DOI: 10.12809/hkmj177095
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
Recommendations on prevention and screening for
colorectal cancer in Hong Kong
Cancer Expert Working Group on Cancer Prevention
and Screening (August 2016 to July 2018)
TH Lam, MD1; KH Wong, MB, BS, FHKAM
(Medicine)2; Karen KL Chan, MBBChir, FHKAM (Obstetrics and
Gynaecology)3; Miranda CM Chan, MB, BS, FHKAM (Surgery)4;
David VK Chao, FRCGP, FHKAM (Family Medicine)5; Annie NY
Cheung, MD, FHKAM (Pathology)6; Cecilia YM Fan, MB, BS, FHKAM
(Family Medicine)7; Judy Ho, MB, BS, FHKAM (Surgery)8;
EP Hui, MD (CUHK), FHKAM (Medicine)9; KO Lam, MB, BS, FHKAM
(Radiology)10; CK Law, FHKCR, FHKAM (Radiology)11;
WL Law, MS, FHKAM (Surgery)12; Herbert HF Loong, MB, BS, FHKAM
(Medicine)13; Roger KC Ngan, FRCR, FHKAM (Radiology)14;
Thomas HF Tsang, MB, BS, FHKAM (Community Medicine)15; Martin
CS Wong, MD, FHKAM (Family Medicine)16; Rebecca MW Yeung, MD,
FHKAM (Radiology)17; Anthony CH Ying, MB, BS, FHKAM (Radiology)18;
Regina Ching, MB, BS, FHKAM (Community Medicine)19
1 School of Public Health, Li Ka Shing
Faculty of Medicine, The University of Hong Kong, Hong Kong
2 Department of Health, Hong Kong
3 The Hong Kong College of Obstetricians
and Gynaecologists, Hong Kong
4 Hospital Authority (Surgical), Hong
Kong
5 The Hong Kong College of Family
Physicians, Hong Kong
6 The Hong Kong College of Pathologists,
Hong Kong
7 Professional Development and Quality
Assurance, Department of Health, Hong Kong
8 World Cancer Research Fund Hong Kong,
Hong Kong
9 Hong Kong College of Physicians, Hong
Kong
10 Department of Clinical Oncology, The
University of Hong Kong, Hong Kong
11 Hong Kong College of Radiologists,
Hong Kong
12 The College of Surgeons of Hong Kong,
Hong Kong
13 Department of Clinical Oncology, The
Chinese University of Hong Kong, Hong Kong
14 Hong Kong Cancer Registry, Hospital
Authority, Hong Kong
15 Hong Kong College of Community
Medicine, Hong Kong
16 The Jockey Club School of Public
Health and Primary Care, The Chinese University of Hong Kong, Hong Kong
17 Hospital Authority (Non-surgical),
Hong Kong
18 The Hong Kong Anti-Cancer Society,
Hong Kong
19 Centre for Health Protection,
Department of Health, Hong Kong
Corresponding author: Dr Regina Ching (regina_ching@dh.gov.hk)
Abstract
Colorectal cancer is the commonest cancer in Hong
Kong. The Cancer Expert Working Group on Cancer Prevention and Screening
was established in 2002 under the Cancer Coordinating Committee to
review local and international scientific evidence, assess and formulate
local recommendations on cancer prevention and screening. At present,
the Cancer Expert Working Group recommends that average-risk individuals
aged 50 to 75 years and without significant family history consult their
doctors to consider screening by: (1) annual or biennial faecal occult
blood test, (2) sigmoidoscopy every 5 years, or (3) colonoscopy every 10
years. Increased-risk individuals with significant family history such
as those with a first-degree relative diagnosed with colorectal cancer
at age ≤60 years; those who have more than one first-degree relative
diagnosed with colorectal cancer irrespective of age at diagnosis; or
carriers of genetic mutations associated with familial adenomatous
polyposis or Lynch syndrome should start colonoscopy screening earlier
in life and repeat it at shorter intervals.
