Hong
Kong Med J 2018 Feb;24(1):38–47 | Epub 22 Dec 2017
DOI: 10.12809/hkmj176238
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Optimising the utility of pleural fluid adenosine
deaminase for the diagnosis of adult tuberculous pleural effusion in Hong
Kong
KC Chang, MSc, FHKAM (Medicine)1; MC
Chan, MB, BS, MRCP (UK)2; WM Leung, MB, ChB, FHKAM (Medicine)1;
FY Kong, MB, BS, FHKAM (Medicine)3; Chloe M Mak, MD, FHKAM
(Pathology)4; Sammy PL Chen, MRes(Med), FHKAM (Pathology)4;
WC Yu, FRCP, FHKAM (Medicine)2
1 Tuberculosis and Chest Service,
Department of Health, Hong Kong
2 Department of Medicine and Geriatrics,
Princess Margaret Hospital, Laichikok, Hong Kong
3 Department of Medicine and Geriatrics,
Yan Chai Hospital, Tsuen Wan, Hong Kong
4 Chemical Pathology Laboratory,
Department of Pathology, Princess Margaret Hospital, Laichikok, Hong Kong
Corresponding author: Dr KC Chang (kc_chang@dh.gov.hk)
Abstract
Introduction: Pleural fluid
adenosine deaminase level can be applied to rapidly detect tuberculous
pleural effusion. We aimed to establish a local diagnostic cut-off value
for pleural fluid adenosine deaminase to identify patients with
tuberculous pleural effusion, and optimise its utility.
Methods: We retrospectively
reviewed the medical records of consecutive adults with pleural fluid
adenosine deaminase level measured by the Diazyme commercial kit
(Diazyme Laboratories, San Diego [CA], United States) during 1 January
to 31 December 2011 in a cluster of public hospitals in Hong Kong. We
considered its level alongside early (within 2 weeks) findings in
pleural fluid and pleural biopsy, with and without applying Light’s
criteria in multiple scenarios. For each scenario, we used the receiver
operating characteristic curve to identify a diagnostic cut-off value
for pleural fluid adenosine deaminase, and estimated its positive and
negative predictive values.
Results: A total of 860 medical
records were reviewed. Pleural effusion was caused by congestive heart
failure, chronic renal failure, or hypoalbuminaemia caused by liver or
kidney diseases in 246 (28.6%) patients, malignancy in 198 (23.0%),
non-tuberculous infection in 168 (19.5%), tuberculous pleural effusion
in 157 (18.3%), and miscellaneous causes in 91 (10.6%). All those with
tuberculous pleural effusion had a pleural fluid adenosine deaminase
level of ≤100 U/L. When analysis was restricted to 689 patients with
pleural fluid adenosine deaminase level of ≤100 U/L and early negative
findings for malignancy and non-tuberculous infection in pleural fluid,
the positive predictive value was significantly increased and the
negative predictive value non-significantly reduced. Using this
approach, neither additionally restricting analysis to exudates by
Light’s criteria nor adding closed pleural biopsy would further enhance
predictive values. As such, the diagnostic cut-off value for pleural
fluid adenosine deaminase is 26.5 U/L, with a sensitivity of 87.3%,
specificity of 93.2%, positive predictive value of 79.2%, negative
predictive value of 96.1%, and accuracy of 91.9%. Sex, age, and
co-morbidity did not significantly affect prediction of tuberculous
pleural effusion using the cut-off value.
Conclusion: We have established
a diagnostic cut-off level for pleural fluid adenosine deaminase in the
diagnosis of tuberculous pleural effusion by restricting analysis to a
level of ≤100 U/L, and considering early pleural fluid findings for
malignancy and non-tuberculous infection, but not Light’s criteria.
New knowledge added by this study
- There are limitations to the use of pleural fluid adenosine deaminase (pfADA) level as a surrogate marker for tuberculous pleural effusion (TBPE); thus, it must be interpreted alongside other findings that help exclude non-tuberculous diseases, thereby increasing the pre-test probability of TBPE and the positive predictive value (PPV).
- We demonstrated that TBPE was unlikely when pfADA level was >100 U/L.
- Restricting analysis to patients with pfADA level of ≤100 U/L and early (within 2 weeks) negative findings for malignancy and non-tuberculous infection in pleural fluid significantly increased PPV and non-significantly reduced the negative predictive value (NPV). Using this approach, neither additionally restricting analysis to exudates by Light’s criteria nor adding closed pleural biopsy would further enhance predictive values of pfADA for TBPE. As such, the local pfADA diagnostic cut-off value is set at 26.5 U/L, with a sensitivity of 87.3%, specificity of 93.2%, PPV of 79.2%, NPV of 96.1%, and accuracy of 91.9%.
- Among patients with pfADA level of ≤100 U/L, when pfADA level is ≥26.5 U/L with early negative findings in pleural fluid for malignancy and non-tuberculous infection, it is probably appropriate to manage the patient as a case of TBPE, without additionally performing pleural biopsy (also a surrogate marker for TBPE), but remain vigilant for a 20.8% (1 minus PPV) chance of mistaking non-tuberculous diseases as TBPE.
- When pfADA level is <26.5 U/L with early negative findings in pleural fluid for malignancy and non-tuberculous infection, tuberculosis is highly unlikely, but caution should be exercised because of a 3.9% (1 minus NPV) chance of mistaking TBPE for another disease.
- Other investigations are always indicated when the clinical progress is incompatible with the working diagnosis.
Introduction
Adenosine deaminase (ADA) is an enzyme involved in
purine metabolism, with its primary function in the development and
maintenance of the immune system. There are at least two ADA isoforms:
ADA1 and ADA2. Whereas ADA1 is found in most body cells (especially
lymphocytes and macrophages), ADA2 is predominantly found in the human
plasma and serum, and co-exists with ADA1 in macrophages. Absence of ADA1
causes severe combined immunodeficiency. Serum ADA2 level is increased in
collagen vascular disease,1 2 and most cancers.
