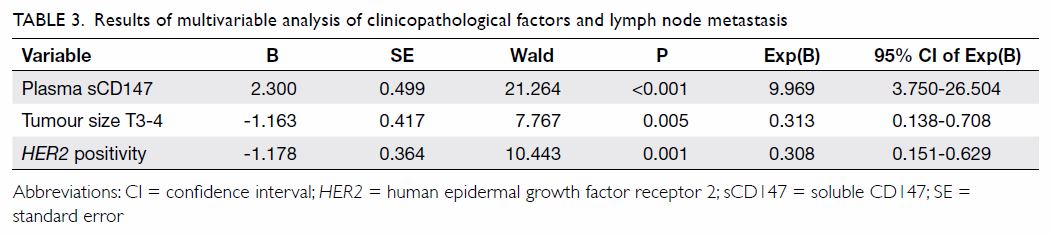Hong Kong Med J 2018 Jun;24(3):252–60 | Epub 25 May 2018
DOI: 10.12809/hkmj176865
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Plasma soluble cluster of differentiation 147 levels
are increased in breast cancer patients and associated with lymph node
metastasis and chemoresistance
YH Kuang, PhD1; YJ Liu, MSc2;
LL Tang, PhD2; SM Wang, PhD2; GJ Yan, BSc2;
LQ Liao, PhD2
1 Department of Dermatology, Xiangya
Hospital, Central South University, Changsha, Hunan, China
2 Department of Breast Surgery, Hunan
Clinical Meditech Research Center for Breast Cancer, Xiangya Hospital,
Central South University, Changsha, Hunan, China
Corresponding author: Dr LQ Liao (aq301981@163.com)
Abstract
Introduction: Cluster of
differentiation 147 (CD147) contributes to breast cancer invasion,
metastasis, and multidrug resistance. Recent studies have shown that
peripheral soluble CD147 (sCD147) is increased in hepatocellular tumour
and multiple myeloma patients and correlated with disease severity. This
study aimed to assess the level, as well as the biological and clinical
significance of sCD147 in breast cancer.
Methods: We tested plasma sCD147
levels in 308 breast cancer patients by enzyme-linked immunosorbent
assay between February 2014 and February 2017. A subset of 165 cases of
benign breast diseases was included as control group at the same period.
We analysed the clinical significance of plasma sCD147 with
relevance to clinicopathological factors of breast cancer patients.
Results: Plasma sCD147 levels
were significantly higher in patients with primary breast cancer than
those with benign breast diseases (P=0.001), in patients with locally
advanced breast cancer (T3-T4 tumour) than those in early breast cancer
(T1-T2 tumour; P=0.001), in patients with lymph node metastasis than in
those without (P<0.001), and in patients with high recurrence risk
than those with medium recurrence risk (P<0.001). Plasma sCD147
levels were also significantly higher in the chemotherapy-resistant
group than in the chemotherapy-sensitive group (P=0.040). Plasma sCD147
was an independent predictor for lymph node metastasis in breast cancer
patients (P=0.001).
Conclusion: This is the first
study to demonstrate that plasma sCD147 levels are elevated in breast
cancer patients. Soluble CD147 is also associated with tumour size,
lymph node metastasis, high recurrent risk, and chemoresistance. Our
findings support that plasma sCD147 is an independent predictive factor
for lymph node metastasis.
New knowledge added by this study
- Plasma sCD147 levels are elevated in breast cancer patients and are associated with tumour size, lymph node metastasis, high recurrent risk, and chemoresistance.
- Plasma sCD147 is an independent predictive factor for lymph node metastasis.
- Plasma sCD147 may be used as the predictive factor to evaluate lymph node metastasis, recurrence risk, and chemoresistance of breast cancer.
- Plasma sCD147 may contribute to the development of optimal adjuvant therapy for individual breast cancer patients.
Introduction
Breast cancer is the most common malignant tumour
and the leading cause of cancer-related deaths among females in developing
countries.1 Breast cancer displays
heterogeneity: it comprises distinct pathologies and histological features
and can have different chemotherapy responses and clinical outcomes.2 The identification of tumour-related factors that can
predict tumour behaviour is important. Predictive factors can help
identify as early as possible not only patients who have a high risk of
recurrence and metastasis, but also patients who can benefit from
different types of adjuvant therapy.3
Conventional predictive factors of high risk of recurrence and metastasis
include relatively large (>5 cm) tumour size and high nuclear grade;
negativity for oestrogen receptor and progesterone receptor; human
epidermal growth factor receptor 2 (HER2) overexpression; and
increased lymph node involvement at the time of breast cancer diagnosis.4 Recent advances in genetic
profiling of tumours have extended our understanding of breast cancer
biology and have allowed the use of several prognostic gene signatures to
select patients at highest risk of early recurrence and those who may
benefit from certain adjuvant treatment.2
5 6
However, despite receiving standard treatments routinely guided by
predictive factors, more than 30% of breast cancer patients develop
metastatic disease and have poor survival.7
8 As such, it is essential and
urgent to identify reliable predictive factors to assist in diagnosis,
staging, evaluation of recurrence risk, and development of new treatment
modalities.
