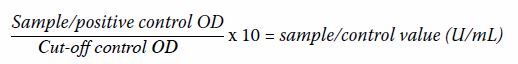Hong Kong Med J 2018 Jun;24(3):261–9 | Epub 25 May 2018
DOI: 10.12809/hkmj177007
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Evaluation of a multiplex flow immunoassay versus
conventional assays in detecting autoantibodies in systemic lupus
erythematosus
Elaine YL Au, MB, BS, FHKAM (Pathology)1;
WK Ip, PhD1; CS Lau, MB, ChB, FHKAM (Medicine)2, YT
Chan, MB, BS, FHKAM (Pathology)1
1 Division of Clinical Immunology,
Department of Pathology, Queen Mary Hospital, Pokfulam, Hong Kong
2 Division of Rheumatology and Clinical
Immunology, Department of Medicine, The University of Hong Kong, Pokfulam,
Hong Kong
Corresponding author: Dr Elaine YL Au (elaineauyl@gmail.com)
Abstract
Introduction: Conventional
diagnostic assays are being replaced with automated multiplex assays,
but their performance needs to be evaluated. We compared a multiplex
flow immunoassay with conventional techniques in the detection of
antinuclear antibodies (ANAs) and antibodies to specific extractable
nuclear antigens (ENAs) in serum samples from patients with systemic
lupus erythematosus.
Methods: A total of 140
consecutive Chinese patients with systemic lupus erythematosus and 41
healthy controls were included. The automated BioPlex 2200 ANA Screen
assay (Bio-Rad Laboratories, Hercules [CA], US) was compared with
indirect immunofluorescence. In addition, use of BioPlex 2200 to detect
anti-ENA antibodies was compared with in-house assays of countercurrent
immunoelectrophoresis (CIEP), enzyme-linked immunosorbent assay (ELISA),
and line blot.
Results: The sensitivity and
specificity of BioPlex in detecting ANAs (91.4% and 95.1%, respectively)
were comparable to those of indirect immunofluorescence (90.7% and
85.4%, respectively). Overall, BioPlex achieved the best agreement with
ELISA in detecting anti-ENA antibodies: agreement was >90% for most
antibody types (κ=0.79-0.94). In contrast, agreement was poorest with
CIEP, ranging from 85.6% (κ=0.33) for anti-Sm antibodies to 93.9%
(κ=0.88) for anti-Ro antibodies. Overall, BioPlex and ELISA had the
highest sensitivity, whereas CIEP had the highest specificity. In terms
of disease association, anti-Sm detected by CIEP had the best positive
predictive value and specificity for lupus nephritis.
Conclusions: In a local lupus
cohort, BioPlex showed comparable sensitivity to indirect
immunofluorescence in detecting ANAs and comparable performance to ELISA
in detecting anti-ENA antibodies. However, CIEP was the best method in
terms of disease specificity.
New knowledge added by this study
- The sensitivity of the BioPlex 2200 ANA Screen was comparable to that of indirect immunofluorescence.
- The BioPlex 2200 multiplex platform has a comparable performance to the enzyme-linked immunosorbent assay in the detection of antibodies to specific extractable nuclear antigens, but it is less specific than conventional gel precipitation (countercurrent immunoelectrophoresis).
- The performance of newer multiplex platforms for autoantibody detection may be different from that of conventional methods, and disease specificity of autoantibodies may change according to the test method.
- This variation may have a significant impact on the interpretation of results and on patient management.
Introduction
Connective tissue disease is a group of disorders
characterised by the presence of antinuclear antibodies (ANAs) and
clinical autoimmune phenomena. The investigations that are performed
depend on both purpose and performance characteristics. For example, to
rule out a diagnosis, a test with high sensitivity is needed, such as
testing for the absence of ANAs to rule out systemic lupus erythematosus
(SLE). In contrast, to establish a diagnosis, a test with high specificity
is more desirable, such as testing for antibodies to double-stranded DNA
(dsDNA) or anti-Sm antigens in SLE. Therefore, after an initial positive
ANA test result, subsequent tests for specific antibodies, such as those
against dsDNA and certain extractable nuclear antigens (ENAs), are
necessary.
Conventionally, ANAs are detected by indirect
immunofluorescence (IIF). This method is sensitive and essentially detects
all antibodies against cellular constituents, with antibody profile having
varying clinical significance. However, it is labour-intensive, and
technical interpretation of the results can be subjective. The
enzyme-linked immunosorbent assay (ELISA), which can be automated and
high-throughput–enabled, is gaining popularity over IIF. When ELISA is
used to screen for ANAs, the source of antigens has major implications on
the sensitivity and specificity of the assay. Although the ELISA technique
has improved with time, concerns over false-negative ANA cases persist.
Therefore, the American College of Rheumatology (ACR) still recommends IIF
as the gold standard in ANA testing.1
To detect antibodies against ENAs, gel
precipitation assays have been used for more than five decades, and
countercurrent immunoelectrophoresis (CIEP) has been accepted as the
reference method for anti-ENA antibody testing. Positive results from CIEP
are highly specific. The majority of the literature on autoantibodies and
disease association has been established with this technique.2 However, other methods such as ELISA, immunoblot, and
line blot are gradually replacing CIEP. In recent years, multiplex assays
have been introduced. The BioPlex 2200 ANA Screen assay (Bio-Rad
Laboratories, Hercules [CA], US) is an automated multiplex immunoassay
using flow cytometry to detect a panel of autoantibodies, including ANAs
and antibodies against ENAs. There are a few published studies showing
reasonable agreement between this system and ELISA.3 4 5 6 7
Conventional assays are being replaced with newer
automated high-throughput assays. However, the performance of the newer
techniques may not be equivalent to that of conventional assays. This
difference will have important implications to clinicians, who may base
their clinical judgement on their knowledge of how conventional assays
perform.8 9 10 11 12 13 14 15 16 In this
study, we evaluate the performance of BioPlex 2200 using serum samples
from a local cohort of SLE patients, and compare it with the performance
of three established techniques (CIEP, ELISA, line blot) in terms of
anti-ENA antibody detection. The sensitivity of BioPlex 2200 ANA Screen
assay was also compared with IIF.
