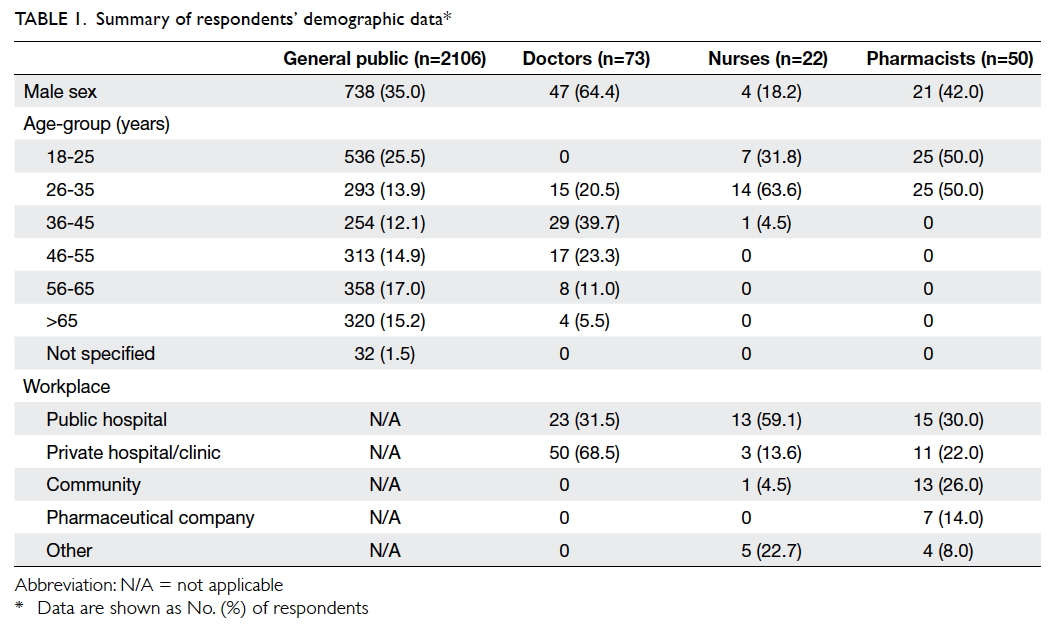
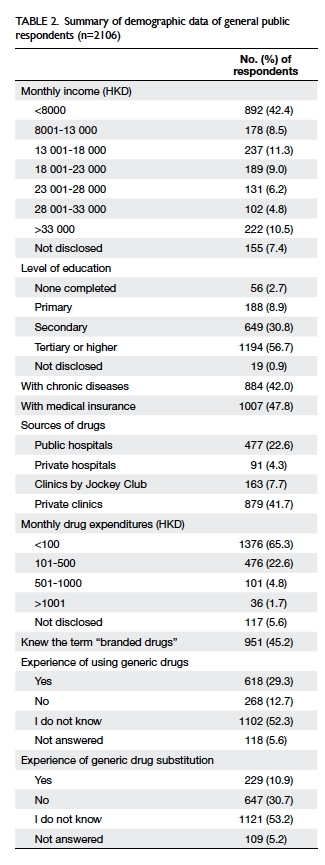
received. Tables 1 and 2 summarise the demographic
data of the 2106 general public respondents, 73
doctor respondents, 22 nurse respondents, and 50
pharmacist respondents.