Cluster of differentiation 147 (CD147), a
transmembrane glycoprotein that belongs to the immunoglobulin superfamily,
can promote tumour invasion and metastasis, and mediate breast cancer drug
resistance.9 10 11 12 13
Expression of CD147 is significantly correlated with axillary lymph node
involvement; tumour, node, and metastasis staging; and shorter
progression-free survival and overall survival.14
Previous data demonstrated that CD147 exists in both membrane-bound and
soluble forms in many solid tumours, and soluble CD147 (sCD147) can be
detected in the conditioned medium of tumour cells and peripheral blood of
cancer patients.15 16 17
Overexpression of the CD147 gene in human breast cancer cells can increase
the sCD147 level, indicating that sCD147 release is correlated with the
degree of CD147 expression in tumour cells.15
16 17
Full-length CD147 may be exported into the microenvironment from tumour
cells by microvesicle shedding or by matrix metalloproteinase
(MMP)–dependent cleavage, thereby stimulating MMP expression in
fibroblasts.18 19 20 In turn,
sCD147 derived from tumour cells acts in a paracrine fashion on stromal
cells that are both adjacent and distant to tumour sites, so as to further
stimulate the production of MMPs and CD147. This additional CD147
consequently contributes to tumour angiogenesis, tumour growth, and
metastasis.16 21 Importantly, several studies investigating the role
of sCD147 level in patients with tumours have suggested that sCD147 may
offer a useful approach in diagnosis, as it is correlated with disease
severity.15 22 However, little is known about the level of sCD147 in
patients with breast cancer. Furthermore, the biological and clinical
significance of sCD147 in breast cancer has not been investigated.
In this study, we measured plasma sCD147 levels in
patients with breast cancer and evaluated the results with respect to
clinicopathological factors. We aimed to demonstrate the association
between plasma sCD147 levels with tumour size, lymph node metastasis,
recurrence risk, and chemoresistance in breast cancer patients.
Methods
Patients and samples
The results of this study are presented in
accordance with the reporting recommendations for tumour marker prognostic
studies.23 We conducted the study
between February 2014 and February 2017 in the Affiliated Xiangya Hospital
of Central South University in Changsha of Hunan Province, China. We
collected peripheral blood samples from consecutive patients with breast
cancer, including primary breast cancer, during their first hospital
admission. To be eligible for this study, patients had to be adult females
who had no other malignant diseases or severe systemic diseases,
especially rheumatic, inflammatory, and cardiovascular diseases. The
peripheral blood of consecutive patients with palpable benign breast
masses, including fibroadenoma and adenopathy, was also collected to serve
as control samples during the same period. All blood samples were
centrifuged at 3000 rpm at 4°C for 5 minutes, and the plasma samples were
stored at -70°C for later plasma sCD147 testing. All the patients’
clinicopathological findings were supplied by the Xiangya Hospital of
Central South University. Breast cancer subtypes were identified according
to the St Gallen Consensus 2013 classification system.24 Recurrence risk of breast cancer was evaluated
according to the St Gallen Consensus 2007 criteria.25
The association between chemotherapy response and
plasma sCD147 level was retrospectively analysed. The patients included in
this analysis had to meet all of the following criteria: (1) had a
confirmed diagnosis of invasive ductal breast carcinoma by pathology and
had consented to undergo neoadjuvant chemotherapy; (2) had operable breast
cancer consisting of a large tumour (>2 cm) that fulfilled the criteria
for breast conserving surgery except tumour size, or triple-negative
breast cancer (TNBC; ie, negative for oestrogen/progesterone receptors and
HER2) with small (T1 stage) tumours; (3) had received no previous
treatment; (4) had received only four cycles of
pirarubicin-cyclophosphamide/epirubicin-cyclophosphamide (AC/EC)–based
neoadjuvant chemotherapy before surgery; and (5) had complete hospital
records that included evaluation of chemotherapy efficacy. Clinical
response to AC/EC-based chemotherapy was evaluated by the decrease in
tumour size and classified according to response evaluation criteria in
solid tumours (RECIST criteria).26
Patients with complete remission or partial remission were classified as
chemotherapy-sensitive, whereas patients with stable disease or
progressive disease were classified as chemotherapy-resistant.
Enzyme-linked immunosorbent assay
The concentrations of plasma sCD147 were measured
by enzyme-linked immunosorbent assay (ELISA). Plasma sCD147 levels were
assessed using the EMMPRIN/CD147 ELISA kit (R&D Systems, Minneapolis
[MN], US) according to the manufacturer’s protocol. The concentration of
the sample in each ELISA well was determined by interpolation from a
standard curve. Each sample was tested in duplicate.
Statistical analysis
The Mann-Whitney U test was used to compare
levels of plasma sCD147 in different groups according to variable
clinicopathological factors. The Chi squared contingency test with Yates
correction was used to determine the relationship between
clinicopathological factors of breast cancer patients and lymph node
status or chemotherapy sensitivity. Binary logistic regression was used to
assess clinicopathological factors (plasma sCD147, tumour size, and HER2)
that were associated with lymph node metastasis or chemoresistance in
invasive breast cancer. All multivariable logistic regression models used
backward stepwise procedures, and only datasets complete for every outcome
analysed were used. Receiver operating characteristic (ROC) curve analysis
was performed to calculate the area under the curve and evaluate the
optimal cut-off point, which was given by the maximum of the Youden index.