Methods
Study setting and participant recruitment
This cross-sectional study was conducted at the
Queen Mary Hospital, Hong Kong, a tertiary university teaching hospital.
Patients were recruited from the hospital’s lupus clinic from 1 December
2013 to 31 December 2013. All patients attending the clinic underwent
routine serology screening during their visit. Of 160 consecutive
patients, 140 with adequate serum stored in the clinical immunology
laboratory were recruited. All patients had an established diagnosis of
SLE, according to the ACR classification criteria.17 Patients who were <18 years or >80 years and
pregnant patients were excluded from the study. Data of serum samples in
41 healthy controls, who were mainly laboratory staff and had given verbal
consent for the blood donation were also included; their age ranged from
18 to 54 years. All stocked serum was stored at -70°C.
Assessment of clinical variables
Electronic and written medical records of the
recruited patients were reviewed, and relevant clinical and laboratory
data were collected. Global disease activity was assessed according to the
SLE disease activity index,18 19 and cumulative organ damage was
assessed in terms of the Systemic Lupus International Collaborating
Clinics/ACR Damage Index score.20
Antinuclear antibody detection
BioPlex 2200 automated system
The BioPlex 2200 ANA Screen system was used to
detect 13 types of autoantibodies simultaneously in one test—namely, those
against dsDNA, chromatin, centromere B, Scl-70, RNP (RNP-A, RNP-68), Sm,
RNP/Sm, Ro (SSA-52, SSA-60), SSB/La, Jo-1, and ribosomal P protein. For
BioPlex results, anti-RNP was reported separately from anti-RNP/Sm; the
kit’s RNP antigen is a recombinant antigen (RNP-A and RNP-68) whereas
RNP/Sm is an affinity-purified antigen, which is similar to the antigen
used for the RNP test in ELISA and line blot in this study.
The presence of anti-dsDNA antibody was classified
as negative if levels were ≤4 IU/mL, indeterminate if 5 to 9 IU/mL, and
positive if ≥10 IU/mL, as recommended by the manufacturer. For the other
autoantibodies, the results were expressed as an antibody index (AI). An
AI of 1.0 was the cut-off concentration that corresponded to approximately
the 99th percentile of values obtained from a nondiseased population in
the manufacturer’s study. Results of ≥1.0 were reported as positive
(range, 0.2-8.0 AI). A test result was considered positive for ANAs if one
or more of the antibody tests in the panel was positive.
Indirect immunofluorescence
The IIF assay was adopted as the reference method
for ANA detection. All serum samples were diluted in 1:80 in
phosphate-buffered saline and tested on slides pretreated with substrate
from a human epithelial cell line (Kallestad HEp-2 Cell Line Substrate
Slides; Bio-Rad Laboratories, Hercules [CA], US) according to the
manufacturer’s instructions. The slides were read using the same
microscope and setting as routine clinical samples by a single observer.
Slides that were negative for ANA by IIF were reviewed by an independent
second observer to confirm negativity. In cases of discrepancy, a third
adjudicator was sought.
Anti–extractable nuclear antigen antibody detection
The performance of BioPlex in the detection of
anti-ENA antibodies was compared with that of the following assays.
Countercurrent immunoelectrophoresis
The CIEP assay used in this study was optimised
in-house. Rabbit thymus extract (ImmunoVision Inc, United States) was used
for typing of anti-Sm, anti-RNP, and anti-La, whereas human spleen extract
(ImmunoVision Inc) was used as a source of Ro antigen.
Line blot
The EUROASSAY test kit (EUROIMMUN, Lübeck, Germany)
was used as the line blot immunoassay in this study. The kit qualitatively
assessed the presence of human immunoglobulin G (IgG) autoantibodies
against six different antigens: RNP, Sm, SS-A, SS-B, SCl-70, and Jo-1. On
the basis of signal intensity, the results were categorised as negative,
borderline, and positive.
Enzyme-linked immunosorbent assay
The QUANTA Lite ENA Profile EIA kit (INOVA
Diagnostics, San Diego [CA], US) was used for ELISA in this study. The kit
qualitatively screened for the presence of IgG autoantibodies against
specific ENAs—namely, SSA (60 and 52 kDa), SSB, Sm, RNP/Sm, Scl-70, and
Jo-1. The results were calculated using the following formula (where OD =
optical density at 450 nm):
Results of <8 U/mL were classified as negative,
8 to 12 U/mL as equivocal, and >12 U/mL as positive.
Statistical analysis
The diagnostic performance of BioPlex versus that
of IIF was compared for the detection of ANAs in the SLE cohort and
controls. Assay sensitivity and specificity were calculated and compared
using a paired McNemar’s test. Cohen’s kappa coefficient and percentage of
observed agreement were also calculated for the two methods.