Introduction
In Hong Kong, the Cancer Coordinating Committee is
a high-level committee chaired by the Secretary for Food and Health to
steer the direction of work on prevention and control of cancer. Under the
Cancer Coordinating Committee, the Cancer Expert Working Group (CEWG) on
Cancer Prevention and Screening was established in 2002 to review local
and international scientific evidence and practices with a view to making
recommendations on cancer prevention and screening suitable for Hong Kong.
This article details the local burden and
prevention of colorectal cancer (CRC), and explains the rationale
underpinning the current CEWG recommendations on CRC screening, which were
reaffirmed and updated in 2017.
Local epidemiology
Colorectal cancer is the commonest cancer in Hong
Kong. According to the Hong Kong Cancer Registry, there were 5036 newly
registered CRC cases in 2015, representing 16.6% of all new cancer cases.1 The age-standardised incidence
rates were 41.5 per 100 000 population for men and 26.2 per 100 000
population for women.1 The median
age at diagnosis of CRC was 68 years in men and 70 years in women.1 The age-specific incidence rates increased
significantly from age 50 years. Colorectal cancer is more common in men
with the male-to-female ratio of 1.3:1 for new cases in 2015.1
The Death Registry registered 2089 deaths caused by
CRC in 2016, representing 14.7% of all cancer deaths and ranking it the
second leading cause of cancer deaths in Hong Kong.2 The age-standardised mortality rates were 18.0 per 100
000 population for men and 10.5 per 100 000 population for women.2 After adjusting for the effect of population ageing,
the age-standardised incidence rates for both sexes still showed an upward
trend, whereas the age-standardised mortality rates for both sexes have
remained stable for more than 30 years.2
Risk factors
Risk factors for developing CRC may be modifiable
or non-modifiable. Modifiable risk factors are those that are related to
lifestyle, such as physical inactivity, low fibre intake, consumption of
red meat or processed meat, overweight or obesity, smoking, and alcohol
use. The World Health Organization’s International Agency for Research on
Cancer classifies consumption of processed meat as “carcinogenic to humans
(Group I),” and consumption of red meat as “probably carcinogenic to
humans (Group 2A)” and indicated that every 50-g portion of processed meat
eaten daily increases the risk of CRC by about 18%.3 Conversely, the risk of CRC is inversely associated
with intake of fibre.4 In addition,
the International Agency for Research on Cancer considered that there is
sufficient evidence to classify alcoholic beverage and tobacco smoking as
carcinogenic to humans in the development of CRC.5
Separately, the World Cancer Research Fund/American Institute for Cancer
Research reported that being overweight or obese can increase the risk of
CRC, whereas increased physical activity is associated with reduction in
risk.6
Non-modifiable risk factors include ageing, male
gender, positive family history, history of familial adenomatous
polyposis, Lynch syndrome (previously known as hereditary non-polyposis
CRC), colonic polyp, and ulcerative colitis.
Based on local epidemiology, CRC is more common in
men and its risk increases significantly from age 50 years.1 Regarding family history, according to a local study,
80% to 90% of CRC cases are sporadic, and the remaining 10% to 20% are
familial cancers.7 The cancer risk
of individuals with a positive family history may vary according to the
age of diagnosis of CRC in the index patient and the number of affected
first-degree relatives. The younger the age of diagnosis of CRC in the
index patient, the higher the risk of CRC of family members would be. In a
meta-analysis, the estimated relative risk of individuals with relatives
diagnosed with CRC at age <50 years was 3.55, whereas that for
relatives diagnosed with CRC at age ≥50 years was 2.18.8
Familial adenomatous polyposis is an autosomal
dominant disorder caused by germline mutation of the adenomatous polyposis
coli gene located on the short arm of chromosome 5 (5q21-22).9 Individuals with this mutation have a 95% chance of
developing CRC by age 50 years.10
Lynch syndrome is another dominantly inherited CRC syndrome. It is caused
by germline mutation in one of the genes responsible for the repair of
mismatches during DNA replication. The lifetime risk of CRC for those
carrying this mutation is estimated to be 50% to 80%.10
Ulcerative colitis has been associated with an
increased risk of developing CRC, likely caused by long-standing chronic
inflammation.11 Moreover, CRC
arises predominantly from adenomatous polyps, which can develop into CRC
after ≥10 years.12 Development of
larger polyps, villous histology, and severe dysplasia are important
indicators for progression into CRC.13
Primary prevention
Primary prevention is of utmost importance for the
prevention of CRC as many of the risk factors are modifiable. For
preventing CRC, the CEWG recommends:
Secondary prevention
Secondary prevention involves screening individuals
without symptoms in order to detect disease or identify individuals who
are at increased risk of disease. Since CRC arises predominantly from
precancerous adenomatous polyps developed over a long latent period, it is
one of the few cancers that can be effectively prevented through organised
and evidence-based screening. In general, for CRC screening, individuals
can be classified into “average risk” and “increased risk” groups.