Statistical significance was set at P<0.05. The GraphPad Prism 6
software (GraphPad Software, La Jolla [CA], US) and SPSS (Windows version
19.0; IBM Corp, Armonk [NY], US) were used for statistical analysis.
Results
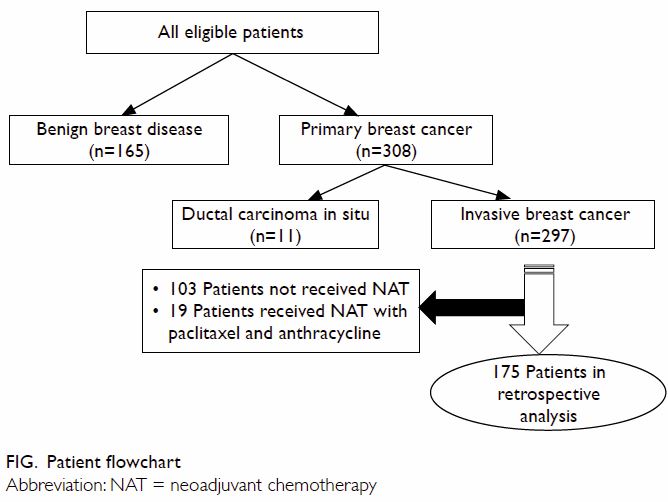
Patient’ characteristics
Among all eligible patients with complete records,
165 had benign breast disease (age range, 22-68 years) and 308 had primary
breast cancer (age range, 24-77 years). There was no significant
difference in age between the two groups (P=0.381). Breast cancer patients
comprised 11 with ductal carcinoma in situ and 297 with invasive ductal
carcinoma. Retrospective analysis of the association of plasma sCD147
level with response to neoadjuvant chemotherapy included 175 patients who
met all the inclusion criteria (Fig)—luminal A in 39, luminal B in 70, HER2-positive
in 28, and TNBC in 38. In all, 170 patients had T2-T4 tumours and five had
T1 TNBC tumours. Using the RECIST criteria, we assigned the 175 patients
to two groups: chemotherapy-sensitive (n=126) and chemotherapy-resistant
(n=49).
Plasma soluble CD147 levels in breast cancer patients
According to ELISA results, plasma sCD147 levels
were significantly higher in patients with primary breast cancer than in
those with benign breast disease (median [interquartile range; IQR],
8629.81 pg/mL [7426.33-10 309.20 pg/mL] vs 7625.99 pg/mL [6739.20-9140.04
pg/mL]; P=0.001). However, there was no significant difference in plasma
sCD147 levels between patients with invasive breast cancer and those with
ductal carcinoma in situ (8618.91 pg/mL [7404.81-10 358.50 pg/mL] vs
9185.79 pg/mL [7671.15-9626.47 pg/mL]; P=0.787). Regarding cancer subtypes
of the 297 patients with invasive breast carcinoma, median (IQR) plasma
sCD147 levels were significantly higher in patients with HER2-positive
breast cancer (10 042.34 pg/mL [7772.01-11 058.48 pg/mL]) than in those
with luminal A tumours (7991.05 pg/mL [7101.72-10 237.4 pg/mL]; P=0.007),
luminal B tumours (8629.81 pg/mL [7200.45-9953.32 pg/mL]; P=0.017), and
TNBC tumours (8585.16 pg/mL [7884.27-10 545.51 pg/mL]; P=0.027).
Association between plasma soluble CD147 and
clinicopathological factors
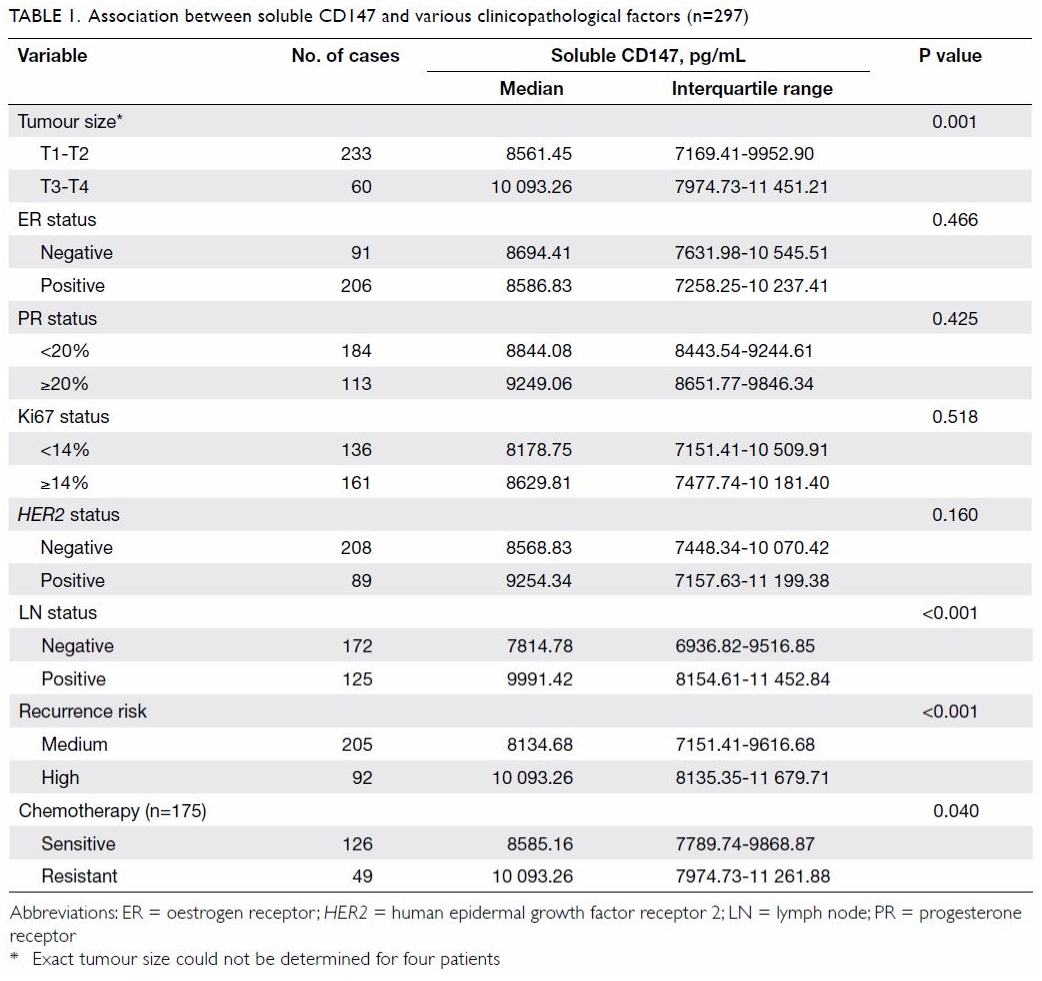
The association between plasma sCD147 level and