For individual anti-ENA antibodies (against RNP,
Sm, Ro, La, Scl-70, and Jo-1), agreement analysis was calculated for the
four laboratory methods. Fleiss’ kappa coefficient with its 95% confidence
interval and the percentage of observed agreement were calculated to
assess overall agreement among the four methods. Pairwise agreement
analysis for the four methods was also performed by calculating Cohen’s
kappa coefficient and percentage of observed agreement. Weak and
borderline results in the ELISA and line blots were treated as negative in
the analysis.
The diagnostic value of anti-ENA antibody detection
to predict various disease manifestations was examined, along with
comparisons between the different methods. In particular, we studied
diagnostic performance for the association of anti-Sm antibodies with
nephritis, anti-RNP antibodies with Raynaud’s phenomenon, and anti-Ro/La
antibodies with photosensitivity, discoid rash, Sicca symptoms,
leukopenia, and lymphopenia.21 22 23
24 25
26 Sensitivity, specificity,
positive and negative predictive values, and diagnostic accuracies were
calculated.
The SPSS (Windows version 20.0; IBM Corp, Armonk
[NY], US) and Microsoft Excel 2010 for Windows were used for statistical
analysis and calculation of confidence intervals, respectively. P values
of <0.05 were regarded as statistical significance. The STARD 2015
guidelines were used during the writing of this article.27
Results
Patients and autoantibodies
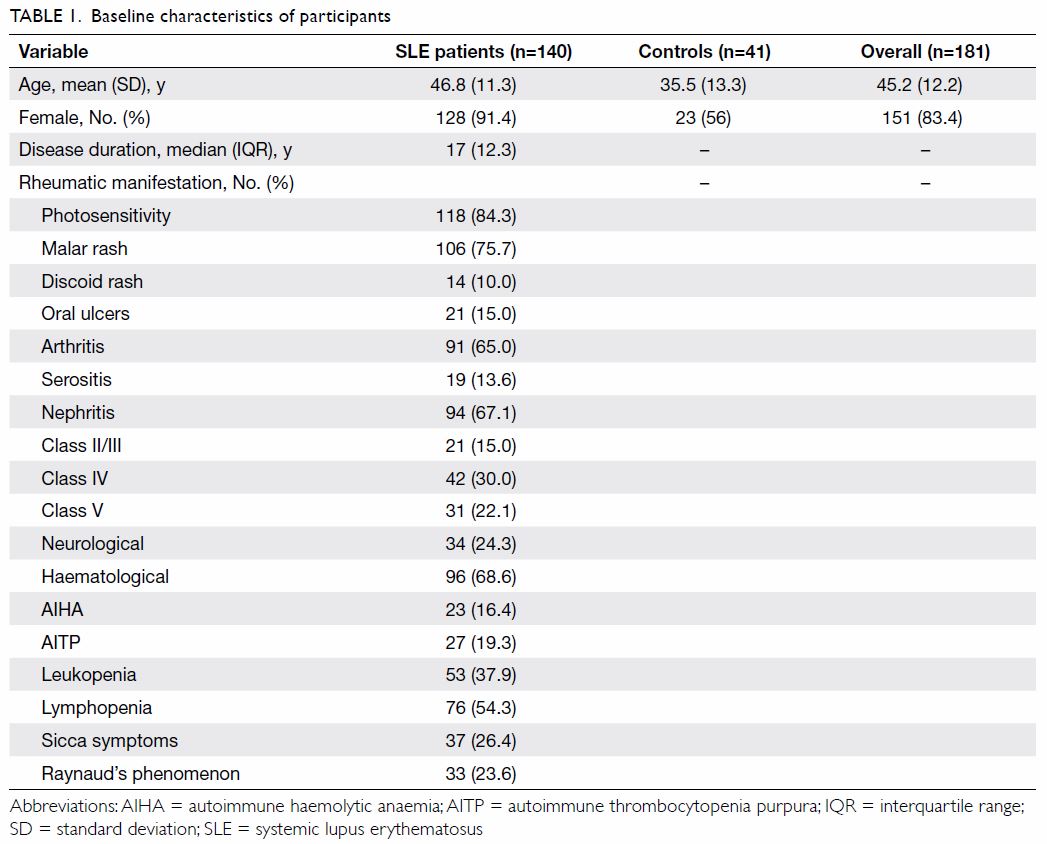
All 140 SLE patients were local Chinese patients,
with a female predominance (n=128, 91.4%). The mean age was 46.8 (range,
24-69) years and the median disease duration was 17 years (Table
1). The majority of our cohort had at least one anti-ENA antibody
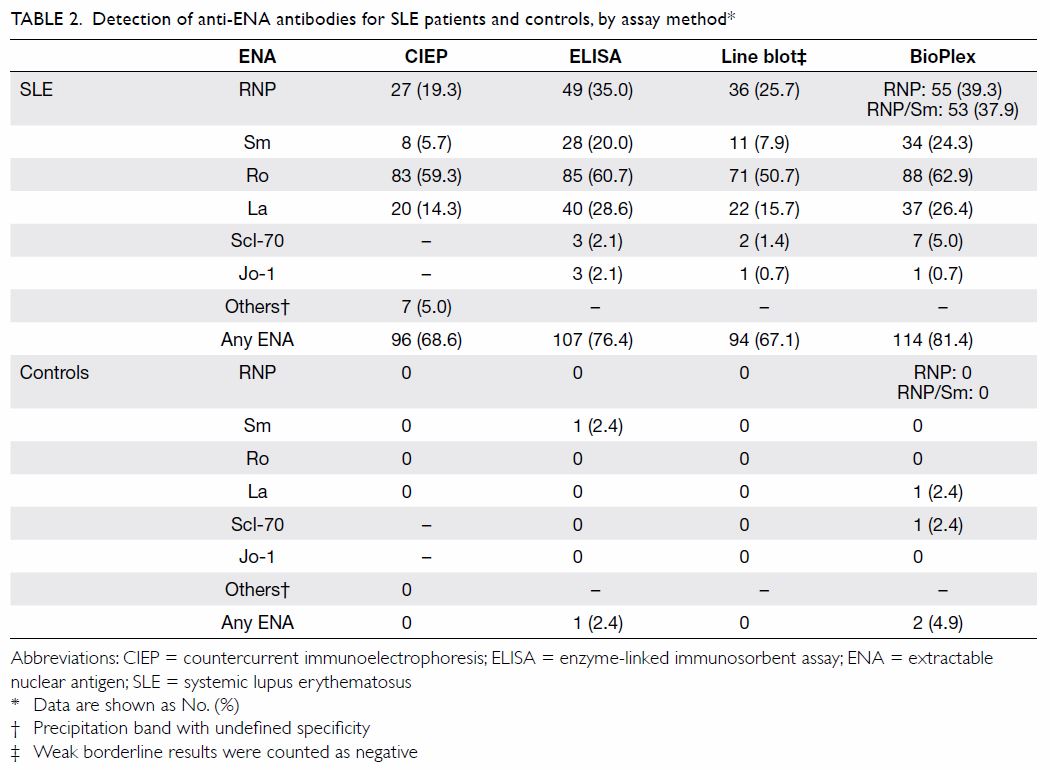
present (n=114, 81.4%) as detected by the BioPlex method (Table
2). Anti-Ro antibody was the most commonly detected antibody,
ranging from 50.7% to 62.9% of the cohort depending on the assay method (Table 2). Methods other than CIEP had a positivity
rate of 1.4% to 5.0% for anti–Scl-70 antibody and 0.7% to 2.1% for
anti–Jo-1 antibody.
BioPlex antinuclear antibody screen versus indirect
immunofluorescence
The sensitivity of the BioPlex 2200 ANA Screen
