Hong Kong Med J 2018 Apr;24(2):98–106 | Epub 9 Feb 2018
DOI: 10.12809/hkmj176871
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Outcomes of a pharmacist-led medication review
programme for hospitalised elderly patients
Patrick KC Chiu, FRCP (Glasg), FHKAM (Medicine)1;
Angela WK Lee, MPharm, RPharmS (Great Britain)2; Tammy YW See,
MClinPharm, RPharmS (Great Britain)2; Felix HW Chan, FRCP
(Edin, Glasg, Irel), FHKAM (Medicine)1
1 Geriatric Medical Unit, Grantham
Hospital, Wong Chuk Hang, Hong Kong
2 Pharmacy, Grantham Hospital, Wong Chuk
Hang, Hong Kong
Corresponding author: Dr Patrick KC Chiu (chiukc@ha.org.hk)
Abstract
Introduction: Elderly patients
are at risk of drug-related problems. This study aimed to determine
whether a pharmacist-led medication review programme could reduce
inappropriate medications and hospital readmissions among geriatric
in-patients in Hong Kong.
Methods: This prospective
controlled study was conducted in a geriatric unit of a regional
hospital in Hong Kong. The study period was from December 2013 to
September 2014. Two hundred and twelve patients were allocated to
receive either routine care (104) or pharmacist intervention (108) that
included medication reconciliation, medication review, and medication
counselling. Medication appropriateness was assessed by a pharmacist
using the Medication Appropriateness Index. Recommendations made by the
pharmacist were communicated to physicians.
Results: At hospital admission,
51.9% of intervention and 58.7% of control patients had at least one
inappropriate medication (P=0.319). Unintended discrepancy applied in
19.4% of intervention patients of which 90.7% were due to omissions.
Following pharmacist recommendations, 60 of 93 medication reviews and 32
of 41 medication reconciliations (68.7%) were accepted by physicians and
implemented. After the program and at discharge, the proportion of
subjects with inappropriate medications in the intervention group was
significantly lower than that in the control group (28.0% vs 56.4%;
P<0.001). The unplanned hospital readmission rate 1 month after
discharge was significantly lower in the intervention group than that in
the control group (13.2% vs 29.1%; P=0.005). Overall, 98.0% of
intervention subjects were satisfied with the programme. There were no
differences in the length of hospital stay, number of emergency
department visits, or mortality rate between the intervention and
control groups.
Conclusions: A pharmacist-led
medication review programme that was supported by geriatricians
significantly reduced the number of inappropriate medications and
unplanned hospital readmissions among geriatric in-patients.
New knowledge added by this study
- This is the first prospective controlled study of the effect of a pharmacist-led medication review programme on medication use and health services utilisation among hospitalised Chinese elderly patients in Hong Kong.
- The medication review programme led by a clinical pharmacist resulted in a substantial reduction in the use of inappropriate medications among hospitalised elderly patients and all-cause unscheduled readmissions at 1 month after hospital discharge.
- A pharmacist-led medication review programme is an important strategy that can enhance the safety and quality of prescription among elderly patients in hospital.
- It is strongly recommended that these programmes be standardised and implemented in all medical and geriatric wards in Hong Kong.
Introduction
Elderly patients have multiple co-morbidities and
they are consequently prone to multiple medication use. Inappropriate
medication use is common among hospitalised older adults. The number of
drugs taken is one of the important determinants of risk for receiving an
inappropriate medication.1 There is
a high prevalence of unnecessary drug use in frail older people. In one
hospital study, 44% of patients were prescribed at least one unnecessary
drug, with the most common reason being lack of indication.2 The most commonly prescribed unnecessary drug classes
were gastrointestinal, central nervous system, and therapeutic
nutrients/minerals.2 Appropriate
use of drugs is particularly important in the frail older people who are
especially at risk of adverse drug reactions.3
It has been shown that implementation of a clinical pharmacist service has
a positive effect on medication use and health care service utilisation
among hospitalised patients.4 5 A local study in a geriatric hospital demonstrated the
effectiveness of a drug rationalisation programme with involvement of a
clinical pharmacist in reducing the incidence of polypharmacy and
inappropriate medications.6
Interacting with the health care team on patient rounds, interviewing
patients, reconciling medications, and providing patient discharge
counselling and follow-up all resulted in improved outcomes.7 It is for this reason that patient safety strategies
encourage the use of medication reconciliation and clinical pharmacists in
health care systems to reduce adverse drug events.8 9 10
There is not much information about the
effectiveness of a medical review programme among hospitalised elderly
patients in Hong Kong. Two recent local reports that examined the effects
of a clinical pharmacist–led medication review on hospital readmissions
showed conflicting results and did not specifically address elderly
patients.11 12 We therefore conducted a prospective controlled study
to investigate the effectiveness of a comprehensive pharmacist
intervention on medication use and hospital readmission among a group of
geriatric in-patients in Hong Kong.