Many studies have suggested that pleural fluid
adenosine deaminase (pfADA) is useful in the diagnosis of tuberculous
pleural effusion (TBPE).3 4 5 6 7 8 9 10 11 12 13 The
merits of using pfADA include its low cost, short turnaround time, and
high sensitivity and specificity.3
12 Notwithstanding possibly better
sensitivity and specificity for detecting TBPE by combining ADA1 or ADA2
in pleural fluid (PF) with other PF biomarkers such as tumour necrosis
factor–alpha, interleukin 27, interferon-gamma and dipeptidyl peptidase
IV,14 15
16 17
it may not be cost-effective to combine pfADA with other PF biomarkers.18 Although ADA2 is predominantly
increased in TBPE, and ADA1 is more commonly associated with pleural
effusion due to pyogenic bacteria,19
determination of ADA1 and ADA2 may not provide a diagnostic advantage over
the use of total pfADA.20
A standardised and automated method (Diazyme
commercial kit; Diazyme laboratories, San Diego [CA], United States) has
been developed to determine pfADA activity. The test performance of pfADA
has largely been evaluated by including all cases with pleural effusion,
and estimating its sensitivity and specificity with reference to an
optimal cut-off value. Some studies fine-tuned the test performance by
restricting the analysis to subjects with lymphocytic exudates9 13 or to young
adults.21 In Hong Kong, pfADA has
been measured centrally by the Chemical Pathology Laboratory at the
Princess Margaret Hospital using the Diazyme commercial kit. In the
absence of a diagnostic cut-off value established from local data, pfADA
level of ≥30 U/L has been used territory-wide in Hong Kong for detecting
TBPE. This is with reference to a retrospective Thai study of 59 (33.1%)
patients with TBPE among 178 patients with predominantly exudative
lymphocytic pleural effusion.22 It
suggested a sensitivity of 82% and specificity of 91% for pfADA level of
≥30 U/L, as measured by the Diazyme commercial kit.22 Corresponding estimates of positive predictive value
(PPV) and negative predictive value (NPV) were 81.4% and 90.8%,
respectively.22
Although pfADA rapidly detects TBPE, it is often
assessed alongside other tests that include sputum bacteriology for
acid-fast bacilli (AFB) and other pathogens, sputum cytology, PF
bacteriology for AFB and other pathogens, PF biochemistry, PF cytology,
and pleural biopsy. Restricting analysis to patients with exudative
pleural effusion may help optimise the utility of pfADA for detecting
TBPE. Excluding patients with an early diagnosis of non-tuberculous
disease, notably malignancy and non-tuberculous infection, may also help
improve the utility of pfADA for TBPE.5
9 23
24 25
26
Diagnostic test accuracy depends on sensitivity and
specificity, which are relatively stable, and pre-test probability that
can be enhanced by selecting appropriate patients. In this study, we aimed
to optimise its utility by increasing the pre-test probability, and
establish a local diagnostic pfADA cut-off value for adult TBPE.
Additionally, we evaluated whether the prediction of TBPE using the pfADA
cut-off value was affected by sex, age, or co-morbidity.
Methods
We retrospectively searched a centralised
computerised database for consecutive PF specimens tested for ADA from 1
January 2011 to 31 December 2011 and assembled a cohort of patients with
exudative pleural effusion. These patients were all managed at a cluster
of public hospitals that served a large population in western Kowloon of
Hong Kong. At least 90% of patients with pleural effusion had PF tested
for ADA. We considered pfADA alongside early (within 2 weeks) findings in
PF and pleural biopsy, with and without applying Light’s criteria27 in multiple scenarios. For each scenario, we used the
receiver operating characteristic (ROC) curve and the Youden Index (the
point of maximal summation of sensitivity and specificity estimates) to
identify an optimal pfADA diagnostic cut-off value for TBPE, and estimated
the corresponding PPV and NPV. The Youden Index maximises the difference
between the true-positive rate (sensitivity) and the false-positive rate
(1 minus specificity), thereby maximising the correct classification rate.
When the Youden Index comprised more than 1 point, we also considered the
point at minimal distance between the ROC curve and the coordinate with
100% specificity and 100% sensitivity.
The following data were collected by review of
medical records that had been created and maintained by clinicians who
were unaware of the study hypothesis: sex, age (at the time of initial
diagnosis), smoking history, drinking history, co-morbidity (chronic
obstructive pulmonary disease, diabetes mellitus, chronic renal failure),
use of immunosuppressive treatment for at least 1 month in the past year,
nature of PF (exudate vs transudate by Light’s criteria), sputum AFB smear
and culture, PF AFB smear and culture, PF bacterial and fungal stain, PF
culture of other bacteria or fungus, pleural biopsy findings, other
significant findings related to initial or definitive diagnosis, the early
diagnosis (within 2 weeks after checking pfADA), and the definitive
diagnosis (by 1 year after checking pfADA).
This study was conducted in accordance with the
amended Declaration of Helsinki, and approved by the Kowloon West Cluster
Research Ethics Committee (IRB approval number: KW/EX-13-139(69-17)) and
the Department of Health Ethics Committee (IRB approval number: L/M
400/2013).
Definitions
The exudative versus transudative nature of PF was
established by reference to Light’s criteria that classify PF as exudative
in the presence of any one of the following: ratio of protein in PF to
serum >0.5, ratio of lactate dehydrogenase (LDH) in PF to serum
>0.6, and PF LDH level of >200 IU/L.27
A definitive diagnosis of TBPE was made when Mycobacterium
tuberculosis complex was isolated in culture of PF or parietal
pleura, or any one of the following in the absence of an alternative
diagnosis by 1 year after pfADA checking: (i) granulomatous inflammation
of parietal pleura, (ii) culture-proven pulmonary tuberculosis (TB) with
pleural effusion and compatible response to TB treatment, (iii) a clinical
diagnosis of TBPE with compatible response to TB treatment, or (iv) AFB
and/or positive findings from nucleic acid amplification tests in PF or
parietal pleura. An early diagnosis of TBPE was made when pleural biopsy
showed granulomatous inflammation in the absence of an alternative cause,
or rarely, the presence of AFB or positive findings from nucleic acid
amplification tests in PF or parietal pleura.