clinicopathological factors in patients with invasive breast cancer is
summarised in Table 1. Plasma sCD147 levels increased with tumour
size: median (IQR) levels were significantly higher in patients with
locally advanced (stage T3-T4) than those with early (stage T1-T2) breast
cancer (10 093.26 pg/mL [7974.73-11 451.21 pg/mL] vs 8561.45 pg/mL
[7169.41-9952.90 pg/mL]; P=0.001). Plasma sCD147 levels were also elevated
in patients with lymph node metastasis compared with those without (median
[IQR], 9991.42 pg/mL [8154.61-11 452.84 pg/mL] vs 7814.78 pg/mL
[6936.82-9516.85 pg/mL]; P<0.001). In addition, plasma sCD147 levels
were significantly higher in patients with a high risk of recurrence than
in those with a medium risk (median [IQR], 10 093.26 pg/mL [8135.35-11
679.71 pg/mL] vs 8134.68 pg/mL [7151.41-9616.68 pg/mL]; P<0.001).
Although plasma sCD147 levels were elevated for the HER2- positive
breast cancer subtype as compared with other breast cancer subtypes, there
was no significant difference between HER2-positive and HER2-negative patients (median [IQR], 9254.34 pg/mL [7157.63-11 199.38 pg/mL]
vs 8568.83 pg/mL [7448.34-10 070.42 pg/mL]; P=0.160).
Plasma soluble CD147 as an independent predictor for
lymph node metastasis
Because plasma sCD147 was associated with lymph
node status and recurrent risk, we speculated that plasma sCD147 may be a
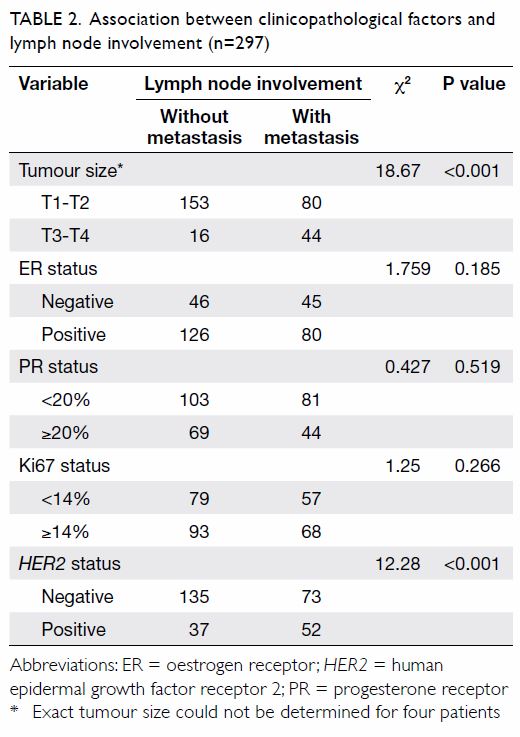
predictor for lymph node metastasis of breast cancer. Univariate analysis
showed that tumour size and HER2 status may be involved in lymph
node metastasis (Table 2). We subsequently used binary logistic
regression analysis to identify clinicopathological factors associated
with lymph node metastasis in invasive breast cancer. Our data showed that
plasma sCD147 (P<0.001), HER2-positive tumours (P=0.001), and
tumour size T3-T4 (P=0.005) were independent predictors of lymph node
metastasis of breast cancer (Table 3). When we analysed ROC curves to evaluate
use of plasma sCD147 as a diagnostic biomarker for lymph node metastasis,
the area under the curve was 0.745 (95% confidence interval, 0.676-0.813)
and the optimal cut-off point of plasma sCD147 was 8577 pg/mL, which
provided a sensitivity of 70.9% and a specificity of 61.7%.