According to screening recommendations made by the
CEWG, increased-risk individuals are those with a significant family
history, such as an immediate relative diagnosed with CRC at age ≤60
years; more than one immediate relatives diagnosed with CRC irrespective
of age at diagnosis; or immediate relatives diagnosed with hereditary
bowel diseases. Average-risk individuals are those aged 50 to 75 years who
do not have the aforesaid family history.
Screening for general population at average risk
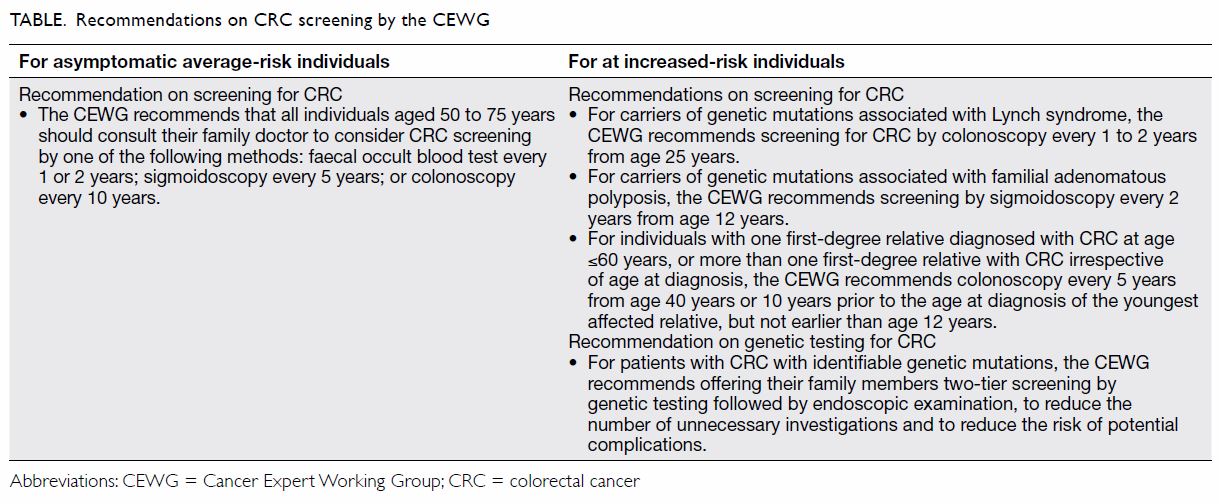
Since 2010, the CEWG has recommended that
average-risk individuals aged 50 to 75 years should consult their doctors
to consider one of the following screening methods:
The CEWG made the above recommendations after
taking into consideration local epidemiology, research evidence, as well
as international guidelines and practices.