assay in the SLE cohort was 91.4%, which was comparable to that of IIF
(90.7%; Table 3). The specificity of BioPlex among healthy
controls was high, reaching 95.1%, compared with 85.4% for IIF, although
the difference was not statistically significant. The agreement between
BioPlex and IIF was moderate (κ=0.657). Eight patients tested positive by
IIF, but negative by BioPlex. The IIF patterns of these cases were either
weak homogeneous or weak fine-speckled. Nine patients were negative by
IIF, but positive by BioPlex; these included two with a low titre of
anti-dsDNA antibodies, one with anti-Sm antibodies, one with anti-RNP
antibodies, and five with anti-Ro antibodies. Four patients tested
ANA-negative by both methods; all four had a long-standing history of SLE
(12-40 years). All had had severe disease manifestations, including
cerebral lupus and lupus nephritis, and all had been taking powerful
immunosuppressants for years, although the disease had become stable and
inactive in recent years. Interestingly, they had been ANA-positive in the
past. Possible changes in their serology after a long period of heavy
immunosuppression for disease control may have accounted for the observed
results.
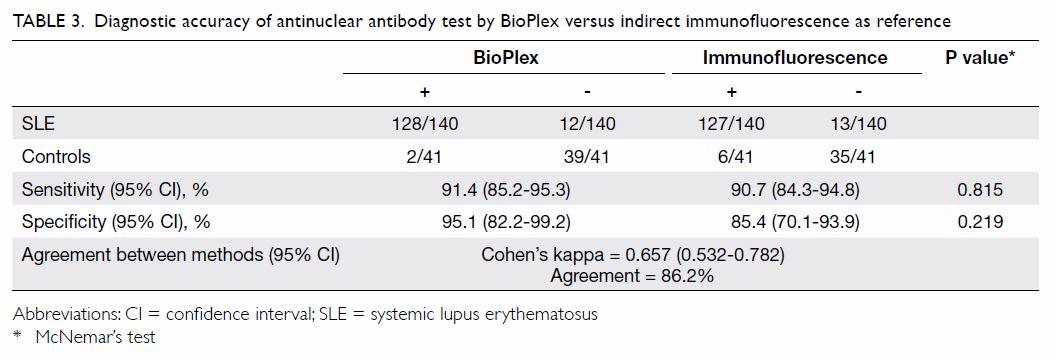
Table 3. Diagnostic accuracy of antinuclear antibody test by BioPlex versus indirect immunofluorescence as reference
Agreement between assays for antibodies to extractable
nuclear antigens
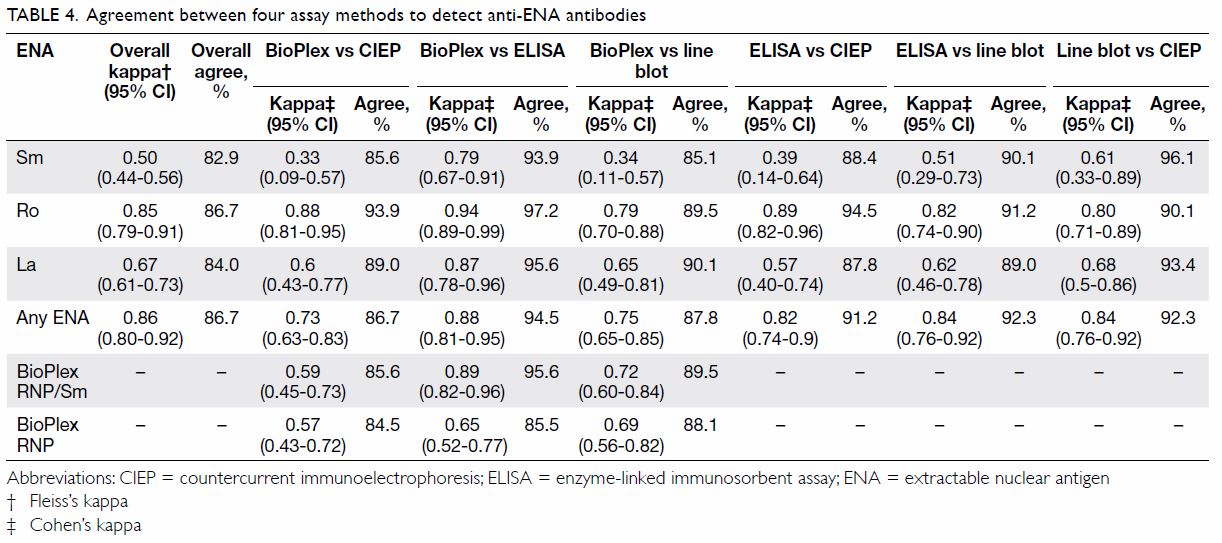
In terms of agreement between different methods,
BioPlex achieved the best agreement with ELISA, of >90% for the
detection of most of the antibodies tested by ELISA (Table
4). The agreement between BioPlex and ELISA was 95.6% for
anti-RNP/Sm antibodies (κ=0.89), 93.9% for anti-Sm (κ=0.79), 97.2% for
anti-Ro (κ=0.94), and 95.6% for anti-La (κ=0.87). In contrast, the
agreement between BioPlex and CIEP was not as strong. Agreement was 84.5%
for detection of anti-RNP antibodies (κ=0.57), 85.6% for anti-Sm (κ=0.33),
93.9% for anti-Ro (κ=0.88), and 89.0% for anti-La (κ=0.6). Overall, the
CIEP tended to agree better with the line blot assay than with ELISA or
BioPlex.
Performance of assays for antibodies to extractable
nuclear antigens