Association of plasma soluble CD147 levels with
chemotherapy response
Table 1 shows that plasma sCD147 levels in the
chemotherapy-resistant group were significantly higher than those in the
chemotherapy-sensitive group (median [IQR], 10 093.26 pg/mL [7974.73-11
261.88 pg/mL] vs 8585.16 pg/mL [7789.74-9868.87 pg/mL]; P=0.040).
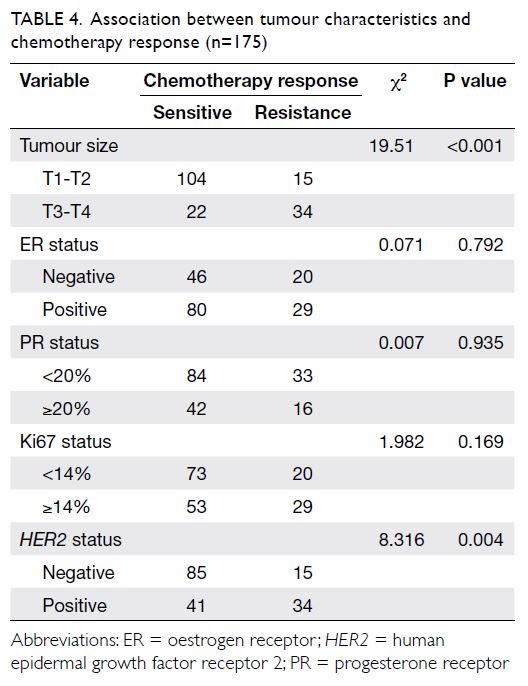
Univariate analysis revealed that tumour size and HER2 status may
be involved in chemotherapy response (Table 4). Binary logistic regression analysis
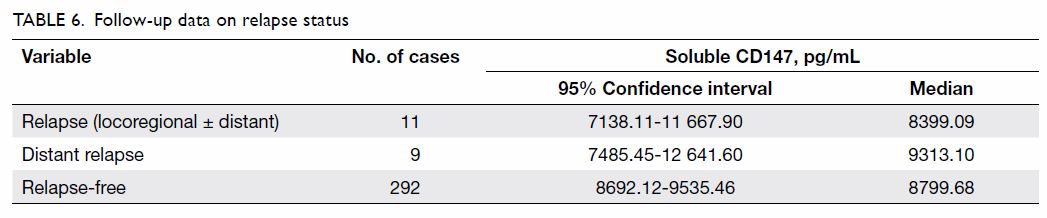
demonstrated that plasma sCD147 was not an independent predictor for
chemotherapy response of breast cancer patients, but tumour size of T3-T4
was (P=0.001) [Table 5].
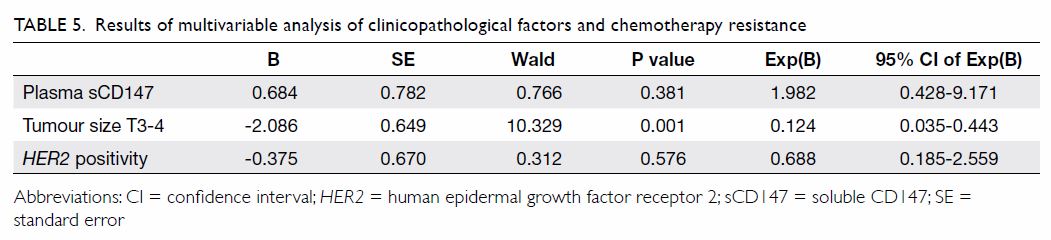
Table 5. Results of multivariable analysis of clinicopathological factors and chemotherapy resistance
Discussion
The tumour microenvironment plays a proactive role
in malignant disease progression, including the transition from ductal
carcinoma in situ to invasive cancer, tumour cell proliferation,
dissemination, and metastasis.27
CD147 has been found to be overexpressed in breast cancer, associated with
tumour size and staging, and predictive of poor prognosis.28 29 30 31 Tumour
cells express molecules, either secreted or presented on the cell surface,
that interact with surrounding stromal cells. Soluble CD147 may be
released from membrane-associated CD147 as a result of both MMP
proteolytic activity and microvesicle shedding in the tumour
microenvironment. Soluble CD147 may then act in a paracrine fashion on
stromal cells to further trigger production of MMPs and CD147; the latter
contributes to tumour angiogenesis, tumour growth, and metastasis.16 19 21
Wu et al15
reported that serum sCD147 enhances the secretion of MMP-2 from
hepatocellular carcinoma cells by activating extracellular
signal-regulated kinase and focal adhesion kinase, as well as
phosphoinositide-3-kinase/Akt signalling, indicating that sCD147 may
contribute to hepatocellular carcinoma progression. Moreover, serum sCD147
was elevated in patients with hepatocellular carcinoma compared with
healthy individuals, and sCD147 level was associated with tumour size and
Child-Pugh score.15 Gross et al22 also reported that sCD147 levels
were elevated in patients with multiple myeloma, and elevated levels were
associated with refractory disease and shortened progression-free
survival, indicating that sCD147 may be a new prognostic factor for
patients with multiple myeloma.