The age range recommended for CRC screening in the
general population should be defined to capture the largest number of CRC
cases while taking into account the efficacy and cost-effectiveness of
screening tests, local epidemiology, and anticipated benefits and harms to
the screened population. In Hong Kong, the risk of CRC increases
significantly from age 50 years.1
Guidelines in US14 15 and Singapore16
recommend starting screening at age 50 years, whereas guidelines in the UK
recommend starting screening at age >50 years.17
Regarding screening modalities, FOBT, sigmoidoscopy
and colonoscopy have been shown to reduce mortality from CRC. Faecal
occult blood tests can decrease CRC mortality by 15% to 33%, according to
findings from large-scale randomised control trials.18 19 20 A Cochrane review showed that screening by FOBT might
reduce CRC mortality in the average risk population by 16%.21 Sigmoidoscopy has been shown to lead to a 28% risk
reduction in overall CRC mortality and a 43% risk reduction in distal CRC
mortality in a meta-analysis.22
Colonoscopy was associated with 61% reduction in CRC mortality in another
meta-analysis.23
International guidelines and practices for CRC
screening in the general population mainly recommend annual or biennial
FOBT, sigmoidoscopy once every 5 years, or colonoscopy once every 10
years.15 16
To reduce the burden arising from CRC, the
government launched the 3-year Colorectal Cancer Screening Pilot Programme
(Pilot Programme) on 28 September 2016 to provide subsidised screening by
phases to average risk Hong Kong residents born in 1946 to 1955 (aged
61-70 years in 2016). The screening workflow comprises two stages.
Participants first receive a subsidised faecal immunochemical test (a new
version of FOBT) from an enrolled primary care doctor. If the faecal
immunochemical test result is positive, the participant receives a
subsidised colonoscopy examination from a colonoscopy specialist enrolled
in the Pilot Programme. In August 2018, the government regularised the
programme and would progressively extend it to cover individuals aged 50
to 75 years. Details are available at http://www.colonscreen.gov.hk.
Screening for increased-risk individuals
In 2017, the CEWG updated the CRC screening
recommendations for increased-risk individuals. The key change was related
to the interval for colonoscopy screening among individuals with
significant family history of CRC but without genetic mutations.
For patients with CRC with identifiable genetic
mutations, including Lynch syndrome and familial adenomatous polyposis,
the CEWG recommends two-tier screening for their family members. Genetic
testing should be conducted first, followed by endoscopic examination at
specified and shorter intervals if the genetic test is positive. This
reduces the number of unnecessary investigations among those with strong
family history but without proven genetic mutation, decreasing the risk of
potential complications arising from repeated endoscopic procedures.
These recommendations were made after considering
the scientific evidence and international guidelines and practices.
Individuals who are carriers of genetic mutations
associated with familial adenomatous polyposis or Lynch syndrome and
individuals with a family history of CRC are at increased risk of CRC.
Colorectal cancer in these individuals tends to be diagnosed at a younger
age and progresses more aggressively than CRC in the general population.24 25
International recommendations emphasise that CRC
screening in increased-risk individuals needs to start earlier in their
lifetime and be repeated at shorter intervals. Recommendations made by
countries and by professional organisations on screening for
increased-risk individuals generally suggest the use of colonoscopy and
sigmoidoscopy as the screening methods.15
16 26
27 28
29 30
31 32
The recommended endoscopic screening method for
carriers of genetic mutations associated with Lynch syndrome is annual or
biennial colonoscopy. It is recommended to start screening at age 20 to 25
years in the US15 26 27 and
Singapore,16 at age 25 years in
UK,28 and at age 25 years or 5
years earlier than the age at diagnosis of the youngest affected member of
the family (whichever is the earliest) in Australia.29 The guidelines issued by the World Gastroenterology
Organization (WGO) recommend that screening should start at age 20 to 25
years or 10 years earlier than the youngest age at CRC diagnosis in the
family, whichever comes first.30
The recommended endoscopic screening method for
carriers of genetic mutations associated with familial adenomatous
polyposis is mainly annual or biennial flexible sigmoidoscopy, or annual
colonoscopy. It is recommended to start screening at age 10 to 12 years in
the US15 and Singapore,16 at age 13 to 15 years in the UK,28 and at age 12 to 15 years (later age is recommended)
in Australia.29 The guidelines
issued by the National Comprehensive Cancer Network suggest screening
should start at age 10 to 15 years,31
32 whereas the WGO recommendation
is to start screening at age 10 to 12 years.30
International guidelines recommend endoscopic
screening for individuals who have one first-degree relative diagnosed
with CRC at age 50 to 60 years.15
16 26
28 29
30 31
32 33
Individuals with more than one first-degree relative with CRC irrespective
of age at diagnosis are considered at increased risk and endoscopic
screening at more frequent intervals is recommended.15 16 26 28 29 30 31 32 For these
individuals, the recommended endoscopic screening method is to receive
colonoscopy every 5 years.15 16 26 29 30 31 32 It is
recommended to start screening at age 40 to 50 years, or 10 years prior to
the age at diagnosis of the youngest affected relative.15 16 26 29 30 31 32
Currently, patients with CRC may be referred for
genetic counselling and testing. These services are provided at centres
run by non-governmental organisation, including the Hereditary
Gastrointestinal Cancer Genetic Diagnosis Laboratory
(http://www.patho.hku.hk/colonreg.htm); the Department of Health’s
Clinical Genetic Service (http://www.dh.gov.hk); and the private sector.