Methods
This prospective controlled study was conducted in
the geriatric unit of a regional hospital in Hong Kong. The unit has 38
in-patient beds and admits older people aged 65 years or above who are
transferred from an acute hospital after initial stabilisation of medical
and/or geriatric problems. The unit admits more than 1000 patients per
year and provides medical treatment, rehabilitation, and discharge
planning services by a multidisciplinary team composed of a geriatrician,
residents, nurses, physiotherapists, occupational therapists, and medical
social workers. All patients admitted to the unit during December 2013 to
September 2014 were included. Patients were excluded if they refused to
participate, were terminally ill with a life expectancy of less than 3
months, or if they had already received pharmacist intervention in another
hospital prior to this admission. Eligible subjects were assigned to an
intervention or control group according to the admission day of the week.
Those who were admitted on Monday through Thursday were assigned to the
intervention group, and those admitted on Friday through Sunday to the
control group. This arrangement was to ensure that pharmacist intervention
could be initiated promptly within 48 hours of patient admission.
Demographic data, functional status, co-morbidities, and number of drugs
on admission were collected at admission.
The intervention was conducted by a pharmacist who
was present in the unit from Monday to Saturday. The pharmacist provided
pharmaceutical care from admission to discharge. Interventions performed
by the pharmacist consisted of the following:
(1) Medication reconciliation on admission to
identify unintended discrepancies between medications prescribed on
admission and the usual medications prior to admission—sources to assist
medication reconciliation included: electronic patient record; patient’s
ward case notes; interview with patient and/or patient carer. The number
and type of unidentified discrepancies were recorded.
(2) Medication review to check for medication
appropriateness on admission and also at discharge—medication
appropriateness was assessed by the Medication Appropriateness Index
(MAI).13 There are 10 criteria to
assess for appropriateness, namely indication, effectiveness, dosage,
correct direction, practical direction, drug-drug interaction,
drug-disease interaction, duplication, duration, and expense. For a drug
item coded as ‘inappropriate’, relative weights for each criterion would
apply. A sum of MAI scores could then be calculated to give a score
ranging from 0 to 18. The higher the score, the more inappropriate the
drug. Recommendations from the pharmacist after the reconciliation and
medication review in the intervention group were then communicated to the
in-charge doctor via a written note in the medical records.
Recommendations were reinforced verbally if deemed appropriate by the
pharmacist.
(3) Pharmacist counselling on admission and also at
discharge was provided to improve patients’ drug knowledge to ensure
proper use of drugs and compliance after discharge. A discharge
counselling service was provided for all patients who returned home. The
counselling included any changes to drug regimen; an explanation of each
drug’s indication; any untoward effects that might occur and when to seek
medical advice; and drug storage and administration instructions. To
ensure patient understanding, written information such as patient
information leaflets were given to patients and their carers to remind
them of the correct drug regimen. If the patient was illiterate, a simple
diagram was drawn on drug labels to demonstrate the time of day and number
of tablets to be taken. If necessary, individualised pictorial schedules
with drug images and administration instructions could be produced for
patients and their carers. The assistance of a family member or external
care services such as a community nurse was enlisted if the patient was
found to have compliance issues.
The control group received routine clinical
services. Records of the control group were retrospectively reviewed by
the pharmacist after patient discharge to check for medication
appropriateness on admission and also at discharge. The primary outcome
measure was the appropriateness of prescription as measured by the MAI.
Secondary outcomes included the acceptance rate by physicians, number of
subjects with unintended discrepancies, patient satisfaction with the
programme (for those home-living only), and unplanned hospitalisations 1
and 3 months after discharge.
A sample size of 98 patients per group was required
to have 85% power to detect an effect size of 0.9 on the MAI. Our sample
size was finally set at 210 patients to account for loss of participants
due to dropout or death. This sample size was comparable with a study by
Spinewine et al14 in which MAI was
used as one of the tools to assess appropriateness of prescribing in an
acute geriatric care unit and 203 patients were recruited. Our study
included 212 patients and was expected to have adequate statistical power
to detect differences between groups. Descriptive analyses were performed
and included the number and types of unintended discrepancies, MAI score
upon admission and at discharge, types of drug-related problems, number of
interventions made by pharmacists, and number of recommendations accepted
by doctors and implemented. Outcomes for the two groups before and after
the programme were compared using the t test and Chi squared test.
The Statistical Package for the Social Sciences (Windows version 17.0;
SPSS Inc, Chicago [IL], United States) was used and a P value of <0.05
was regarded as statistically significant. The study was approved by the
Cluster Research Ethics Committee of the Hospital Authority Hong Kong West
Cluster. Written consent was obtained from the patient or their caregiver.
The absence of pharmacist intervention in the control group was considered
acceptable because a pharmacy service was not a part of routine care at
the institution.