A previous study demonstrated that CD147 was
overexpressed in human breast cancer.10
In this study, we measured plasma sCD147 levels by ELISA and found that
plasma sCD147 levels were significantly elevated in breast cancer patients
compared with control patients who had benign breast diseases. We also
found that plasma sCD147 was significantly elevated in lymph node
metastasis in breast cancer patients. Taken together, these data show that
plasma sCD147 may be released from tumour cells and promote lymph node
metastasis of breast cancer. Some studies have reported that sCD147 has
been detected in patients with inflammatory diseases31 or cardiovascular diseases.32
33 To eliminate interference from
other diseases and conditions, we excluded patients with inflammatory or
cardiovascular diseases and ensured patients in each group had a similar
age distribution.
Previous studies have shown that membrane-bound
CD147 may correlate with HER2 expression. Yan et al34 reported that CD147 induces angiogenesis by
stimulating vascular endothelial growth factor production, invasiveness by
stimulating MMP production, and multidrug resistance by
hyaluronan-mediated upregulation of HER2 signalling. Xue et al30 reported that CD147 expression was positively
correlated with HER2 overexpression. In a recent study, CD147
knockdown was shown to improve the antitumour efficacy of trastuzumab in HER2-positive
breast cancer cells.35 Although we
found that plasma sCD147 levels were significantly higher in the HER2-positive
breast cancer subtype than in luminal A, luminal B, and TNBC subtypes,
plasma sCD147 had no association with expression of HER2 or
oestrogen/progesterone receptors in breast cancer. The reason for this
finding is that there are four breast cancer subtypes—luminal A, luminal
B, HER2-positive, and TNBC—according to oestrogen/progesterone
receptor, HER2, and Ki67 status. The luminal B subtype includes
some breast cancers that are positive for oestrogen/progesterone receptor
and HER2. Hence, patients who are HER2-positive (Table
1) include those with HER2-positive subtype and also luminal
B subtype; plasma sCD147 levels in patients who were ‘HER2-positive’
were different from those with a HER2-positive subtype.
It is essential to establish predictive factors to
allow evaluation of the recurrence risk of breast cancer, so that optimal
adjuvant therapy can be selected for individual patients.3 36 Larger
tumour size at diagnosis, high proliferation factors, absence of
oestrogen/progesterone receptors and HER2 overexpression, and
lymph node metastasis are related to a high risk of recurrence and poor
survival, and are commonly recognised as prognostic and predictive factors
for breast cancer recurrence risk.4
37 38
Consistent with these findings, we found that plasma sCD147 levels were
significantly increased in patients with locally advanced lymph node
metastasis and a high risk of breast cancer recurrence. We also found that
plasma sCD147 was positively associated with tumour size, lymph node
metastasis, and high recurrence risk of invasive breast cancer.
Lymph node status, which confers different
strategies for patients at different tumour stages, is critical
information for the treatment of breast cancer, and the accurate
prediction of lymph node status is a prerequisite for treatment decision.
Our binary logistic regression analysis showed that plasma sCD147, HER2
positive subtype, and tumour size (T3-T4) were independent predictors for
lymph node metastasis of breast cancer patients. Taken together, these
data suggest that plasma sCD147 may be a new factor for the evaluation of
breast cancer recurrence risk. Our ROC analysis demonstrated that plasma
sCD147 could be a biomarker for distinguishing breast cancer patients with
lymph node metastasis from those without; however, the sensitivity and
specificity were not high (70.9% and 61.7%, respectively). The relatively
low sensitivity and specificity suggest that using plasma sCD147 as the
sole biomarker may result in substantial numbers of false positives and
false negatives. Therefore, it may be necessary to investigate whether the
combination of plasma sCD147 and other biomarkers can improve efficacy.
According to the data of 303 patients who were
followed up for 3 to 38 months (median, 20 months), 11 patients had
relapse: two had local recurrences and nine had distant metastases. The
mean time of recurrence/metastasis was 23.6 months, with no difference
between patients with relapse and those without (Table 6). We were not able to investigate the
relationship between plasma sCD147 and disease-free survival or overall
survival, because of the short median follow-up period.
Previous data have shown that CD147 is one of the
apoptosis-related proteins and it may mediate adriamycin chemoresistance
in breast cancer by affecting the cellular localisation and dimerisation
of the protein ABCG2 (ATP-binding cassette subfamily G member 2).10 In this study, we studied the relationship between
plasma sCD147 and chemotherapy response in invasive breast cancer. All
patients were given four cycles of AC/EC-based chemotherapy. We also found
that plasma sCD147 levels were significantly higher in the
chemotherapy-resistant group than in the chemotherapy-sensitive group, and
such levels were positively associated with chemotherapy resistance.
Although our data also showed that plasma sCD147, tumour size (T3-T4), and
HER2 positive subtype may be involved in chemotherapy response,
binary logistic regression demonstrated that tumour size (T3-T4) was an
independent predictor for chemotherapy response of breast cancer patients,
but plasma sCD147 was not. Owing to the small number of cases in the
chemotherapy-resistant group, the statistical analysis of data may be
underpowered.