Overall, BioPlex and ELISA had a higher sensitivity
in detecting autoantibodies in the SLE cohort than the other two methods (Table 2). There were a few positive cases of
anti–Scl-70 and anti–Jo-1 antibody detection in the cohort by all assays
except CIEP, although the clinical significance of these antibodies in
patients with SLE is uncertain.
In the healthy control group, CIEP had the highest
specificity; none of the healthy subjects had anti-ENA autoantibodies when
tested by CIEP (Table 2). With BioPlex, however, 2.4% (1/41) of the
controls for each were positive for anti-La and anti–Scl-70 antibodies.
For the line blot, if a weak borderline band were considered positive,
then 4.9% (2/41) of the subjects were positive for anti-La antibodies
(data not shown). If a weak borderline band were considered negative, then
none of the healthy controls tested positive. For ELISA, if borderline
results were counted negative then 2.4% (1/41) of the healthy controls
still tested positive for anti-Sm antibodies.
Antibodies to extractable nuclear antigens and disease
manifestations
Among the panel of anti-ENA autoantibodies tested,
anti-Sm antibody had the best predictive value for the presence of lupus
nephritis. However, the predictive value was method-dependent (Table
5). Anti-Sm antibody detection by CIEP had the best positive
predictive value for lupus nephritis, reaching 87.5%. The specificity of
anti-Sm antibody detection by CIEP for lupus nephritis was high, reaching
98.6%, although the sensitivity was only 10.4%. Anti-Sm antibody detection
by BioPlex in nephritis had a higher sensitivity of 26.9%, however, the
specificity and positive predictive value were lower than those achieved
by CIEP (78.1% and 52.9%, respectively).
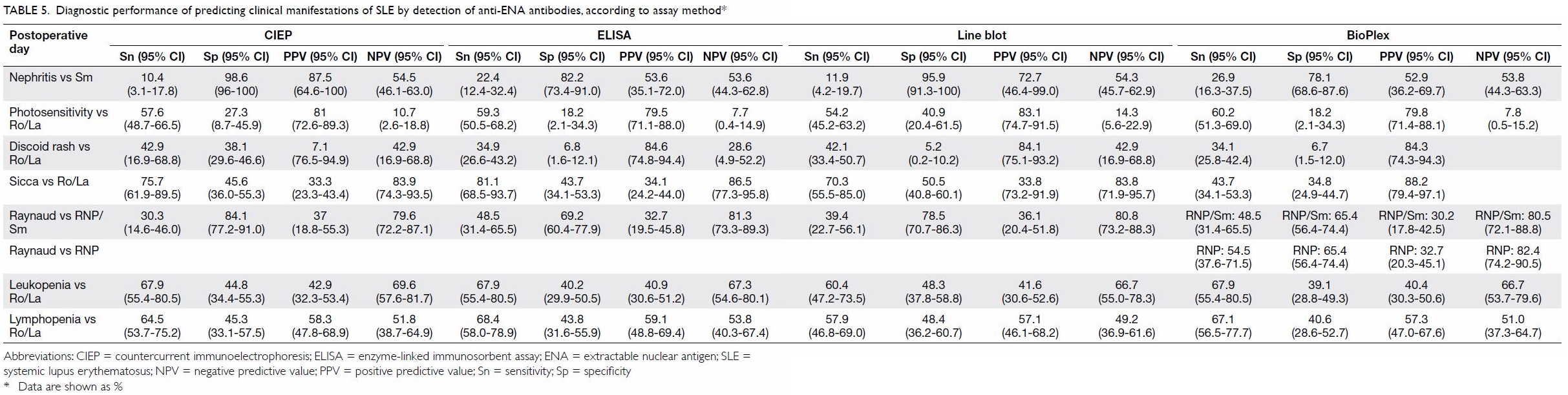
Table 5. Diagnostic performance of predicting clinical manifestations of SLE by detection of anti-ENA antibodies, according to assay method
Anti-RNP antibody detection by CIEP had a
specificity of 84.1% for Raynaud’s phenomenon, whereas the specificity by
other methods was lower (69.2% for ELISA, 78.5% for line blot, and 65.4%
for BioPlex). As the prevalence of Raynaud’s phenomenon in the cohort was
not high, the positive predictive value was at best 37.0% only, by CIEP.