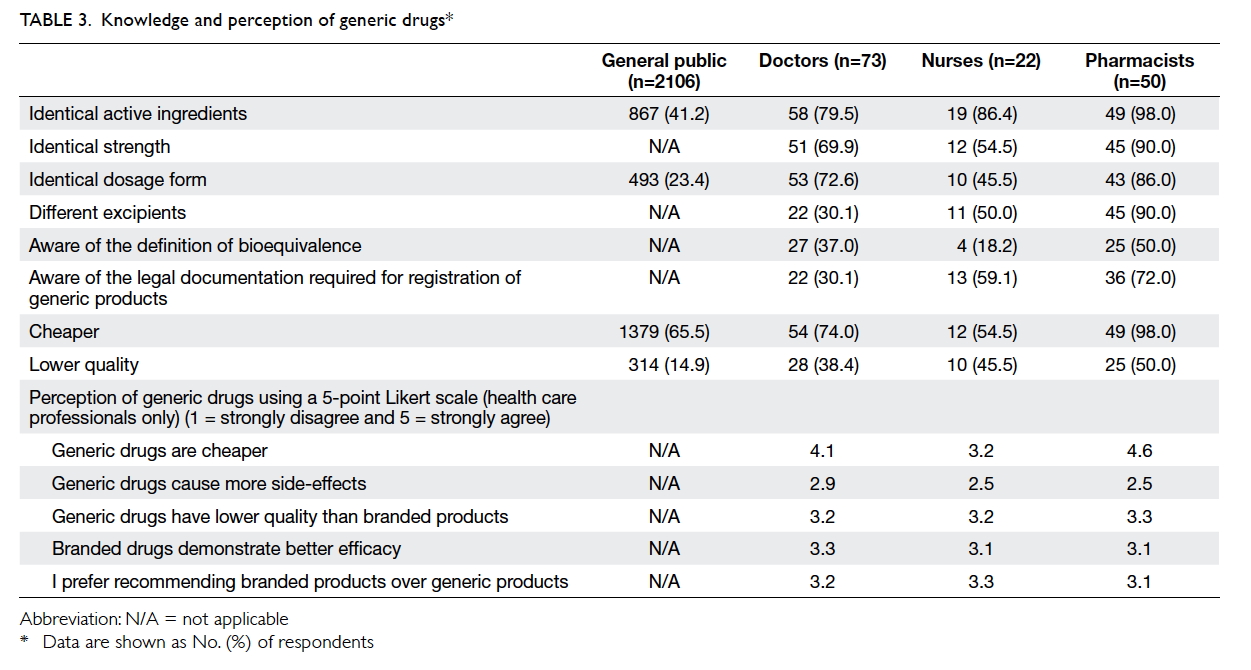
Only 41.2% and 23.4% of the general public
respondents were aware that generic drugs have
the same active ingredients and dosage forms,
respectively, as branded drugs. However, 65.5%
of the respondents thought generic drugs were
cheaper; only 14.9% believed that they were of lower
quality. In contrast, the majority of the health care
professionals were aware that generic drugs have
the same active ingredients (doctors: 79.5%; nurses:
86.4%; pharmacists: 98.0%) and strength (doctors:
69.9%; nurses: 54.5%; pharmacists: 90.0%) as the
branded product at a cheaper price (doctors: 74.0%;
nurses: 54.5%; pharmacists: 98.0%). However, they
were not aware of the definition of bioequivalence
(doctors: 37.0%; nurses: 18.2%; pharmacists: 50.0%).
Table 3 summarises the knowledge and perceptions
of generic drugs among the different groups of
respondents.
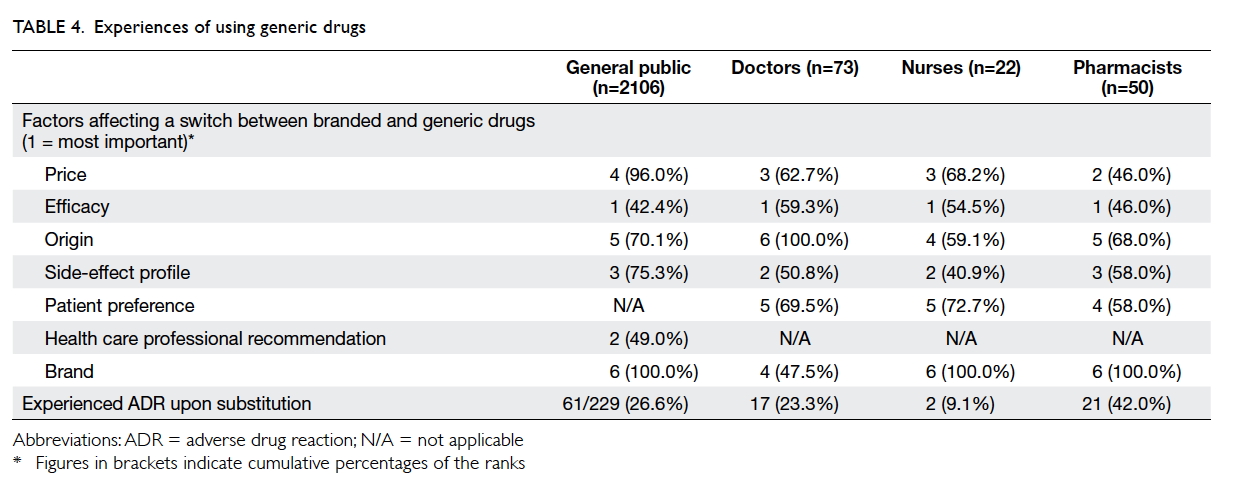
All four groups of respondents ranked “efficacy”
as their primary concern when considering generic
substitution. Other factors that were considered
are detailed in Table 4. Moreover, a substantial
number of respondents reported that they or their
patients experienced adverse drug reactions upon
generic substitution (general public: 26.6%; doctors:
23.3%; nurses: 9.1%; pharmacists: 42.0%), primarily
comprising cardiovascular, gastrointestinal,
psychiatric, and respiratory medications. The
preferences of patients with chronic illnesses for
branded products are illustrated in the Figure. Most
respondents would opt for branded products as long
as the price was not more than 1.4-times that of
generic products.