Results
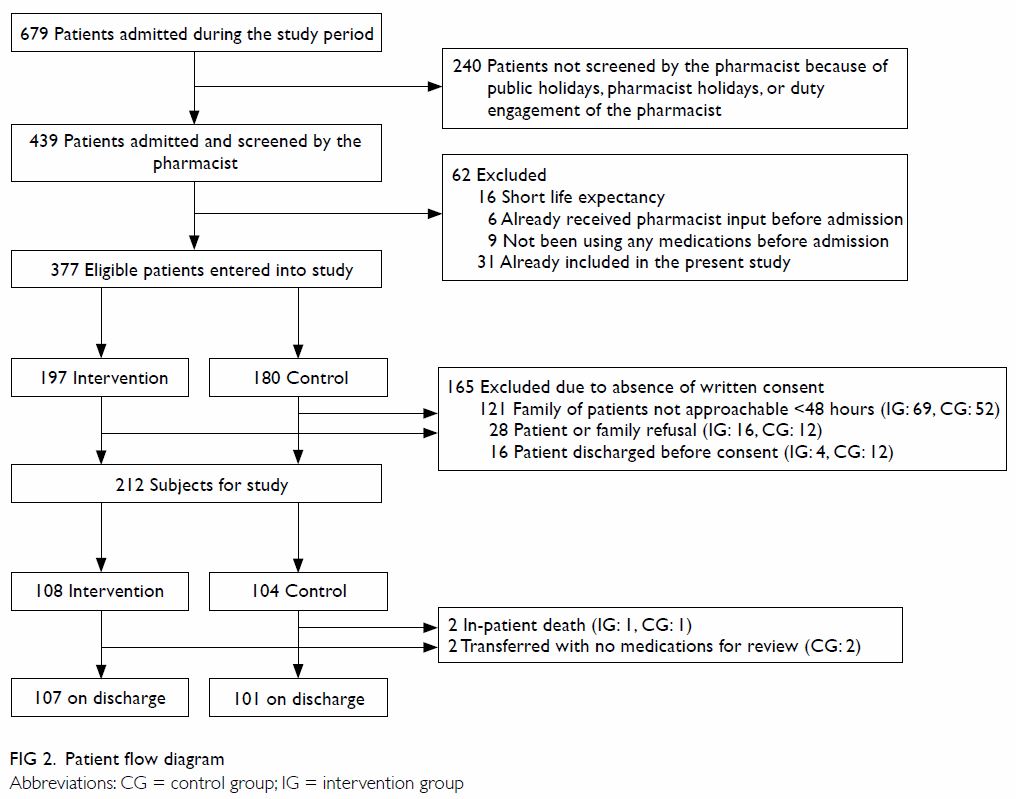
Figure 1 summarises the patient flow from
recruitment to hospital discharge, the components of the medication review
programme, and the planned outcome measures. A total of 212 patients were
recruited. There were 108 subjects in the intervention group and 104 in
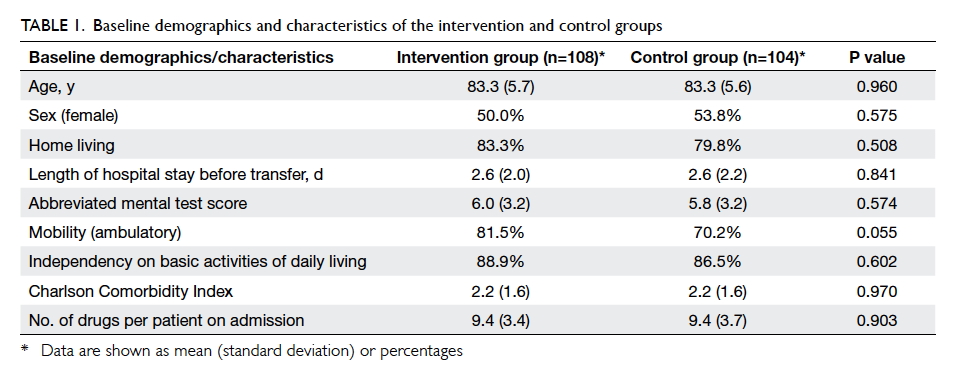
the control group (Fig 2). There were no statistical differences in the
baseline characteristics of patients (Table 1).