Parapneumonic effusion refers to any pleural
effusion secondary to pneumonia or lung abscess.28
The PF is often exudative with a predominance of neutrophils.28 It can be ‘simple’ (with sterile exudate) or
‘complicated’ (with progression to a fibrinopurulent state), characterised
by pH <7.2, glucose level of <2.2 mmol/L, and LDH level of >1000
IU/L.29 Empyema thoracis is a
complicated parapneumonic effusion with frank pus.28 A definitive diagnosis of simple non-tuberculous
parapneumonic effusion was made if the PF was exudative and sterile with
LDH level of ≤1000 IU/L and if there was a compatible clinical response to
empirical antibiotic treatment, in the absence of an alternative diagnosis
by 1 year after the first attempt of diagnostic thoracentesis. Without an
identifiable non-tuberculous pathogen, we considered it impossible to
confidently make an early diagnosis of simple non-tuberculous
parapneumonic effusion. A definitive diagnosis of complicated
non-tuberculous parapneumonic effusion, or empyema thoracis in the
presence of frank pus or compatible radiological signs on chest computed
tomographic scan was made if the PF was exudative with a non-tuberculous
pathogen (demonstrated by positive stain/culture) in PF or parietal
pleura, or LDH level of >1000 IU/L, and compatible clinical response to
empirical antibiotic treatment and/or drainage, in the absence of an
alternative diagnosis by 1 year after the first attempt of diagnostic
thoracentesis. An early diagnosis of complicated non-tuberculous
parapneumonic effusion, or empyema thoracis in the presence of frank pus
or compatible radiological signs, was made when a non-tuberculous pathogen
could be identified in PF or parietal pleura.
Malignant pleural effusion refers to the presence
of malignant cells in PF and/or parietal pleura.30
A definitive diagnosis of malignant pleural effusion was made if malignant
cells were found in PF and/or parietal pleura, or clinical/radiological
findings were compatible with malignant pleural effusion in the absence of
an alternative diagnosis by 1 year after the first attempt of diagnostic
thoracentesis. An early diagnosis of malignant pleural effusion was made
when malignant cells could be demonstrated in PF or parietal pleura.
Statistical analysis
Chi squared test (for categorical data), Fisher’s
exact test (for categorical data), McNemar’s test (for paired data),
Student’s t test (for continuous variables normally distributed),
and Mann-Whitney U test (for continuous variables not normally
distributed) were used as appropriate to evaluate the association between
TBPE and the pfADA cut-off as well as demographic factors and
co-morbidity. Factors with a P value of <0.25 by univariate analysis
were forced into a logistic regression model after considering
multicollinearity.
Laboratory methods
Throughout the study period, ADA activity was
measured by the same automated method, the Diazyme commercial kit in the
Beckman Coulter UniCel DxC 800 Synchron Clinical System. The automated
Diazyme method has been validated.31
Results
Search from the computerised database of ADA assay
from 1 January to 31 December 2011 identified a total of 903 independent
PF specimens from 903 patients. We evaluated 860 patients with pleural
effusion and pfADA after excluding 42 cases that were peritoneal rather
than PF and one case with no medical record. Table 1 shows their definitive diagnoses and the
corresponding pfADA value. Pleural effusion was caused by congestive heart
failure, chronic renal failure, hypoalbuminaemia, or nephrotic
syndrome/nephropathy with membranous glomerulonephritis in 246 (28.6%)
cases, malignancy in 198 (23.0%), non-tuberculous infection (simple
non-tuberculous parapneumonic effusion, complicated non-tuberculous
parapneumonic effusion other than empyema, and non-tuberculous empyema
thoracis) in 168 (19.5%), TBPE in 157 (18.3%), and miscellaneous or
unknown causes in 91 (10.6%). By Light’s criteria, 626 (72.8%) cases were
classified as exudative, 222 (25.8%) as transudative, and 12 (1.4%) as
indeterminate (lack of data).
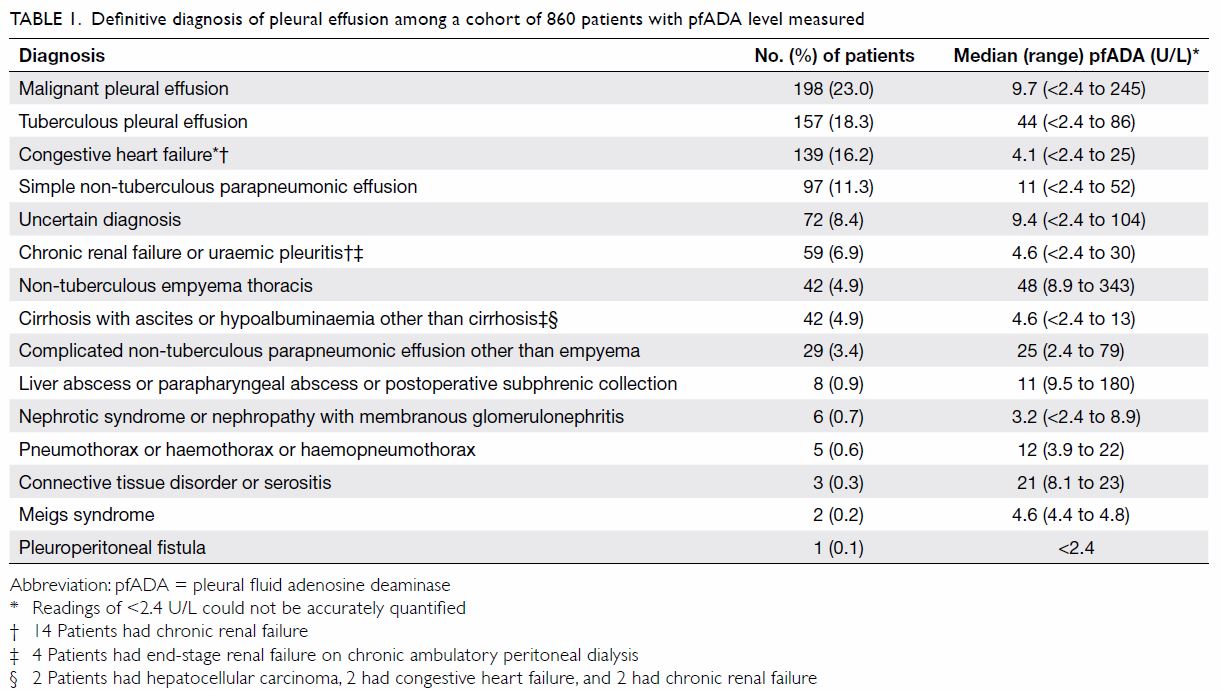
Table 1. Definitive diagnosis of pleural effusion among a cohort of 860 patients with pfADA level measured
Among the 198 patients with malignant pleural
effusion, an early diagnosis could be established by detecting malignant
cells in PF in 136 (68.7%), including 21 also detected by pleural biopsy
(20 closed and 1 open), and seven (3.5%) by pleural biopsy alone (5 closed
and 2 open). Malignant pleural effusion was caused by lung cancer in 152
(76.8%) patients, lymphoid or haematological malignancy in 12 (6.1%),
unknown primary in nine (4.5%), gastric cancer in five (2.5%), ovarian
cancer in four (2.0%), breast cancer in four (2.0%), liver cancer in two
(1.0%), pancreatic cancer in two (1.0%), and one (0.5%) each by cancer in
the nasopharynx, tongue, oesophagus, unspecified gastrointestinal tract,
kidney, urinary bladder, prostate, and nerve.