BioPlex generally had a higher sensitivity than the
other methods, with the trade-off of lower specificity. However, CIEP
generally performed better than BioPlex in disease-antibody associations (Table 6). The superiority of CIEP over BioPlex was
most obvious in the diagnostic accuracy of linking anti-RNP antibody
detection to Raynaud’s phenomenon (71.4% for CIEP vs 62.9% for BioPlex;
P<0.001). Detection of antibodies to RNP (recombinant) and RNP/Sm by
BioPlex did not differ significantly in diagnostic accuracy for
association with Raynaud’s phenomenon.
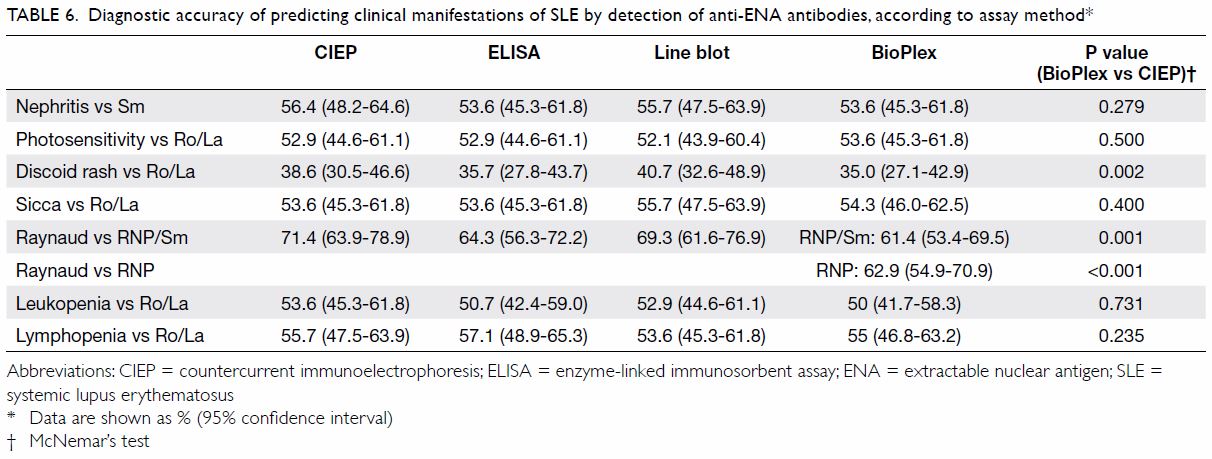
Table 6. Diagnostic accuracy of predicting clinical manifestations of SLE by detection of anti-ENA antibodies, according to assay method
Discussion
In recent years, the multiplex method has been
introduced in ANA testing. However, on the basis of the existing
literature, this method is considered suboptimal in sensitivity compared
with IIF, and its false-negative rate is similar to that of ELISA, ranging
from 0.2% to 41.5% in the different populations studied.4 7 28 29 30 When Tozzoli et al31
compared the detection of ANAs between IIF using a 1:80 cut-off and
BioPlex 2200 ANA Screen in a cohort of 95 SLE patients, they found that
IIF had superior sensitivity over BioPlex (85/95 [89.5%] positive vs 77/95
[81.1%] positive, respectively). Generally, multiplex methods are
considered to be simple to operate, have potential for automated and
high-throughput processing, and can detect multiple specific antibodies
simultaneously. Nonetheless, the main limitation is that such methods do
not detect all the autoantibodies that can be detected by IIF. Hence,
multiplex systems are considered to be insufficient in sensitivity and
negative predictive value, and IIF remains the reference method of ANA
testing.32 33
For our cohort, the BioPlex system demonstrated
good sensitivity (91.4%), comparable to that of IIF (90.7%), with an
agreement of 86.2% (κ=0.657). The specificity of BioPlex was slightly
higher than that of IIF (95.1% vs 85.4%) but the difference did not reach
statistical significance, perhaps because of the relatively small control
group. Notably, a large proportion (6 of 9 cases) of the BioPlex-positive
IIF-negative cases were actually positive for anti-Ro antibodies. Although
IIF is the preferred method for ANA screening, as recommended by the ACR
and the European Autoimmunity Standardisation Initiative, inconsistency
among IIF assays exists.33 Slides
from different vendors vary in sensitivity, especially for anti-SSA/Ro
antibody detection.34 Moreover,
the reading and interpretation of slides are reader- and skill-dependent.