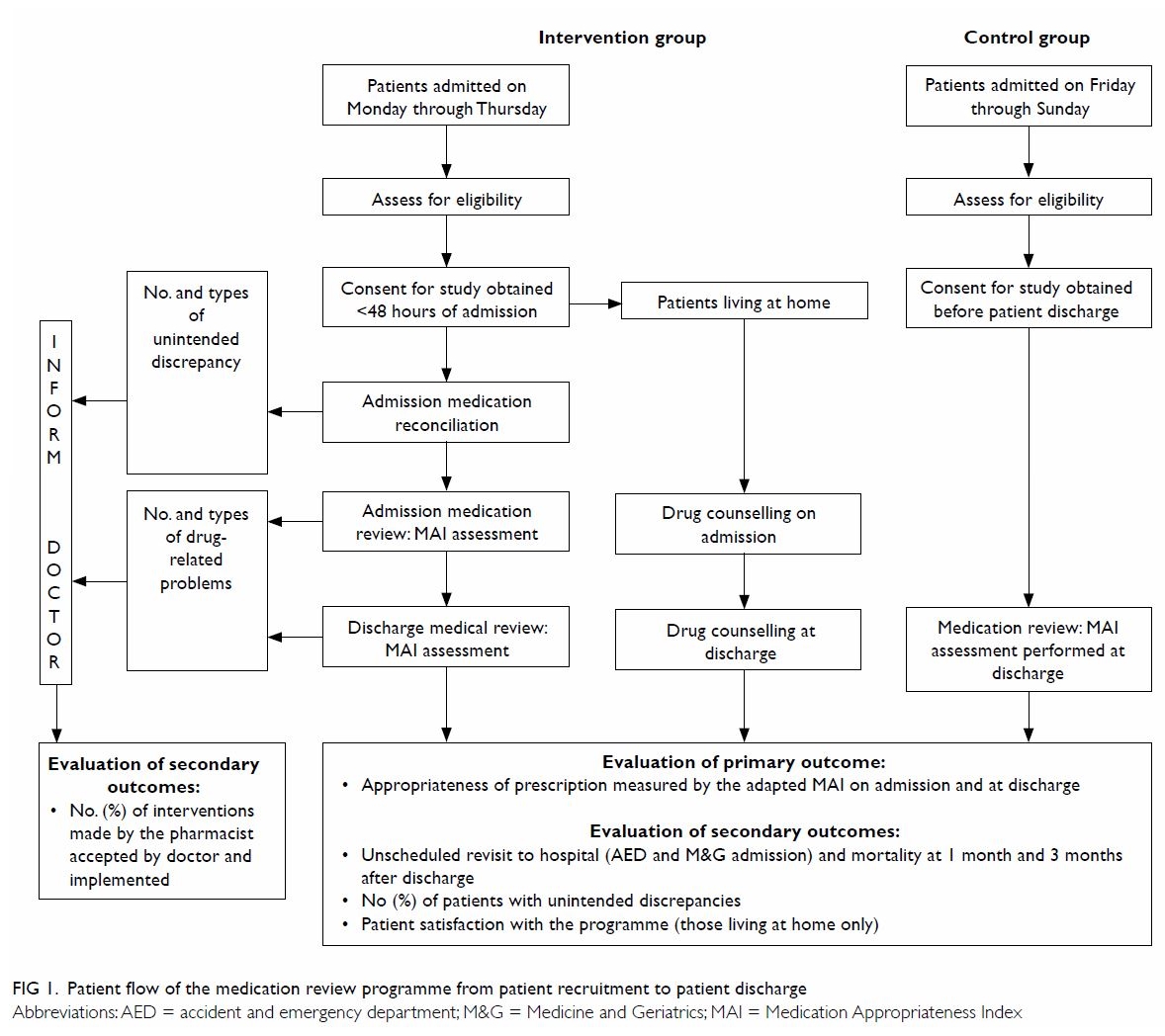
Figure 1. Patient flow of the medication review programme from patient recruitment to patient discharge
Appropriateness of prescription
On admission, 51.9% (56/108) of the intervention
group and 58.7% (61/104) of the control group had at least one drug
classified as inappropriate (P=0.319). Overall, 1996 drug items were
reviewed by a pharmacist on admission of which 1020 were from the
intervention group and 976 from the control group. Among them, 9.3% and
11.1% of the drugs, respectively, were classified as inappropriate
(P=0.282). In the intervention group, 93 recommendations were made by the
pharmacist of which 60 (64.5%) were accepted by the physicians and
implemented. The mean (standard deviation) MAI score per patient was
2.19 (3.03) in the intervention group and 2.28 (3.09) in the control group
(P=0.841). The mean MAI score per drug was 0.23 (0.30) in the intervention
group and 0.25 (0.31) in the control group (P=0.628) [Table
2].
After the program and at discharge, the proportion
of subjects with inappropriate medications in the intervention group was
significantly lower than that in the control group (28.0% vs 56.4%;
P<0.001). Among the 1999 drug items reviewed by the pharmacist on
patient discharge, 3.5% (37 of 1048) of the intervention group and 9.7%
(92 of 951) of the control group were classified as inappropriate
(P<0.001). The intervention group also had a significantly lower MAI
score per patient (0.95 (2.02) vs 2.02 (2.53); P<0.001) and MAI score
per drug (0.09 (0.17) vs 0.24 (0.30); P<0.001) implying a significant
reduction in medication inappropriateness after the pharmacist medication
review (Table 2).
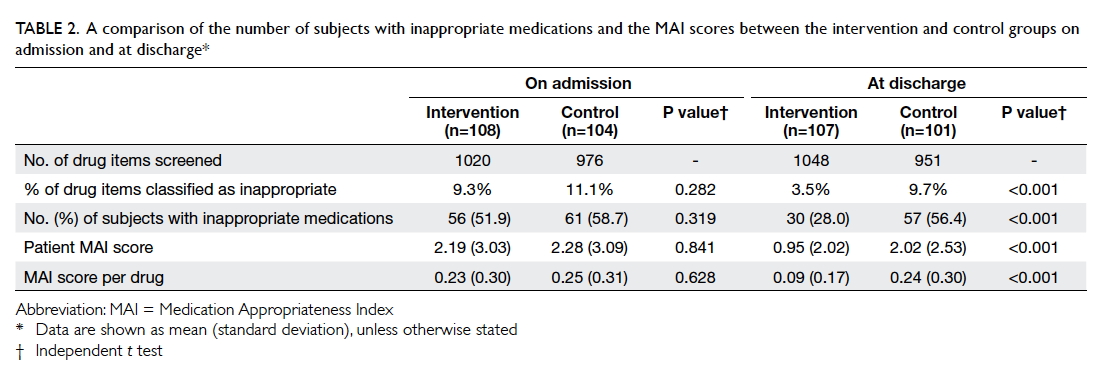
Table 2. A comparison of the number of subjects with inappropriate medications and the MAI scores between the intervention and control groups on admission and at discharge
Types of inappropriateness according to the MAI in
the two groups are illustrated in Figure 3. In both the intervention and control
groups, the common causes were indication, effectiveness, dosage,
practical direction, duration, and expense. After the programme, there was
a significant reduction in the number of these drug-related problems in
the intervention group.