Among the 168 patients with non-tuberculous
infection, infection was bacteriologically confirmed by PF culture in 21
(12.5%) cases with non-tuberculous empyema thoracis (including 19 with
early diagnosis), and 10 (6.0%) with complicated non-tuberculous
parapneumonic effusion (including 9 with early diagnosis).
Among the 157 patients with TBPE, the diagnosis was
(1) bacteriologically confirmed by PF culture in 62 (39.5%) including four
also confirmed by pleural tissue culture and 26 also suggested by pleural
biopsy; (2) bacteriologically confirmed by pleural tissue culture in 12
(7.6%) including four also confirmed by PF culture and nine also suggested
by pleural biopsy; (3) histologically suggested by pleural biopsy in 74
(69 closed and 5 open; 47.1%) including 26 also confirmed by PF culture
and nine also confirmed by pleural tissue culture; (4) clinically
suggested by pulmonary TB in 44 (28.0%) including 26 solely by clinical
correlation with radiological progress; and (5) clinically suggested by
culture-proven TB ascites in one (0.6%). Sputum AFB smear was positive in
nine (5.7%) patients, with M tuberculosis complex isolated in the
sputum culture of 51 (32.5%). An early diagnosis could be established in
65 (41.4%), using pleural biopsy in 64 and polymerase chain reaction in PF
in one. The majority (n=152) of patients with TBPE were Chinese.
Among 90 patients with TBPE and closed pleural
biopsy performed, TBPE was detected by pleural biopsy in 69 (76.7%) and
pfADA cut-off level in 85 (94.4%). The difference was statistically
significant (P<0.005 by McNemar’s test).
Table 2 shows the distribution of pfADA levels
stratified by the nature of PF and tuberculous versus non-tuberculous
pleural effusion. The prevalence (pre-test probability) of TBPE was
significantly higher among exudative (24.3%) than transudative (2.3%)
cases. All cases with TBPE had pfADA level of ≤86 U/L. With pfADA level of
>86 U/L, all cases (n=18) with exudative non-tuberculous pleural
effusion had pfADA level of >100 U/L of whom 13 patients had
non-tuberculous empyema thoracis, two had lymphoma, one had plasmacytoma,
one had liver abscess, and one had an uncertain diagnosis.
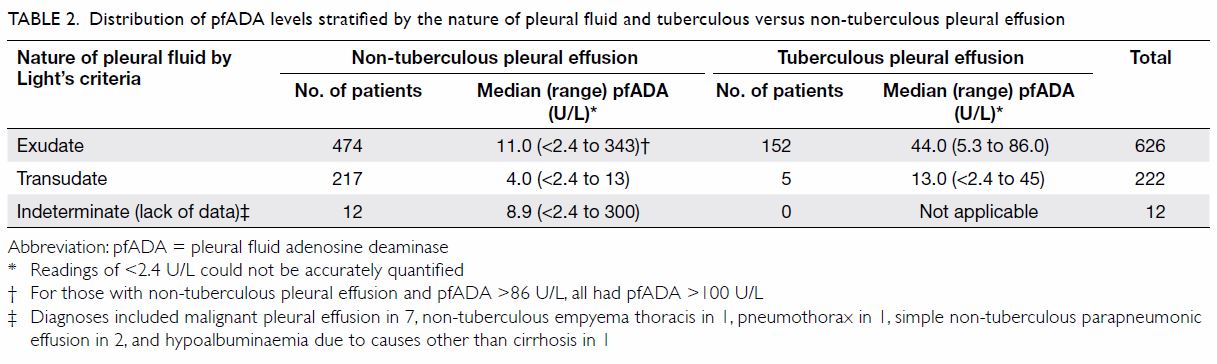
Table 2. Distribution of pfADA levels stratified by the nature of pleural fluid and tuberculous versus non-tuberculous pleural effusion
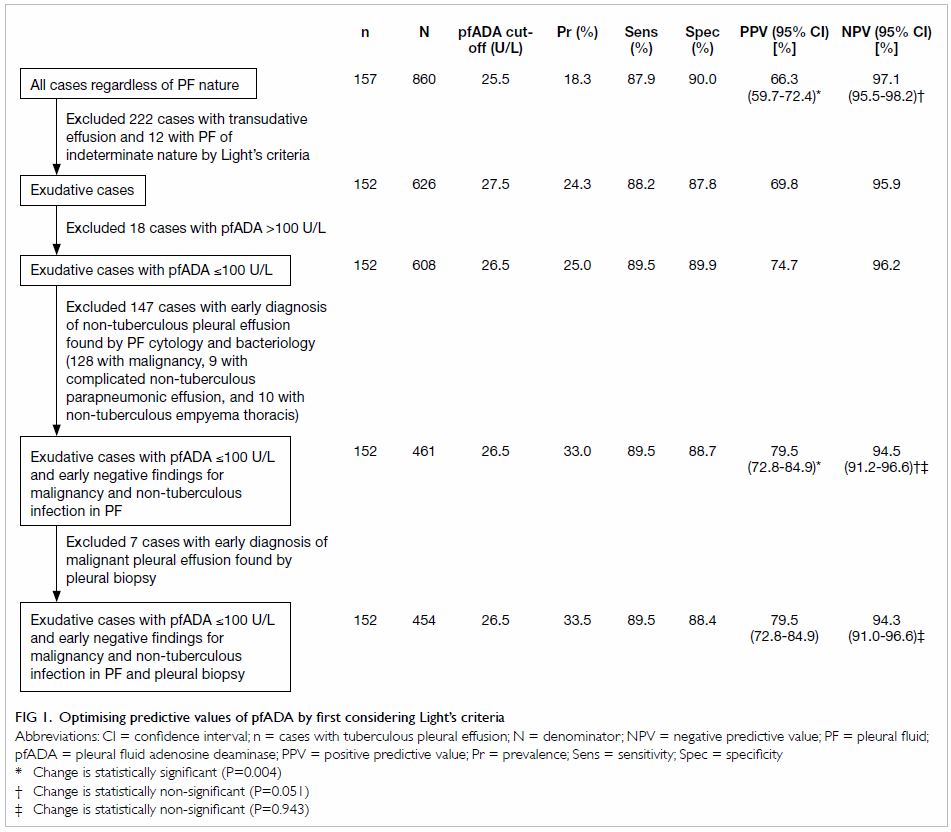
Figure 1 shows how we proceeded to increase the
pre-test probability of TBPE by first excluding transudates, and then
stepwise excluding non-tuberculous patients to further increase the
pre-test probability. For each scenario, the pfADA cut-off value was
tabulated alongside estimates of sensitivity, specificity, PPV, and NPV.