Although referral criteria for testing may differ among these testing
services, commonly adopted criteria include strong family history,
occurrence of multiple cancers in a single individual, early onset of
disease, presence of pathogenic mutation in the cancer predisposition
gene, and clinically suspected hereditary cancer syndrome.
Emerging evidence for colorectal cancer screening
In the past 2 years, new evidence supporting the
effectiveness of CRC screening has emerged which reinforces the CEWG
recommendations.
A systematic review reported that sigmoidoscopy is
associated with a 27% reduction in CRC-specific mortality in four
randomised controlled trials and that biennial FOBT screening reduced
CRC-specific mortality by 9% to 22% at 19.5 to 30 years of follow-up in
five randomised controlled trials compared with no screening in the
average-risk population.34 35
Separately, a prospective study in Sweden found
that colonoscopic surveillance for increased-risk individuals with
significant family history is a cost-effective intervention to prevent
CRC.36
In addition, the US Preventive Services Task Force,37 the US Multi-Society Task Force
on Colorectal Cancer Screening,38
and the American Cancer Society39
40 updated their recommendations
for CRC screening in 2016 and 2017. All continue to recommend annual FOBT,
sigmoidoscopy every 5 to 10 years, or colonoscopy every 10 years as
appropriate screening modalities for average-risk individuals.
Conclusion
After considering local epidemiology, scientific
evidence, and local and international screening guidelines and practices,
the CEWG reaffirms in 2017 the CRC screening recommendations for
average-risk individuals and updates the screening interval relating to
the recommendations for increased-risk individuals with significant family
history of CRC, as summarised in the Table. A leaflet and booklet on the CEWG
recommendations are available
(http://www.chp.gov.hk/en/content/9/25/31932.html) for downloading and
dissemination to the general public to help them make informed choices.
The CEWG will continue to monitor emerging local and international
evidence and practice to ensure evidence-based CRC prevention and
screening recommendations are up to date.
Author contributions
All authors have made substantial contributions to
the concept or design; acquisition of data; analysis or interpretation of
data; drafting of the article; and critical revision for important
intellectual content.
Declaration
As editors of this journal, DVK Chao, HHF Loong,
and MCS Wong were not involved in the peer review process of this article.
All authors have no conflicts of interest to disclose. All authors had
full access to the data, contributed to the study, approved the final
version for publication, and take responsibility for its accuracy and
integrity. An earlier version of this article was published online on the
Centre for Health Protection website
(https://www.chp.gov.hk/files/pdf/cewg_crc_professional_hp.pdf).
References
1. Hong Kong Cancer Registry. Colorectal
cancer in 2015. 2016. Available from:
http://www3.ha.org.hk/cancereg/pdf/factsheet/2015/colorectum_2015.pdf.
Accessed 31 Oct 2017.