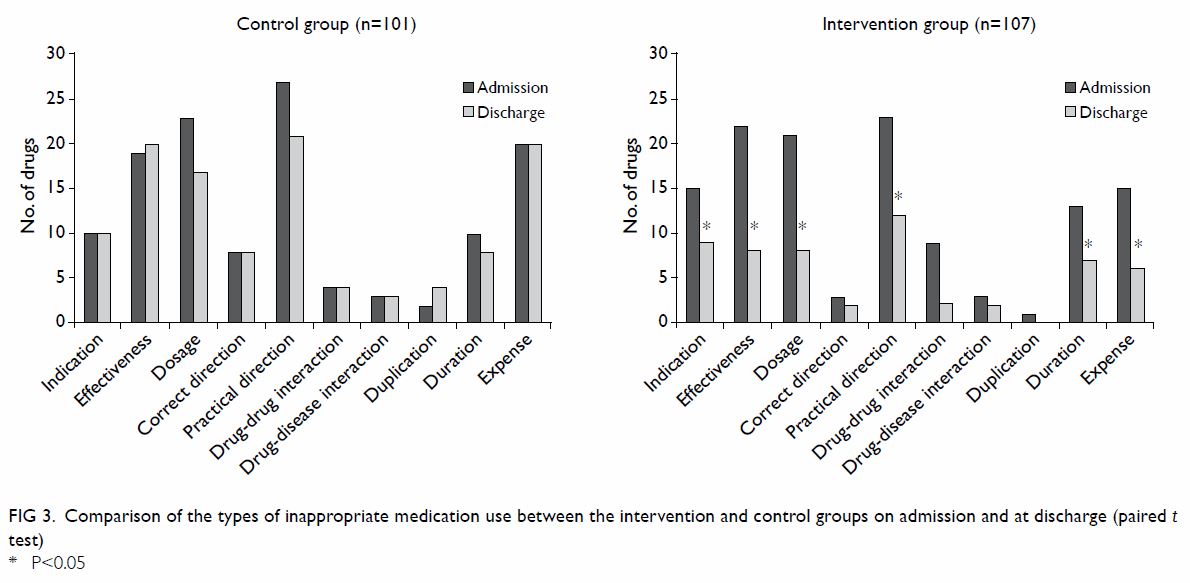
Figure 3. Comparison of the types of inappropriate medication use between the intervention and control groups on admission and at discharge (paired t test)
Unintended discrepancy of medications
Among the 108 subjects in the intervention group,
19.4% had at least one unintended discrepancy in medications, involving a
total of 43 drug factors. The majority (90.7%) of these factors were
omission of drugs, and 4.6% were due to inappropriate dosages. Of all the
drug factors involved, 69.8% involved prescribed drugs from hospitals,
25.6% were from a private clinic, and 4.6% were over-the-counter drugs.
Overall, 41 recommendations were made, of which 32 (78.0%) were accepted
by physicians and implemented.
Patient satisfaction
Contact was made with 50 of the 90
non-institutionalised subjects 1 month after discharge to assess
satisfaction with the programme. Of those contacted, 98.0% were satisfied
with the programme and only one (2.0%) patient expressed a neutral
opinion.
Impact on health care services utilisation and
mortality
There were no statistical differences in the length
of hospital stay, in-patient mortality, or mortality at 1 month or 3
months after discharge. There was also no statistical difference in the
number of attendances at the accident and emergency department 1 month or
3 months after discharge or in the unplanned hospital readmission rate at
3 months after discharge. The unplanned hospital readmission rate 1 month
after discharge, however, was significantly lower in the intervention
group than that in the control group (13.2% vs 29.1%; P=0.005) [Table
3].