Restricting analysis to 461 patients with exudative pleural effusion,
pfADA level of ≤100 U/L, and early negative findings for non-tuberculous
infection and malignancy in PF significantly increased PPV from 66.3% to
79.5% and non-significantly reduced NPV from 97.1% to 94.5%. Further
excluding seven patients with an early diagnosis of malignancy by pleural
biopsy resulted in no change to PPV and a non-significant decrease in NPV.
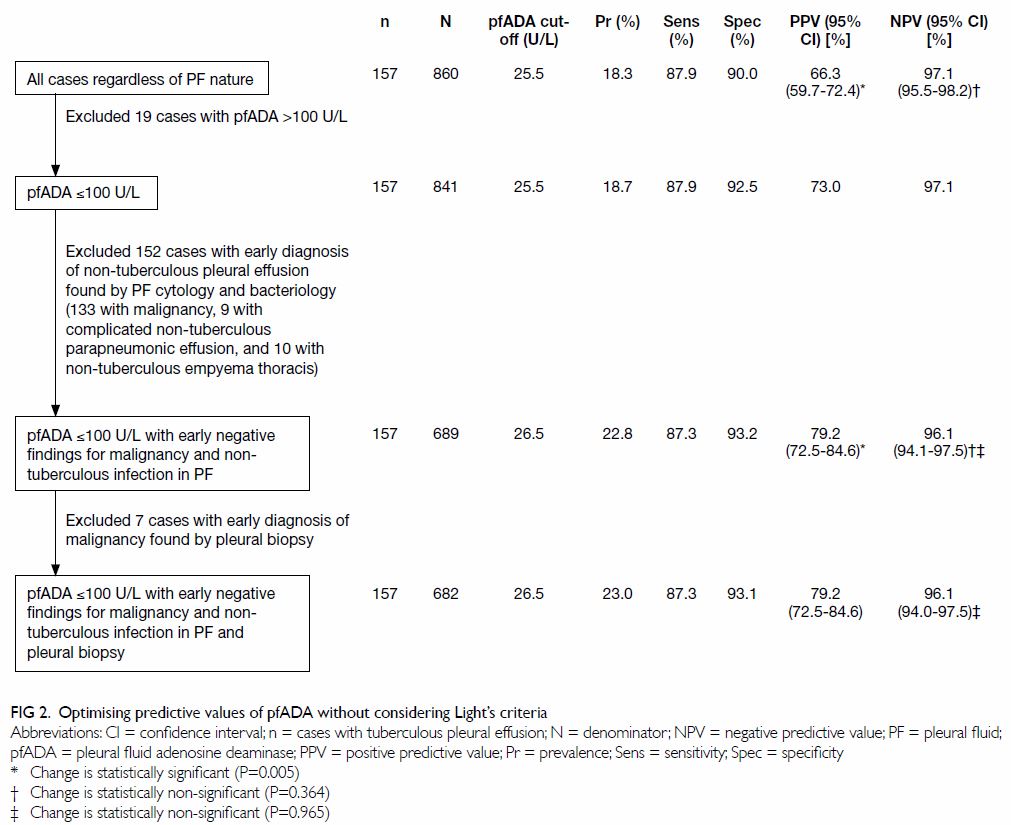
Figure 2 shows an alternative approach that
disregards Light’s criteria. Restricting analysis to 689 patients with
pfADA level of ≤100 U/L and early negative findings for non-tuberculous
infection and malignancy in PF also significantly increased PPV from 66.3%
to 79.2% and non-significantly reduced NPV from 97.1% to 96.1%. Further
excluding seven patients with an early diagnosis of malignancy by pleural
biopsy resulted in no change to PPV and a non-significant decrease in NPV
from 96.12% to 96.07%. With no significant difference in PPV (P=0.938) or
NPV (P=0.279) between the two approaches, the utility of pfADA may be
optimised by applying a diagnostic cut-off among patients with pfADA level
of ≤100 U/L and early negative findings for malignancy and non-tuberculous
infection in PF, without considering Light’s criteria or pleural biopsy.
As such, pfADA level of ≥26.5 U/L, ascertained from the ROC curve using
the Youden Index, detected TBPE with a sensitivity of 87.3%, specificity
of 93.2%, PPV of 79.2%, NPV of 96.1%, and accuracy of 91.9% (Fig
3).