Overall, BioPlex as used in our study showed a
higher performance when compared with other studies. This difference could
be due to different cohort characteristics, disease activities, and
ethnicities. For example, the vast majority of the literature reports on
studies of Caucasian populations, and studies of Chinese populations are
scarce. In addition, we recruited only patients with SLE, but not other
autoimmune diseases, thereby precluding direct comparisons. We also had a
relatively small number of SLE cases; hence, some ANA staining patterns
(eg, nuclear dots, proliferating cell nuclear antigen, nuclear lamina)
were not encountered in the cohort, which limits the evaluation. Given the
available literature and international recommendations, IIF remains the
preferred method for ANA test until more supportive data for BioPlex are
available.
In our study, CIEP performed best in terms of
specificity, with none of the healthy controls testing positive for
anti-ENA antibodies. In contrast, the specificity of the other platforms,
especially ELISA and BioPlex, was less optimal, and positivity for
antibodies to Sm (ELISA), Scl-70 (BioPlex), and La (BioPlex) was recorded.
In addition, anti–Scl-70 and anti–Jo-1 antibodies were detected in assays
other than CIEP in the SLE group. If appropriate disease controls, such as
vasculitis, rheumatoid arthritis, and chronic infections are included, the
performance of these assays would be better characterised.
There were several important limitations in our
study. First, this was a cross-sectional study, and the clinical features
and manifestations were retrospectively reviewed. The reviewer of the
medical records was not blinded to the results of assays, which may have
led to potential bias in record review and data extraction. Second, the
performance of BioPlex was not evaluated in other rheumatic or autoimmune
disease groups, which limits the generalisability of the results in other
settings. Third, the number of participants included, especially that of
healthy controls, was relatively small; disease controls were not
included; and the controls were not age- and sex- matched with cases.
These limitations may have led to bias in the evaluation of anti-ENA
antibody assays. Finally, autoantibodies may precede clinical
manifestations for years. A prospective study with parallel assessment of
cases referred to the laboratory for ANA and anti-ENA antibody assessment
by different techniques, as well as follow-up of the clinical
manifestations and diagnosis, may provide a better assessment of the
BioPlex system.
Conclusions
The BioPlex 2200 ANA Screen demonstrated comparable
sensitivity to IIF in a local SLE cohort. The detection of specific
antibodies, including those against ENAs, by the BioPlex system was more
sensitive than that by CIEP, although with less specificity. Overall
performance of BioPlex resembled that of the conventional ELISA technique,
but with higher speed and turnaround time. Hence, BioPlex can be
considered as a high-throughput ELISA-like assay for the detection of
anti-ENA antibodies in SLE.
Author contributions
All authors have made substantial contributions to
the concept or design of this study; acquisition of data; analysis or
interpretation of data; drafting of the article; and critical revision for
important intellectual content.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Declaration
All authors have no conflicts of interest to
disclose. All authors had full access to the data, contributed to the
study, approved the final version for publication, and take responsibility
for its accuracy and integrity.
Ethical approval
This study was approved by The University of Hong
Kong/Hospital Authority Hong Kong West Cluster Institutional Review Board
(Ref No. UW14-442). The requirement for patient consent was waived by the
ethics board.
References
1. Meroni PL, Schur PH. ANA screening: an
old test with new recommendations. Ann Rheum Dis 2010;69:1420-2. Crossref
2. Clotet B, Guardia J, Pigrau C, et al.
Incidence and clinical significance of anti-ENA antibodies in systemic
lupus erythematosus. Estimation by counterimmunoelectrophoresis. Scand J
Rheumatol 1984;13:15-20. Crossref
3. Hanly JG, Su L, Farewell V, Fritzler MJ.
Comparison between multiplex assays for autoantibody detection in systemic
lupus erythematosus. J Immunol Methods 2010;358:75-80. Crossref
4. Hanly JG, Thompson K, McCurdy G, Fougere
L, Theriault C, Wilton K. Measurement of autoantibodies using multiplex
methodology in patients with systemic lupus erythematosus. J Immunol
Methods 2010;352:147-52. Crossref
5. Kim Y, Park Y, Lee EY, Kim HS.
Comparison of automated multiplexed bead-based ANA screening assay with
ELISA for detecting five common anti-extractable nuclear antigens and
anti-dsDNA in systemic rheumatic diseases. Clin Chim Acta 2012;413:308-11.