At least half of the general public (50.0%)
and health care professionals (nurses: 63.6%;
pharmacists: 92.0%), except doctors (42.5%),
considered that patients should be given a choice for
generic substitution. However, fewer than one-fifth
of doctors and nurses, and approximately one-third
of pharmacists, considered that patient consent was
necessary prior to generic substitution, compared
with approximately two-thirds of the general public.
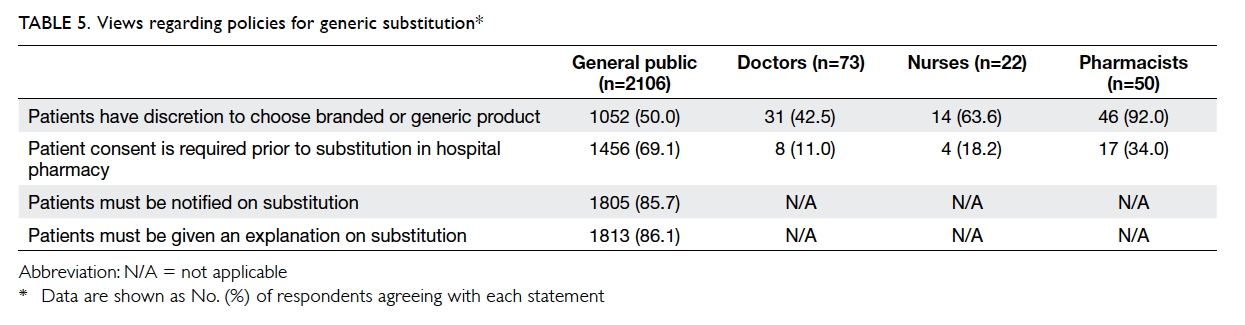
Views regarding policies of generic substitution
among the different stakeholders are listed in Table 5.
Discussion
A Japanese study showed that most patient
respondents declined the use of generic drugs.6 In
contrast to that report, the current study showed
that 53.2% of the general public in Hong Kong
was unaware whether they were using branded
medications or generic substitutes. The proportion of
awareness of “branded drugs” was also significantly
lower in the current study than in the Japanese
study (45.2% vs 68.4%).6 This difference could be
attributed to the lower literacy in Hong Kong. More
than 40% of respondents did not attend tertiary
school or higher education, and more than 10%
only completed primary school or lower. This low
level of literacy may act as a barrier against effective
communication when discussing the use of generic
substitutes.
From our findings, generic substitution
remains controversial in Hong Kong. Although most
general public respondents believed that generic
substitutes are not of lower quality, they demanded
notification from health care professionals and
wished to be given the option to consent to generic
substitution; most health care professionals were
reluctant to follow this approach. The reluctance
may be attributed to the lack of understanding about
generic drugs. Indeed, most doctors, nurses, and
pharmacists tended to believe that generic drugs were
less expensive because of their lower quality. Thus,
it is unsurprising that the health care professionals
exhibited a slight tendency to recommend branded
products over generic substitutes (5-point Likert
scale score = 3.1-3.3). Hence, education combined
with more stringent registration requirements (eg,
mandatory pharmacokinetic data) is essential for
greater acceptance of generic drugs and maintenance
of a sustainable health care system.