In addition to the small sample of study and short
median follow-up period, there were other limitations in this study. This
study was conducted in one centre, and the researchers who extracted the
data and conducted the analysis were not blinded to the study hypothesis.
There may have increased selection and information bias. Furthermore, as
the design of this study was relatively simple, there may be insufficient
control for potential confounding factors in the multivariable analysis.
In conclusion, our study found that plasma sCD147
levels were elevated in breast cancer patients compared with controls with
benign breast disease, and plasma sCD147 level was associated with tumour
size, lymph node metastasis, high recurrence risk, and AC/EC-based
chemoresistance. Moreover, our study supports that plasma sCD147 is an
independent predictive factor for lymph node metastasis and is a feasible
marker to distinguish breast cancer patients with lymph node metastasis
from patients without.
Author contributions
Concept or design: LL Tang, LQ Liao.
Acquisition of data: YJ Liu, YH Kuang, SM Wang, GJ Yan.
Analysis or interpretation of data: LL Tang, LQ Liao.
Drafting of the article: YH Kuang, LQ Liao.
Critical revision for important intellectual content: YH Kuang, LQ Liao.
YH Kuang, YJ Liu, and LL Tang contributed equally to this study.
Acquisition of data: YJ Liu, YH Kuang, SM Wang, GJ Yan.
Analysis or interpretation of data: LL Tang, LQ Liao.
Drafting of the article: YH Kuang, LQ Liao.
Critical revision for important intellectual content: YH Kuang, LQ Liao.
YH Kuang, YJ Liu, and LL Tang contributed equally to this study.
Funding/support
This study was supported by two grants from the
National Natural Science Foundation of China (No. 81101654, awarded to LQ
Liao, and No. 81573049, awarded to YH Kuang).
Declaration
The authors have no conflicts of interest to
disclose. All authors had full access to the data, contributed to the
study, approved the final version for publication, and take responsibility
for its accuracy and integrity.
Ethical approval
The research protocols for the use of human tissue
were approved by and conducted in accordance with the policies of the
Institutional Review Boards at Central South University (Ref No.
201403152), which were formulated based on the 1964 Helsinki Declaration
and its later amendments. Written informed consent was obtained from all
participants.
References
1. Torre LA, Bray F, Siegel RL, et al.
Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. Crossref
2. Rivenbark AG, O’Connor SM, Coleman WB.
Molecular and cellular heterogeneity in breast cancer: challenges for
personalized medicine. Am J Pathol 2013;183:1113-24. Crossref
3. Goldhirsch A, Wood WC, Coates AS, et al.
Strategies for subtypes—dealing with the diversity of breast cancer:
highlights of the St. Gallen International Expert Consensus on the Primary
Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736-47. Crossref
4. Soerjomataram I, Louwman MW, Ribot JG,
et al. An overview of prognostic factors for long-term survivors of breast
cancer. Breast Cancer Res Treat 2008;107:309-30. Crossref
5. Adaniel C, Jhaveri K, Heguy A, et al.
Genome-based risk prediction for early stage breast cancer. Oncologist
2014;19:1019-27. Crossref
6. Weigelt B, Peterse JL, van ’t Veer LJ.
Breast cancer metastasis: markers and models. Nat Rev Cancer
2005;5:591-602. Crossref
7. Redig AJ, McAllister SS. Breast cancer
as a systemic disease: a view of metastasis. J Intern Med 2013;274:113-26.
Crossref
8. O’Shaughnessy J. Extending survival with
chemotherapy in metastatic breast cancer. Oncologist 2005;10 Suppl 3:20-9.
Crossref
9. Kuang YH, Chen X, Su J, et al. RNA
interference targeting the CD147 induces apoptosis of multi-drug resistant
cancer cells related to XIAP depletion. Cancer Lett 2009;276:189-95. Crossref
10. Zhou S, Liao L, Chen C, et al. CD147
mediates chemoresistance in breast cancer via ABCG2 by affecting its
cellular localization and dimerization. Cancer Lett 2013;337:285-92. Crossref
11. Yang JM, Xu Z, Wu H, et al.
Overexpression of extracellular matrix metalloproteinase inducer in
multidrug resistant cancer cells. Mol Cancer Res 2003;1:420-7.
12. Marieb EA, Zoltan-Jones A, Li R, et
al. Emmprin promotes anchorage-independent growth in human mammary
carcinoma cells by stimulating hyaluronan production. Cancer Res
2004;64:1229-32. Crossref
13. Nabeshima K, Iwasaki H, Koga K, et al.
Emmprin (basigin/CD147): matrix metalloproteinase modulator and
multifunctional cell recognition molecule that plays a critical role in
cancer progression. Pathol Int 2006;56:359-67. Crossref
14. Zhao S, Ma W, Zhang M, et al. High
expression of CD147 and MMP-9 is correlated with poor prognosis of
triple-negative breast cancer (TNBC) patients. Med Oncol 2013;30:335. Crossref
15. Wu J, Hao ZW, Zhao YX, et al.
Full-length soluble CD147 promotes MMP-2 expression and is a potential
serological marker in detection of hepatocellular carcinoma. J Transl Med
2014;12:190. Crossref
16. Tang Y, Kesavan P, Nakada MT, et al.
Tumor-stroma interaction: positive feedback regulation of extracellular
matrix metalloproteinase inducer (EMMPRIN) expression and matrix
metalloproteinase-dependent generation of soluble EMMPRIN. Mol Cancer Res
2004;2:73-80.