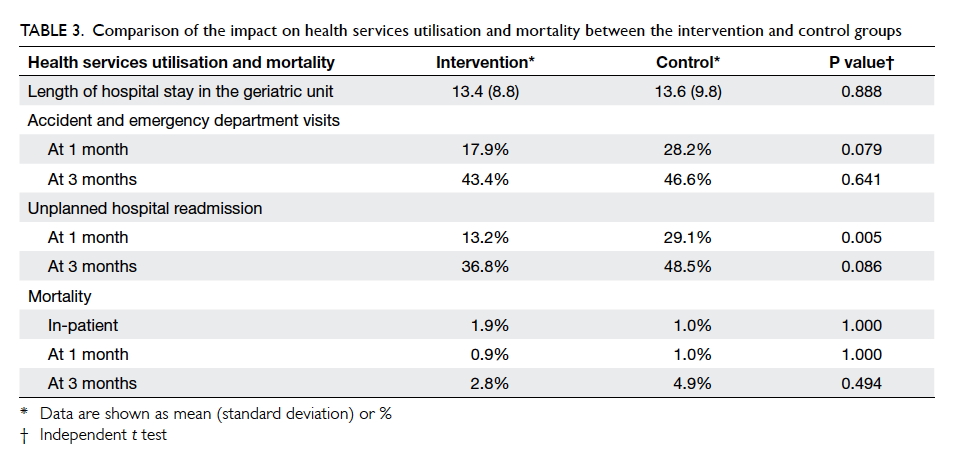
Table 3. Comparison of the impact on health services utilisation and mortality between the intervention and control groups
Discussion
To the best of our knowledge, this is the first
local prospective controlled study to investigate the effectiveness of a
pharmacist-led medication review programme on medication appropriateness
and clinical outcomes among geriatric in-patients in Hong Kong. This study
has demonstrated superior outcomes that favour a pharmacist-led
intervention. There was a substantial reduction in the use of
inappropriate medications and all-cause unscheduled readmissions 1 month
after hospital discharge. Nonetheless, analysis of length of hospital
stay, number of all-cause emergency department visits, and mortality rate
favoured neither the intervention nor the usual pharmacist care.
This study showed that one in five geriatric
in-patients had an unintended medication discrepancy on admission. This
figure was slightly higher than that found in a group of 3317 hospitalised
medical patients (13%) over 1 year in an acute hospital in Hong Kong by
Kwok et al.15 Subjects in our
study were all elderly patients, whereas those in Kwok et al’s study were
adults of all ages. Elderly subjects tend to have polypharmacy and thus
are more vulnerable to unintended medication discrepancy when they move in
and out of hospital or are transferred to another health care unit for
further care. Unjustifiable medication discrepancies account for more than
half of the medication errors that occur during transition of care and up
to one third have the potential to cause harm.16
17 This does not bode well for our
elderly patients with multiple co-morbidities.
Up to 30% of the discrepancies in our study
involved medications that had been prescribed by private practitioners or
purchased over-the-counter. Unlike medications prescribed from the
Hospital Authority, these medications might be overlooked unless the
admitting doctor specifically asks for a detailed drug history from the
patient. Knowing the medication history and hence resuming these
medications are important if new health problems are to be prevented.
Pharmacist-led medication reconciliation is therefore a critical process
that can enhance patient medication safety by compiling a complete and
accurate medication list for patients in hospital.
This study revealed that more than half of the
subjects (55.2%) received inappropriate medications. The majority of
reasons for inappropriateness related to effectiveness, dosage, practical
directions, and expense as reflected by the MAI. The inappropriate dosage
and the questionable effectiveness might lead to not only failed
pharmacological effects, but also potentially an untoward adverse drug
reaction, especially in elderly individuals with pre-existing organ
dysfunction.18 When a medication
is not used according to the practical directions, it may lead to patient
non-compliance. Optimising outcomes while reducing costs are the keys for
medication management in today’s health care environment.19 Often there are several choices of drugs available to
treat a disease or health condition and some are more expensive than
others. The involvement of a ward-based pharmacist to review medication
can enhance the use of appropriate medications in hospitalised patients
and potentially reduce medication costs.
Following the medication review (60/93) and
medication reconciliation (32/41), there were 92 recommendations that were
accepted by the physician. The overall acceptance rate by physicians and
the implementation of pharmacist recommendations in our study was 68.7%
(92/134). This figure ranged from 39% to 100% in a previous systematic
review of 32 studies.4 Our study
did not specifically record recommendations accepted by physicians but not
implemented. Hence the acceptance rate in our study may be underestimated.
Nevertheless, the clinical pharmacist is encouraged to discuss the
medication-related problems in person with the physician as well as
contacting the patient in order to enhance the implementation rate.4
Pharmacy departments within the public hospital
system in Hong Kong have strived to implement the aforementioned patient
safety strategies in different specialties. Nonetheless, this is not a
standard practice simply because of insufficient pharmacist staffing
resources. In some hospitals, a pharmacist service is provided in wards,
but this does not apply in all cases and is not standardised. Experience
of a pharmacist-led medication reconciliation service from an acute
teaching hospital in Hong Kong showed promising results over a 1-year
trial run with high acceptance and recognition by other health care
professionals.16 It is hoped that
the clinical role of clinical pharmacists in patient medication management
in hospitals can be encouraged. Another local study has demonstrated a
positive impact on medication safety in patients with diabetes by
pharmacists’ intervention in collaboration with a multidisciplinary team.20 The feasibility of incorporating
a pharmacist as part of a multidisciplinary team of health care
professionals must be explored in geriatric wards in Hong Kong. With
increasing life expectancy, the expanding elderly population will equate
to an increase in morbidity and mortality owing to drug-related problems
where the need for trained health care professionals to perform medication
reviews will be in even greater demand. To enhance safe drug use with
limited resources, a systematic approach must be adopted to cover all
aspects that affect drug therapy.