Despite being ranked as a primary concern
regarding generic substitution, the efficacy of generic
drugs cannot be guaranteed in Hong Kong due to
a lack of pharmacokinetic data. Indeed, over 40%
of the health care professional respondents stated
that generic medications were of lower quality.
Very recently, the Hong Kong government began to
include BABE studies as legal requirements for the
registration of certain generic drugs (eg, antiepileptic
drugs and drugs with narrow therapeutic ranges), in
addition to existing good manufacturing practice
requirements.16 With the implementation of
BABE requirements, this fundamental step may
support increased quality of generic products, thus
addressing the concerns of both the general public
and health care professionals.20
Adverse drug reactions were also encountered
upon generic substitution among various categories
of commonly prescribed medications. Similar
results have been reported in a Norway study, in
which approximately one-third of patients reported
negative experiences upon switching; there was no
correlation between the adverse reaction and age,
gender, or complexity of medical regimen.10 The lack
of BABE studies could be a possible explanation, but
further investigation is warranted.
Concerning preferences for branded products
with respect to changes in price, it was surprising
that not all respondents opted for branded products,
even when the price was identical to that of generic
products. Furthermore, the preference for branded
products did not linearly increase with price.
These results could be attributed to the design of
the questionnaire and to the perceptions of the
respondents, because the questionnaire did not
specify the nature of the hypothetical medications
that were substituted (eg, short-term or long-term
administration), the equivalence of the products, or
the actual price of the products. These factors may be
significant to respondents when making a decision.
As indicated by the low response rate,
selection bias is a major limitation of the current
study due to its self-administered questionnaire
nature and the convenient sampling method used to
distribute the questionnaire. The unexpected high
proportion of respondents with tertiary education
or higher may be explained by an increased level of
health consciousness among individuals who attend
outreach services and public lectures organised
by the university, as well as the complexity of the
questionnaire. The survey results in this study
potentially overestimated the knowledge of the
general public with respect to generic drugs; thus,
the generalisability of the results to the whole
population may be limited. Further, as indicated
from the demographic data, the sample size of health
care professionals was relatively small and may not
be representative of the overall population of health
care professionals.
Conclusion
Although generic medications have been commonly
used in Hong Kong, knowledge and perception of
these medications has remained low, both in the
general public and among health care professionals.
This knowledge deficit could potentially lead to
conflicting perspectives among stakeholders in
terms of “generic substitution.”
Author contributions
Concept and design of study: VWY Lee.
Acquisition, analysis, and interpretation of data: FYH Fong,
EEN Ng, LLH Lo, LYS Ngai, ASM Lam.
Drafting of the article: FWT Cheng.
Critical revision of important intellectual content: VWY Lee, FWT Cheng.
Acquisition, analysis, and interpretation of data: FYH Fong,
EEN Ng, LLH Lo, LYS Ngai, ASM Lam.
Drafting of the article: FWT Cheng.
Critical revision of important intellectual content: VWY Lee, FWT Cheng.
Funding/support
This research received no specific grant from any funding
agency in the public, commercial, or not-for-profit sectors.
Declaration
The authors have no conflicts of interest to disclose. The
authors had full access to the data, contributed to the
study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Ethical approval
This study was approved by the Survey and Behavioural
Research Ethics of The Chinese University of Hong Kong and
followed the Standards for Reporting Qualitative Research
guidelines.
References
1. Center for Drug Evaluation and Research, US Food
and Drug Administration. Facts About Generic Drugs.
Available from: https://www.fda.gov/downloads/drugs/resourcesforyou/consumers/buyingusingmedicinesafely/understandinggenericdrugs/ucm305908.PDF. Accessed 25
Aug 2017.
2. King DR, Kanavos P. Encouraging the use of generic
medicines: implications for transition economies. Croat
Med J 2002;43:462-9.
3. Medicines for Europe. Our 5 pillars. Available from: http://www.medicinesforeurope.com/generic-medicines/our-5-
pillars/. Accessed 25 Aug 2017.
4. Audit Commission, Hong Kong SAR Government. Report
No. 67, Chapter 5, Hospital Authority’s Drug Management.