2. Department of Health and Census and
Statistics Department, Hong Kong SAR Government. Mortality Statistics;
2016.
3. World Health Organization. Q&A on
the carcinogenicity of the consumption of red meat and processed meat.
2015. Available from: http://www.who.int/features/qa/cancerred-meat/en/.
Accessed 6 Sep 2017.
4. Bradbury KE, Appleby PN, Key TJ. Fruit,
vegetable, and fiber intake in relation to cancer risk: findings from the
European Prospective Investigation into Cancer and Nutrition (EPIC). Am J
Clin Nutr 2014;100(Suppl l):394S-398S. Crossref
5. International Agency for Research on
Cancer, World Health Organization. List of classifications by cancer sites
with sufficient or limited evidence in humans, Vol 1 to 121. 2018.
Available from: https://monographs.iarc.fr/ENG/Classification/Table4.pdf.
Accessed 6 Sep 2017.
6. World Cancer Research Fund and American
Institute for Cancer Research. Diet, Nutrition, Physical Activity and
Colorectal Cancer. World Cancer Research Fund International; 2017.
7. Ho JW, Yuen ST, Lam TH. A case-control
study on environmental and familial risk factors for colorectal cancer in
Hong Kong: chronic illnesses, medication and family history. Hong Kong Med
J 2006;12(Suppl 1):S14-6.
8. Butterworth AS, Higgins JP, Pharoah P.
Relative and absolute risk of colorectal cancer for individuals with a
family history: a meta-analysis. Eur J Cancer 2006;42:216-27. Crossref
9. Half E, Bercovich D, Rozen P. Familial
adenomatous polyposis. Orphanet J Rare Dis 2009;4:22. Crossref
10. Samadder NJ, Jasperson K, Burt RW.
Hereditary and common familial colorectal cancer: evidence for colorectal
screening. Dig Dis Sci 2015;60:734-47. Crossref
11. Castaño-Milla C, Chaparro M, Gisbert
JP. Systematic review with meta-analysis: the declining risk of colorectal
cancer in ulcerative colitis. Aliment Pharmacol Ther 2014;39:645-59. Crossref
12. Winawer SJ. Natural history of
colorectal cancer. Am J Med 1999;106:3S-6S. Crossref
13. Terry MB, Neugut AI, Bostick RM, et
al. Risk factors for advanced colorectal adenomas: a pooled analysis.
Cancer Epidemiol Biomarkers Prev 2002;11:622-9.
14. Agency for Healthcare Research and
Quality, Department of Health & Human Services, US Government. US
Preventive Services Task Force. Screening for Colorectal Cancer: Summary
of Recommendations. October 2008. Available from:
http://www.ahrq.gov/clinic/uspstf/uspscolo.htm. Accessed 8 Oct 2008.
15. Levin B, Lieberman DA, McFarland B, et
al. Screening and surveillance for the early detection of colorectal
cancer and adenomatous polyps, 2008: a joint guideline from the American
Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and
the American College of Radiology. CA Cancer J Clin 2008;58:130-60. Crossref
16. Ministry of Health, Singapore
Government. Cancer Screening: MOH Clinical Practice Guidelines 1/2010,
February 2010.
17. The UK NSC recommendation on bowel
cancer screening. Available from:
https://legacyscreening.phe.org.uk/bowelcancer. Accessed 10 Sep 2018.
18. Hardcastle JD, Chamberlain JO,
Robinson MH, et al. Randomised controlled trial of faecal-occult-blood
screening for colorectal cancer. Lancet 1996;348:1472-7. Crossref
19. Kronborg O, Fenger C, Olsen J,
Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal
cancer with faecal-occult-blood test. Lancet 1996;348:1467-71. Crossref
20. Mandel JS, Bond JH, Church TR, et al.
Reducing mortality from colorectal cancer by screening for fecal occult
blood. Minnesota colon cancer control study. N Engl J Med
1993;328:1365-71. Crossref
21. Hewitson P, Glasziou P, Irwig L,
Towler B, Watson E. Screening for colorectal cancer using the faecal
occult blood test, Hemoccult. Cochrane Database Syst Rev
2007;(1):CD001216.