In terms of the impact on health care services
utilisation, a recent systematic review and meta-analysis of the
effectiveness of a pharmacist-led medication reconciliation programme
revealed a substantial reduction in the rate of all-cause readmissions
(19%), all-cause emergency department visits (28%), and adverse drug
event–related hospital visits (67%).21
Our study revealed a significant reduction in unplanned hospital
admissions (all-cause admission) at 1 month but not at 3 months. This
implication might be due to an inadequate sample size to show the
difference at 3 months. Alternatively, it might also imply that pharmacist
intervention needs to be continued after patient discharge in order to
have a sustained effect. This is supported by a study by Schnipper et al22 in which pharmacist intervention
after patient discharge was associated with a lower rate of preventable
adverse drug events 30 days after hospital discharge.
During the pharmacist review, cost-effectiveness of
drug use was assessed through MAI. Alternative options such as
less-expensive formulations or drugs but of the same quality would be
recommended to the doctor in-charge. This was a means of encouraging
cost-effective use of drugs in a hospital. Furthermore, the reduction in
unscheduled hospital readmissions in the intervention group implies a
potential saving in hospital costs. Although a detailed analysis was not
performed in this study, a rough estimation is that nearly HK$2 million
may be saved annually as a result of lower drug costs and reduced hospital
admissions, even after considering the cost of employing a pharmacist. The
estimation was based on the following calculations. The estimated drug
saving as a result of a switch to a more cost-effective alternative was
HK$7500 among the 108 patients in the intervention group. This can be
projected to a saving of about HK$69 500 in a unit that admits 1000
patients annually. In the current study, there were 16 fewer readmissions
in the intervention group compared with the control group. Assuming a
daily cost of an acute hospital bed is HK$4680 and the mean length of
hospital stay is 3 days, this equates to a potential saving of about HK$2
080 000 per year in a unit that admits 1000 patients annually. If it is
assumed that a pharmacist spends 30 minutes for each patient at an hourly
salary of HK$433, the projected cost of an additional pharmacist to run
the intervention would be HK$216 500 per year. The net annual saving of
this programme to serve 1000 patients in this unit would thus still be
close to HK$2 million.
This study had several limitations. First, a
substantial proportion (35%) of all the admitted patients were not
screened by a pharmacist on admission. This was due to a temporary pause
in subject recruitment when patients were admitted on public holidays,
when the pharmacist was on holiday or when she had to relieve another
pharmacist in the hospital. Moreover, a substantial proportion (44%) of
eligible subjects were not included owing to no consent or refusal. These
factors might have resulted in selection or self-selection bias. Second,
subject recruitment was not randomised, but done according to the day of
admission. This might be a source of bias. Nevertheless, this would have
minimal influence on the outcomes, as the baseline characteristics of the
intervention and control groups were comparable. Third, the pharmacist who
carried out the review and data extraction was not blinded to the study
hypothesis and the group status of the subjects. This could potentially
lead to information bias, although this might be partially offset by the
fact that the majority of the information or data on the outcome measures
were taken with reference to a well-established and validated tool.
Fourth, this study was performed in a single unit, so generalisation to
other settings is not possible. Fifth, MAI is an implicit tool that is
subjective. A single pharmacist as the rater might limit the reliability
of the assessment results. Nevertheless, the more explicit tools of
STOPP/START criteria23 had also
been referred to in addition to the MAI during the review process. Sixth,
this study only addressed appropriateness of drug use, whereas underuse of
drugs was not investigated. Finally, this study could not conclude a
causal relationship between the reduction in inappropriate medications and
the reduction in unscheduled hospital readmissions because there were
several components in the intervention that included a medication review,
medication reconciliation, and discharge counselling. It is difficult to
be certain which of these components alone or in combination gave rise to
the positive outcome of this study.
On the other hand, there were several strengths in
this study. This was the first prospective controlled study of the effect
of a pharmacist-led medication review programme on medication use and
health services utilisation involving over 200 Chinese elderly patients in
Hong Kong. Second, a well-validated tool was used to assess medication
appropriateness. The use of the MAI tool focused on the patient and the
entire medication regimen. Third, there was a comprehensive review of
outcomes including quality of prescribing, health services utilisation,
mortality, length of hospital stay, and patient satisfaction.
Conclusions
This study supported the role of a hospital-based
clinical pharmacist to enhance appropriate medication use among elderly
Chinese in-patients. A systematic medication review programme in a
geriatric unit resulted in a reduced number of drug omissions and fewer
inappropriate medications. The service provided by the clinical pharmacist
and supported by geriatricians was welcomed by patients and their carers.
Together with the potential to reduce hospital readmissions and their
associated cost, it is hoped that an in-hospital pharmacist-led medication
review programme can be recognised as one of the important strategies to
enhance the safety and quality of prescription among elderly patients in
hospitals. It is strongly recommended that these programmes be
standardised and implemented in all medical and geriatric wards in Hong
Kong. Future studies should recruit a larger sample size in a randomised
controlled design in other geriatric hospital settings to reiterate our
findings. Furthermore, these studies might consider including adverse drug
event–related hospital visits as one of the outcome measures.
Declaration
All authors have disclosed no conflicts of
interest.