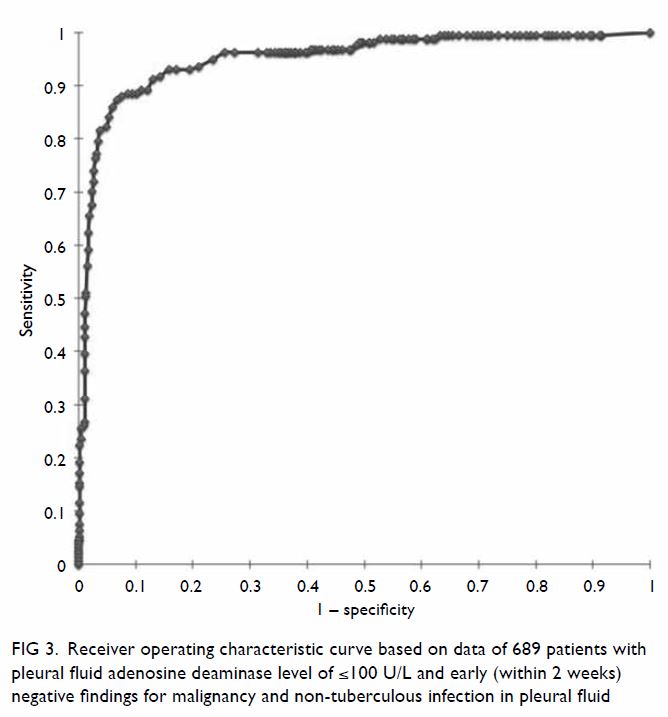
Figure 3. Receiver operating characteristic curve based on data of 689 patients with pleural fluid adenosine deaminase level of ≤100 U/L and early (within 2 weeks) negative findings for malignancy and non-tuberculous infection in pleural fluid
Table 3 shows different causes of pleural effusion
above and below the diagnostic pfADA cut-off value of 26.5 U/L among the
689 patients with pfADA level of ≤100 U/L, and early negative findings for
malignancy and non-tuberculous infection in PF. It is noteworthy that in
the seven (4.0%) patients with pfADA level of ≥26.5 U/L and 64 (12.4%)
patients with pfADA level of <26.5 U/L, the diagnosis was uncertain.
Among 157 patients with TBPE, 137 (87.3%, sensitivity) tested positive
(pfADA ≥26.5 U/L), with false-negative results in 20 (12.7%, the
false-negative rate or 1 minus sensitivity). Among 532 patients with
non-tuberculous pleural effusion, 496 (93.2%, specificity) tested negative
(pfADA <26.5 U/L), with false-positive results in 36 (6.8%,
false-positive rate or 1 minus specificity). Among 173 patients who tested
positive, 137 (79.2%, PPV) were true-positive, and 36 (20.8%, 1 minus PPV)
were false-positive with non-tuberculous diseases mistaken for TBPE: 10
with complicated non-tuberculous parapneumonic effusion, 10 with
non-tuberculous empyema thoracis, seven with uncertain diagnosis, four
with malignant pleural effusion, four with simple non-TB parapneumonic
effusion, and one with chronic renal failure. Among 516 patients tested
negative, 496 (96.1%, NPV) were true-negative, and 20 (3.9%, 1 minus NPV)
were false-negative with TBPE mistaken for non-tuberculous pleural
effusion. Among 689 test results, 633 (91.9%) were accurate and comprised
137 true-positive and 496 true-negative results.
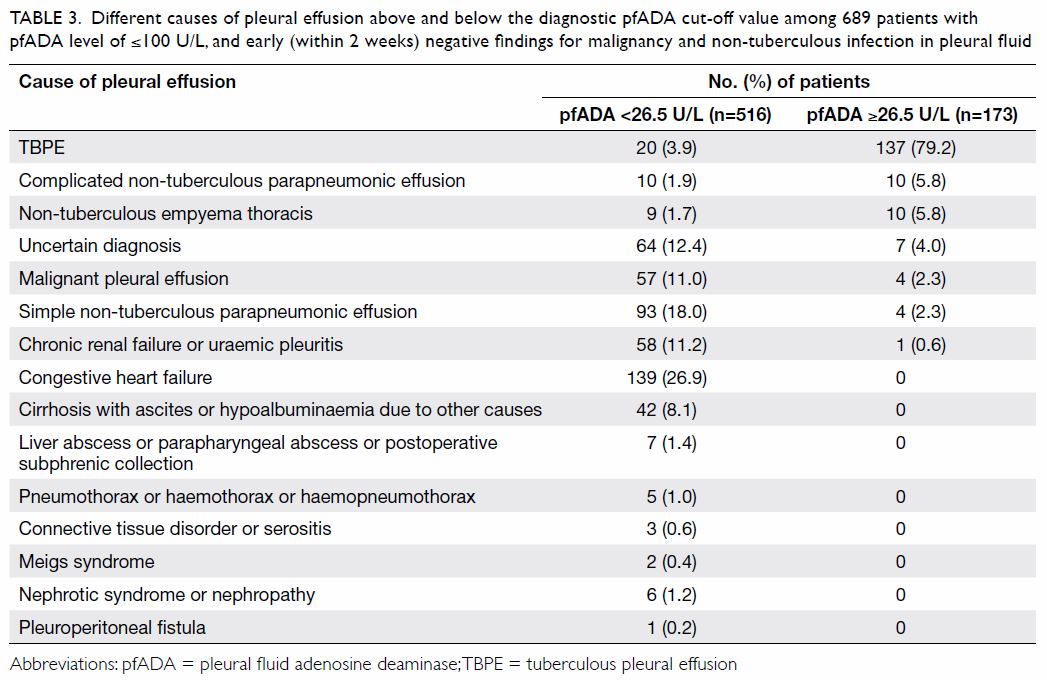
Table 3. Different causes of pleural effusion above and below the diagnostic pfADA cut-off value among 689 patients with pfADA level of ≤100 U/L, and early (within 2 weeks) negative findings for malignancy and non-tuberculous infection in pleural fluid
A logistic regression model that considered sex,
age, and co-morbidity alongside the pfADA diagnostic cut-off value
identified pfADA level of ≥26.5 U/L as the only significant predictive
variable of TBPE (Table 4).