22. Shroff J, Thosani N, Bartra S, Singh
H, Guha S. Reduced incidence and mortality from colorectal cancer with
flexible-sigmoidoscopy screening: a meta-analysis. World J Gastroenterol
2014;20:18466-76. Crossref
23. Pan J, Xin L, Ma YF, Hu LH, Li ZS.
Colonoscopy reduces colorectal cancer incidence and mortality in patients
with non-malignant findings: a meta-analysis. Am J Gastroenterol
2016;111:355-65. Crossref
24. Winawer SJ, Fletcher RH, Miller L, et
al. Colorectal cancer screening: clinical guidelines and rationale.
Gastroenterology 1997;112:594-642. Crossref
25. Rose P, Dunlop M, Burton H, Haites N.
Screening for late onset genetic disorders colorectal cancer. The UK
National Screening Committee; October 2000.
26. Rex DK, Johnson DA, Anderson JC, et
al. American College of Gastroenterology guidelines for colorectal cancer
screening 2009 [corrected]. American J Gastroenterol 2009;104:739-50. Crossref
27. Giardiello FM, Allen JI, Axilbund JE,
et al. Guidelines on genetic evaluation and management of Lynch syndrome:
a consensus statement by the US Multi-Society Task Force on Colorectal
Cancer. Am J Gastroenterol 2014;109:1159-79. Crossref
28. Cairns SR, Scholefield JH, Steele RJ,
et al. Guidelines for colorectal cancer screening and surveillance in
moderate and high risk groups (update from 2002). Gut 2010;59:666-89. Crossref
29. National Health and Medical Research
Council, Australia Government. Clinical Practice Guidelines: The
Prevention, Early Detection and Management of Colorectal Cancer; December
2005.
30. Winawer S, Classen M, Lambert R, et
al. World Gastroenterology Organisation/International Digestive Cancer
Alliance Practice Guidelines: Colorectal Cancer Screening. South African
Gastroenterology Review 2008;6.
31. National Comprehensive Cancer Network.
NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer Version 2;
2016.
32. National Comprehensive Cancer Network.
NCCN Clinical Practice Guidelines in Oncology: Colon Cancer Version 1;
2017.
33. Sung JJ, Ng SC, Chan FK, et al. An
updated Asia Pacific consensus recommendations on colorectal cancer
screening. Gut 2015;64:121-32. Crossref
34. Lin JS, Piper MA, Perdue LA, et al.
Screening for colorectal cancer: updated evidence report and systematic
review for the US preventive services task force. JAMA 2016;315:2576-94. Crossref
35. Lin JS, Piper MA, Perdue LA, et al.
Screening for colorectal cancer: a systematic review for the US preventive
services task force. Evidence synthesis No. 135. Agency for Healthcare
Research and Quality; 2016.
36. Sjöström O, Lindholm L, Melin B.
Colonoscopic surveillance—a cost-effective method to prevent hereditary
and familial colorectal cancer. Scand J Gastroenterol 2017;52:1002-7. Crossref
37. US Preventive Services Task Force,
Bibbins-Domingo K, Grossman DC, et al. Screening for colorectal cancer: US
preventive services task force recommendation statement. JAMA
2016;315:2564-75. Crossref
38. Rex DK, Boland CR, Dominitz JA, et al.
Colorectal cancer screening: recommendations for physicians and patients
from the US multi-society task force on colorectal cancer.
Gastroenterology 2017;153:307-23. Crossref
39. American Cancer Society. American
Cancer Society recommendations for colorectal cancer early detection. July
2017. Available from:
https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/acsrecommendations.html.
Accessed 6 Sep 2017.
40. Smith RA, Andrews KS, Brooks D, et al.
Cancer screening in the United States, 2017: a review of current American
Cancer Society guidelines and current issues in cancer screening. CA
Cancer J Clin 2017;67:100-21. Crossref