References
1. Onder G, Landi F, Cesari M, Gambassi G,
Carbonin P, Bernabei R; Investigators of the GIFA Study. Inappropriate
medication use among hospitalized older adults in Italy: results from the
Italian Group of Pharmacoepidemiology in the Elderly. Eur J Clin Pharmacol
2003;59:157-62. Crossref
2. Hajjar ER, Hanlon JT, Sloane RJ, et al.
Unnecessary drug use in frail older people at hospital discharge. J Am
Geriatr Soc 2005;53:1518-23. Crossref
3. Routledge PA, O’Mahony MS, Woodhouse KW.
Adverse drug reactions in elderly patients. Br J Clin Pharmacol
2004;57:121-6. Crossref
4. Graabaek T, Kjeldsen LJ. Medication
reviews by clinical pharmacists at hospitals lead to improved patient
outcomes: a systematic review. Basic Clin Pharmacol Toxicol
2013;112:359-73. Crossref
5. Gillespie U, Alassaad A, Henrohn D, et
al. A comprehensive pharmacist intervention to reduce morbidity in
patients 80 years or older: a randomized controlled trial. Arch Intern Med
2009;169:894-900. Crossref
6. Chan FH, Pei CK, Chiu KC, Tsang EK.
Strategies against polypharmacy and inappropriate medication—are they
effective? Australas J Ageing 2001;20:85-9. Crossref
7. Kaboli PJ, Hoth AB, McClimon BJ,
Schnipper JL. Clinical pharmacists and inpatient medical care: a
systematic review. Arch Intern Med 2006;166:955-64. Crossref
8. The ACHS EQuIP5 (5th edition of the ACHS
Evaluation and Quality Improvement Program) clinical standard 1.5,
criterion 1.5.1.: Medication are managed to ensure safe and effective
consumer/patient outcomes. Sydney: The Australian Council on Health Care
Standards; 2010.
9. Medication reconciliation. Patient
Safety Network. Agency for Healthcare Research and Quality. US Department
of Health & Human Services. Available from:
http://psnet.ahrq.gov/primer.aspx?primerID=1. Accessed 11 Jun 2017.
10. Gurwitz J, Monane M, Monane S, Avorn
J. Polypharmacy. In: Morris JN, Lipsitz LA, Murphy K, Bellville-Taylor P,
editors. Quality care in the nursing home. St. Louis, MO: Mosby-Year Book;
1997: 13-25.
11. Wong CY, Lee TO, Lam MP. Pharmacist
discharge intervention programme to reduce unplanned hospital use in
patients with polypharmacy. Hong Kong Pharm J 2017;24(Suppl 1):S18.
12. Chan HH. To reduce avoidable
readmission of patients who are categorized as high risk by 30-day
hospital readmission model at medical ward of United Christian Hospital
through medication reconciliation and discharge counseling. Hong Kong
Pharm J 2017;24(Suppl 1):S21.
13. Hanlon JT, Schmader KE, Samsa GP, et
al. A method for assessing drug therapy appropriateness. J Clin Epidemiol
1992;45:1045-51. Crossref
14. Spinewine A, Swine C, Dhillon S, et
al. Effect of a collaborative approach on the quality of prescribing for
geriatric inpatients: a randomized, controlled trial. J Am Geriatr Soc
2007;55:658-65. Crossref
15. Kwok CC, Mak WM, Chui CM.
Implementational experience of medical reconciliation in Queen Mary
Hospital. Hong Kong Pharm J 2009;16:50-2.
16. Rozich JD, Howard RJ, Justeson JM,
Macken PD, Lindsay ME, Resar RK. Standardization as a mechanism to improve
safety in health care. Jt Comm J Qual Saf 2004;30:5-14. Crossref
17. Cornish PL, Knowles SR, Marchesano R,
et al. Unintended medication discrepancies at the time of hospital
admission. Arch Intern Med 2005;165:424-9. Crossref
18. Cameron KA. Caregivers’ guide to
medications and aging. National Center on Caregiving, Family Caregiver
Alliance 2004. Available from:
https://www.caregiver.org/caregiver%CA%BCs-guide-medications-and-aging.
Accessed 11 Jun 2017.
19. Splawski J, Minger H. Value of the
pharmacist in the medication reconciliation process. P T 2016;41:176-8.
20. Chung AY, Anand S, Wong IC, et al.
Improving medication safety and diabetes management in Hong Kong: a
multidisciplinary approach. Hong Kong Med J 2017;23:158-67. Crossref
21. Mekonnen AB, McLachlan AJ, Brien JA.
Effectiveness of pharmacist-led medication reconciliation programmes on
clinical outcomes at hospital transitions: a systematic review and
meta-analysis. BMJ Open 2016;6:e010003. Crossref
22. Schnipper JL, Kirwin JL, Cotugno MC,
et al. Role of pharmacist counseling in preventing adverse drug events
after hospitalization. Arch Intern Med 2006;166:565-71. Crossref
23. Lam MP, Cheung BM. The use of
STOPP/START criteria as a screening tool for assessing the appropriateness
of medications in the elderly population. Expert Rev Clin Pharmacol
2012;5:187-97. Crossref