2016. Available from: http://www.aud.gov.hk/pdf_e/e67ch05.pdf. Accessed 3 Oct 2017.
5. Chong CP, March G, Clark A, Gilbert A, Hassali MA, Bahari
MB. A nationwide study on generic medicines substitution
practices of Australian community pharmacists and patient
acceptance. Health Policy 2011;99:139-48. Crossref
6. Kobayashi E, Karigome H, Sakurada T, Satoh N, Ueda S.
Patients’ attitudes towards generic drug substitution in
Japan. Health Policy 2011;99:60-5. Crossref
7. Andersson K, Sonesson C, Petzold M, Carlsten A, Lönnroth
K. What are the obstacles to generic substitution? An
assessment of the behaviour of prescribers, patients and
pharmacies during the first year of generic substitution in
Sweden. Pharmacoepidemiol Drug Saf 2005;14:341-8. Crossref
8. Himmel W, Simmenroth-Nayda A, Niebling W, et al. What
do primary care patients think about generic drugs? Int J
Clin Pharmacol Ther 2005;43:472-9. Crossref
9. Iosifescu A, Halm EA, McGinn T, Siu AL, Federman
AD. Beliefs about generic drugs among elderly adults in
hospital-based primary care practices. Patient Educ Couns
2008;73:377-83. Crossref
10. Kjoenniksen I, Lindbaek M, Granas AG. Patients’ attitudes
towards and experiences of generic drug substitution in
Norway. Pharm World Sci 2006;28:284-9. Crossref
11. Babar ZU, Grover P, Stewart J, et al. Evaluating
pharmacists’ views, knowledge, and perception regarding
generic medicines in New Zealand. Res Social Adm Pharm
2011;7:294-305. Crossref
12. Rodríguez-Calvillo JA, Lana A, Cueto A, Markham WA,
López ML. Psychosocial factors associated with the
prescription of generic drugs. Health Policy 2011;101:178-84. Crossref
13. Privitera MD, Welty TE, Gidal BE, et al. Generic-to-generic
lamotrigine switches in people with epilepsy: the
randomised controlled EQUIGEN trial. Lancet Neurol
2016;15:365-72. Crossref
14. Ting TY, Jiang W, Lionberger R, et al. Generic lamotrigine
versus brand-name Lamictal bioequivalence in patients
with epilepsy: A field test of the FDA bioequivalence
standard. Epilepsia 2015;56:1415-24. Crossref
15. Williamson IJ, Reid A, Monie RD, Fennerty AG, Rimmer
EM. Generic inhaled salbutamol versus branded
salbutamol. A randomised double-blind study. Postgrad
Med J 1997;73:156-8. Crossref
16. Food and Health Bureau, Hong Kong SAR Government.
Report of the Review Committee on Regulation of
Pharmaceutical Products in Hong Kong. 2009. http://www.fhb.gov.hk/download/press_and_publications/otherinfo/100105_pharm_review/en_full_report.pdf.
Accessed 25 Aug 2017.
17. Guidance for Industry. Bioavailability and Bioequivalence
Studies for Orally Administered Drug Products—General
Considerations. US Department of Health and Human
Services, Food and Drug Administration, Center for Drug
Evaluation and Research; 2003.
18. New Zealand Medicines and Medical Devices Safety
Authority. New Zealand regulatory guidelines for
medicines. Part A: when is an application for approval
of a new or changed medicine required? 2014. Available
from: http://www.medsafe.govt.nz/regulatory/Guideline/Full%20-%20NZ%20Regulatory%20Guidelines%20for%20
Medicines.pdf. Accessed 3 Oct 2017.
19. Pharmacy and Poisons Board Hong Kong. Implementation
plan of Phase 2 requirement of bioavailability and
bioequivalence studies for the registration of generic
drugs. 2016. Available from: http://drugoffice.gov.hk/eps/upload/eps_news/26420/EN/1/Letter%20to%20Trade%20-%20Implementation%20Plan%20of%20Phase%202%20Requirement_v.5.pdf Accessed 25 Aug 2017.
20. Chua GN, Hassali MA, Shafie AA, Awaisu A. A survey
exploring knowledge and perceptions of general
practitioners towards the use of generic medicines in the
northern state of Malaysia. Health Policy 2010;95:229-35. Crossref