Crossref
6. Shovman O, Gilburd B, Barzilai O, et al.
Evaluation of the BioPlex 2200 ANA screen: analysis of 510 healthy
subjects: incidence of natural/predictive autoantibodies. Ann N Y Acad Sci
2005;1050:380-8. Crossref
7. Desplat-Jego S, Bardin N, Larida B,
Sanmarco M. Evaluation of the BioPlex 2200 ANA screen for the detection of
antinuclear antibodies and comparison with conventional methods. Ann N Y
Acad Sci 2007;1109:245-55. Crossref
8. Orton SM, Peace-Brewer A, Schmitz JL,
Freeman K, Miller WC, Folds JD. Practical evaluation of methods for
detection and specificity of autoantibodies to extractable nuclear
antigens. Clin Diagn Lab Immunol 2004;11:297-301. Crossref
9. Lock RJ, Unsworth DJ. Antibodies to
extractable nuclear antigens. Has technological drift affected clinical
interpretation? J Clin Pathol 2001;54:187-90. Crossref
10. Phan TG, Wong RC, Adelstein S.
Autoantibodies to extractable nuclear antigens: making detection and
interpretation more meaningful. Clin Diagn Lab Immunol 2002;9:1-7. Crossref
11. Kumar Y, Bhatia A, Minz RW.
Antinuclear antibodies and their detection methods in diagnosis of
connective tissue diseases: a journey revisited. Diagn Pathol 2009;4:1. Crossref
12. Emlen W, O’Neill L. Clinical
significance of antinuclear antibodies: comparison of detection with
immunofluorescence and enzyme-linked immunosorbent assays. Arthritis Rheum
1997;40:1612-8. Crossref
13. González C, Martin T, Arroyo T,
García-Isidoro M, Navajo JA, González-Buitrago JM. Comparison and
variation of different methodologies for the detection of autoantibodies
to nuclear antigens (ANA). J Clin Lab Anal 1997;11:388-92. Crossref
14. Bruner BF, Guthridge JM, Lu R, et al.
Comparison of autoantibody specificities between traditional and
bead-based assays in a large, diverse collection of patients with systemic
lupus erythematosus and family members. Arthritis Rheum 2012;64:3677-86. Crossref
15. Egner W. The use of laboratory tests
in the diagnosis of SLE. J Clin Pathol 2000;53:424-32. Crossref
16. Wiik AS, Gordon TP, Kavanaugh AF, et
al. Cutting edge diagnostics in rheumatology: the role of patients,
clinicians, and laboratory scientists in optimizing the use of autoimmune
serology. Arthritis Rheum 2004;51:291-8. Crossref
17. Tan EM, Cohen AS, Fries JF, et al. The
1982 revised criteria for the classification of systemic lupus
erythematosus. Arthritis Rheum 1982;25:1271-7. Crossref
18. Petri M, Hellmann D, Hochberg M.
Validity and reliability of lupus activity measures in the routine clinic
setting. J Rheumatol 1992;19:53-9.
19. Urowitz MB, Gladman DD. Measures of
disease activity and damage in SLE. Baillieres Clin Rheumatol
1998;12:405-13. Crossref
20. Gladman D, Ginzler E, Goldsmith C, et
al. The development and initial validation of the systemic lupus
international collaborating clinics/American College of Rheumatology
damage index for systemic lupus erythematosus. Arthritis Rheum
1996;39:363-9. Crossref
21. Tan EM, Fritzler MJ, McDougal JS, et
al. Reference sera for antinuclear antibodies. I. Antibodies to native
DNA, Sm, nuclear RNP, and SS-B/La. Arthritis Rheum 1982;25:1003-5. Crossref
22. Isenberg DA, Maddison PJ. Detection of
antibodies to double stranded DNA and extractable nuclear antigen. J Clin
Pathol 1987;40:1374-81. Crossref
23. McCain GA, Bell DA, Chodirker WB,
Komar RR. Antibody to extractable nuclear antigen in the rheumatic
diseases. J Reumatol 1978;5:399-406.
24. Hamburger M, Hodes S, Barland P. The
incidence and clinical significance of antibodies to extractable nuclear
antigens. Am J Med Sci 1977;273:21-8. Crossref
25. Clark G, Reichlin M, Tomasi TB Jr.
Characterization of a soluble cytoplasmic antigen reactive with sera from
patients with systemic lupus erythematosus. J Immunol 1969;102:117-22.
26. Tan EM, Kunkel HG. Characteristics of
a soluble nuclear antigen precipitating with sera of patients with
systemic lupus erythematosus. J Immunol 1966;96:464-71.
27. Bossuyt PM, Reitsma JB, Bruns DE, et
al. STARD 2015: an updated list of essential items for reporting
diagnostic accuracy studies. BMJ 2015;351:h5527. Crossref
28. Jaskowski TD, Schroder C, Martins TB,
Mouritsen CL, Litwin CM, Hill HR. Screening for antinuclear antibodies by
enzyme immunoassay. Am J Clin Pathol 1996;105:468-73. Crossref
29. Damoiseaux J, Vaessen M, Knapen Y, et
al. Evaluation of the FIDIS vasculitis multiplex immunoassay for diagnosis
and follow-up of ANCA-associated vasculitis and Goodpasture’s disease. Ann
N Y Acad Sci 2007;1109:454-63. Crossref
30. Bonilla E, Francis L, Allam F, et al.
Immunofluorescence microscopy is superior to fluorescent beads for
detection of antinuclear antibody reactivity in systemic lupus
erythematosus patients. Clin Immunol 2007;124:18-21. Crossref
31. Tozzoli R, Bonaguri C, Melegari A,
Antico A, Bassetti D, Bizzaro N. Current state of diagnostic technologies
in the autoimmunology laboratory. Clin Chem Lab Med 2013;51:129-38. Crossref
32. Op De Beéck K, Vermeersch P,
Verschueren P, et al. Antinuclear antibody detection by automated
multiplex immunoassay in untreated patients at the time of diagnosis.
Autoimmun Rev 2012;12:137-43. Crossref
33. Agmon-Levin N, Damoiseaux J,
Kallenberg C, et al. International recommendations for the assessment of
autoantibodies to cellular antigens referred to as anti-nuclear
antibodies. Ann Rheum Dis 2014;73:17-23. Crossref
34. Copple SS, Giles SR, Jaskowski TD,
Gardiner AE, Wilson AM, Hill HR. Screening for IgG antinuclear
autoantibodies by HEp-2 indirect fluorescent antibody assays and the need
for standardization. Am J Clin Pathol 2012;137:825-30. Crossref