17. Bordador LC, Li X, Toole B, et al.
Expression of emmprin by oral squamous cell carcinoma. Int J Cancer
2000;85:347-52.
18. Taylor PM, Woodfield RJ, Hodgkin MN,
et al. Breast cancer cell-derived EMMPRIN stimulates fibroblast MMP2
release through a phospholipase A(2) and 5-lipoxygenase catalyzed pathway.
Oncogene 2002;21:5765-72. Crossref
19. Sidhu SS, Mengistab AT, Tauscher AN,
et al. The microvesicle as a vehicle for EMMPRIN in tumor-stromal
interactions. Oncogene 2004;23:956-63. Crossref
20. Egawa N, Koshikawa N, Tomari T, et al.
Membrane type 1 matrix metalloproteinase (MT1-MMP/MMP-14) cleaves and
releases a 22-kDa extracellular matrix metalloproteinase inducer (EMMPRIN)
fragment from tumor cells. J Biol Chem 2006;281:37576-85. Crossref
21. Hanata K, Yamaguchi N, Yoshikawa K, et
al. Soluble EMMPRIN (extra-cellular matrix metalloproteinase inducer)
stimulates the migration of HEp-2 human laryngeal carcinoma cells,
accompanied by increased MMP-2 production in fibroblasts. Arch Histol
Cytol 2007;70:267-77. Crossref
22. Gross Z, Udd K, Ghermezi M, et al.
Serum CD147 levels are increased in multiple myeloma patients and elevated
levels are associated with refractory disease and shortened progression
free survival. Am Soc Hematology 2016;128:5652.
23. McShane LM, Altman DG, Sauerbrei W, et
al. Reporting recommendations for tumor marker prognostic studies
(REMARK). J Natl Cancer Inst 2005;97:1180-4. Crossref
24. Goldhirsch A, Winer EP, Coates AS, et
al. Personalizing the treatment of women with early breast cancer:
highlights of the St Gallen International Expert Consensus on the Primary
Therapy of Early Breast Cancer 2013. Ann Oncol 2013;24:2206-23. Crossref
25. Goldhirsch A, Wood WC, Gelber RD, et
al. Progress and promise: highlights of the international expert consensus
on the primary therapy of early breast cancer 2007. Ann Oncol
2007;18:1133-44. Crossref
26. Eisenhauer EA, Therasse P, Bogaerts J,
et al. New response evaluation criteria in solid tumours: revised RECIST
guideline (version 1.1). Eur J Cancer 2009;45:228-47. Crossref
27. Liotta LA, Kohn EC. The
microenvironment of the tumour-host interface. Nature 2001;411:375-9. Crossref
28. Dalberg K, Eriksson E, Enberg U, et
al. Gelatinase A, membrane type 1 matrix metalloproteinase, and
extracellular matrix metalloproteinase inducer mRNA expression:
correlation with invasive growth of breast cancer. World J Surg
2000;24:334-40. Crossref
29. Reimers N, Zafrakas K, Assmann V, et
al. Expression of extracellular matrix metalloproteases inducer on
micrometastatic and primary mammary carcinoma cells. Clin Cancer Res
2004;10:3422-8. Crossref
30. Xue S, Li SX, Wu ZS, et al. Expression
of CD147, matrix metalloproteinases and transforming growth factor beta1
in breast cancer [in Chinese]. Zhonghua Bing Li Xue Za Zhi 2009;38:524-8.
31. Yanaba K, Asano Y, Tada Y, et al.
Increased serum soluble CD147 levels in patients with systemic sclerosis:
association with scleroderma renal crisis. Clin Rheumatol 2012;31:835-9. Crossref
32. Major TC, Liang L, Lu X, et al.
Extracellular matrix metalloproteinase inducer (EMMPRIN) is induced upon
monocyte differentiation and is expressed in human atheroma. Arterioscler
Thromb Vasc Biol 2002;22:1200-7. Crossref
33. Schmidt R, Bultmann A, Fischel S, et
al. Extracellular matrix metalloproteinase inducer (CD147) is a novel
receptor on platelets, activates platelets, and augments nuclear factor
kappaB-dependent inflammation in monocytes. Circ Res 2008;102:302-9. Crossref
34. Yan L, Zucker S, Toole BP. Roles of
the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour
progression. Thromb Haemost 2005;93:199-204.
35. Xiong L, Ding L, Ning H, et al. CD147
knockdown improves the antitumor efficacy of trastuzumab in HER2-positive breast cancer cells. Oncotarget 2016;7:57737-51. Crossref
36. Cianfrocca M, Goldstein LJ. Prognostic
and predictive factors in early-stage breast cancer. Oncologist
2004;9:606-16. Crossref
37. Harris L, Fritsche H, Mennel R, et al.
American Society of Clinical Oncology 2007 update of recommendations for
the use of tumor markers in breast cancer. J Clin Oncol 2007;25:5287-312.
Crossref
38. Taneja P, Maglic D, Kai F, et al.
Classical and novel prognostic markers for breast cancer and their
clinical significance. Clin Med Insights Oncol 2010;4:15-34. Crossref