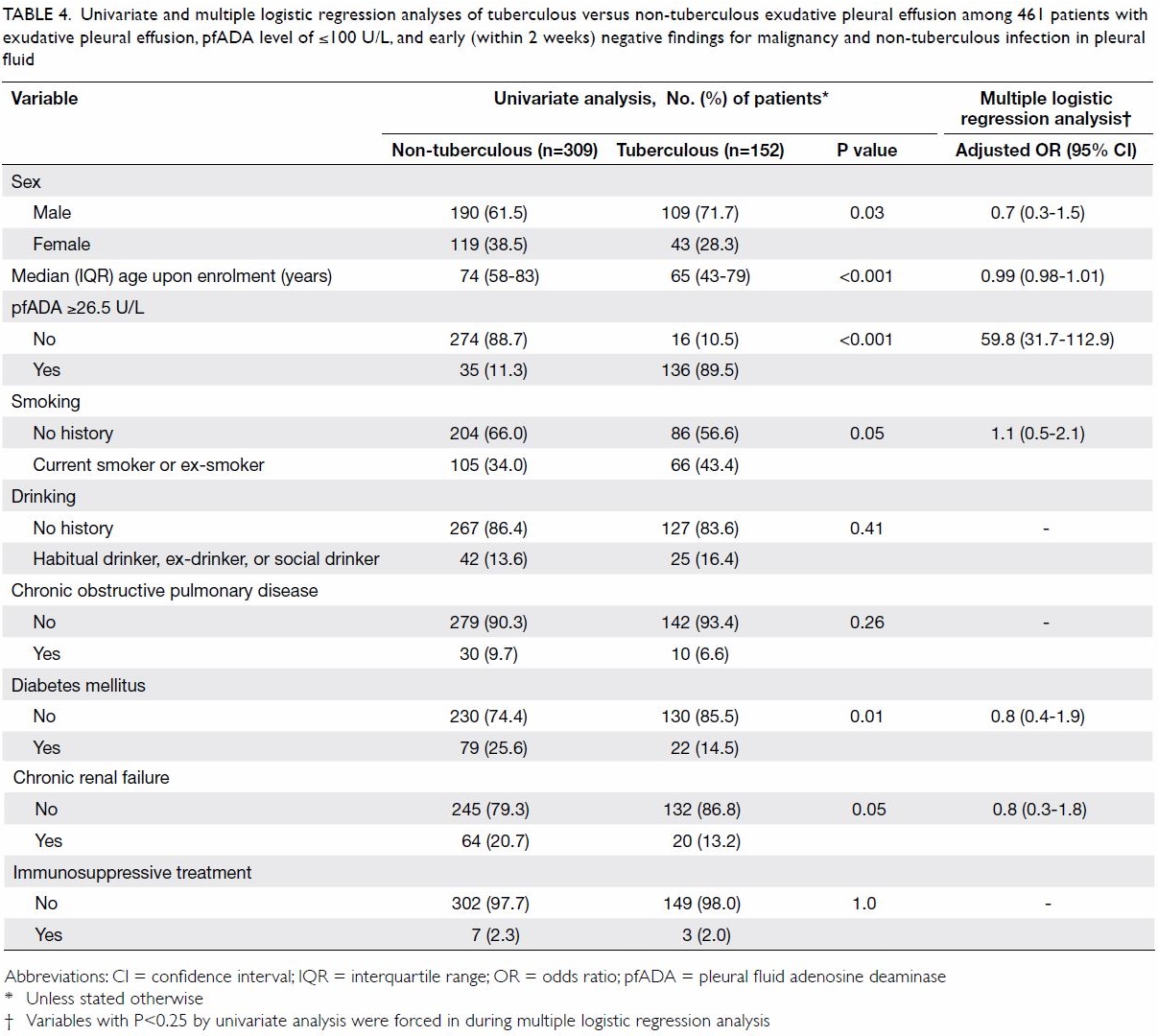
Table 4. Univariate and multiple logistic regression analyses of tuberculous versus non-tuberculous exudative pleural effusion among 461 patients with exudative pleural effusion, pfADA level of ≤100 U/L, and early (within 2 weeks) negative findings for malignancy and non-tuberculous infection in pleural fluid
Discussion
As a limited surrogate marker for TBPE, pfADA level
must be interpreted alongside other clinical, radiological, and laboratory
findings that help exclude non-tuberculous diseases, thereby increasing
the pre-test probability of TBPE and the PPV. Using local data as measured
by the Diazyme commercial kit, we demonstrated that TBPE was unlikely when
pfADA level was >100 U/L. Restricting analysis to patients with pfADA
level of ≤100 U/L and early (within 2 weeks) negative findings for
malignancy and non-tuberculous infection in PF significantly increased the
PPV and non-significantly reduced the NPV. Using this approach, neither
additionally restricting analysis to exudates by Light’s criteria, nor
adding closed pleural biopsy, would further enhance the predictive value
of pfADA for TBPE. This might be explained by the fact that pfADA level of
>13 U/L excluded all non-tuberculous transudative cases (Table
2), and that pfADA was significantly more sensitive than closed
pleural biopsy for TBPE. A recent study, which demonstrated a need to
suspect empyema or lymphoma when the pfADA level was extremely high,32 corroborated our findings regarding the low
likelihood of TBPE when pfADA level was >100 U/L. Furthermore, we
demonstrated that the prediction of TBPE using the pfADA diagnostic
cut-off value was not affected by sex, age, or co-morbidity. Another study
that developed a predictive model for TBPE also failed to show any
significant association between TBPE and either age or sex.33
Among patients with pfADA level of ≤100 U/L, when
pfADA level is ≥26.5 U/L with early negative findings in PF for malignancy
and non-tuberculous infection, it is probably appropriate to manage the
patient as a case of TBPE, without additionally performing pleural biopsy
(also a surrogate marker for TBPE). Nonetheless, it is important to remain
vigilant due to a 20.8% (1 minus PPV) chance of mistaking non-tuberculous
diseases for TBPE and prescribing unnecessary TB treatment. When pfADA
level is <26.5 U/L with early negative findings in PF for malignancy
and non-tuberculous infection, TB is highly unlikely. Again caution should
be exercised in the presence of a 3.9% (1 minus NPV) chance of mistaking
TBPE for other diseases. Tuberculosis is potentially fatal although
effective treatment can reduce morbidity and mortality. Yet standard TB
treatment is not without harmful side-effects that include hepatotoxicity.
This occurs in 1% to 3% of patients on average and becomes more prevalent
among the elderly people and those with underlying liver disease.34 35
Additionally, treating non-tuberculous disease as TB may also delay the
diagnosis of other diseases including malignancy. It is important to
balance the benefits of TB treatment against the risks when using a pfADA
cut-off value to diagnose TBPE. In general, if the test suggests TBPE, and
the risk of morbidity or mortality from untreated TB is substantial, it is
prudent to promptly start TB treatment, and closely monitor treatment
progress, with further investigations for other diseases conducted
concurrently or as soon as treatment response is considered suboptimal.
Other investigations are always indicated when the clinical progress is
incompatible with the working diagnosis.
A major drawback of this study was its
retrospective nature and related selection and misclassification bias.
Selection bias may be modest as public hospitals provide approximately 90%
of hospital care in Hong Kong, and we included every consecutive and
non-duplicated PF sample from all patients managed during the study period
in a large public hospital cluster in which at least 90% patients with
pleural effusion had PF tested for ADA. Misclassification bias may occur.
Efforts made by clinicians to confirm TB disease may be selectively
affected by knowledge about the association between pfADA and TB.
Non-tuberculous infection could have been misclassified as TB, thereby
overestimating PPV or underestimating NPV. On the other hand, TBPE could
also have been misclassified as non-TB, thereby underestimating PPV or
overestimating NPV. Another possible source of misclassification bias was
uncertain diagnosis (Table 3), which was considered as non-tuberculous
during analysis. Of note, TBPE labelled as uncertain diagnosis could have
caused an underestimation of PPV or overestimation of NPV. Nonetheless,
the lack of a definitive diagnosis by 1 year might suggest a low
likelihood of TBPE, thereby reducing the impact of this misclassification
bias.
We have established a pfADA diagnostic cut-off
value for TBPE by restricting analysis to patients with pfADA level of
≤100 U/L, and considering early PF findings for malignancy and
non-tuberculous infection, but not Light’s criteria.
Declaration
All authors have disclosed no conflicts of
interest.
References
1. Sari RA, Taysi S, Yilmaz O, Bakan N.
Correlation of serum levels of adenosine deaminase activity and its
isoenzymes with disease activity in rheumatoid arthritis. Clin Exp
Rheumatol 2003;21:87-90.
2. Stancíková M, Lukác J, Istok R,
Cristalli G, Rovensky J. Serum adenosine deaminase activity and its
isoenzyme pattern in patients with systemic lupus erythematosus. Clin Exp
Rheumatol 1998;16:583-6.
3. Gupta DK, Suri JC, Goel A. Efficacy of
adenosine deaminase in the diagnosis of pleural effusions. Indian J Chest
Dis Allied Sci 1990;32:205-8.
4. Bañales JL, Pineda PR, Fitzgerald JM,
Rubio H, Selman M, Salazar-Lezama M. Adenosine deaminase in the diagnosis
of tuberculous pleural effusions. A report of 218 patients and review of
the literature. Chest 1991;99:355-7. Crossref
5. Valdés L, Alvarez D, San José E, et al.
Value of adenosine deaminase in the diagnosis of tuberculous pleural
effusions in young patients in a region of high prevalence of
tuberculosis. Thorax 1995;50:600-3. Crossref
6. Burgess LJ, Maritz FJ, Le Roux I,
Taljaard JJ. Combined use of pleural adenosine deaminase with
lymphocyte/neutrophil ratio. Increased specificity for the diagnosis of
tuberculous pleuritis. Chest 1996;109:414-9. Crossref
7. Riantawan P, Chaowalit P, Wongsangiem M,
Rojanaraweewong P. Diagnostic value of pleural fluid adenosine deaminase
in tuberculous pleuritis with reference to HIV coinfection and a Bayesian
analysis. Chest 1999;116:97-103. Crossref
8. Reechaipichitkul W, Kawamatawong T,
Teerajetgul Y, Patjanasoontorn B. Diagnostic role of pleural fluid
adenosine deaminase in tuberculous pleural effusion. Southeast Asian J
Trop Med Public Health 2001;32:383-9.
9. Lee YC, Rogers JT, Rodriguez RM, Miller
KD, Light RW. Adenosine deaminase levels in nontuberculous lymphocytic
pleural effusions. Chest 2001;120:356-61. Crossref
10. Jiménez Castro D, Díaz Nuevo G,
Pérez-Rodríguez E, Light RW. Diagnostic value of adenosine deaminase in
nontuberculous lymphocytic pleural effusions. Eur Respir J 2003;21:220-4.
Crossref
11. Chen ML, Yu WC, Lam CW, Au KM, Kong
FY, Chan AY. Diagnostic value of pleural fluid adenosine deaminase
activity in tuberculous pleurisy. Clin Chim Acta 2004;341:101-7. Crossref
12. Baba K, Hoosen AA, Langeland N,
Dyrhol-Riise AM. Adenosine deaminase activity is a sensitive marker for
the diagnosis of tuberculous pleuritis in patients with very low CD4
counts. PLoS ONE 2008;3:e2788. Crossef
13. Garcia-Zamalloa A, Taboada-Gomez J.
Diagnostic accuracy of adenosine deaminase and lymphocyte proportion in
pleural fluid for tuberculous pleurisy in different prevalence scenarios.
PLoS ONE 2012;7:e38729. Crossref
14. Küpeli E, Karnak D, Elgün S, Argüder
E, Kayacan O. Concurrent measurement of adenosine deaminase and dipeptidyl
peptidase IV activity in the diagnosis of tuberculous pleural effusion.
Diagn Microbiol Infect Dis 2009;65:365-71. Crossref
15. Wu YB, Ye ZJ, Qin SM, Wu C, Chen YQ,
Shi HZ. Combined detections of interleukin 27, interferon-γ, and adenosine
deaminase in pleural effusion for diagnosis of tuberculous pleurisy. Chin
Med J (Engl) 2013;126:3215-21.
16. Keng LT, Shu CC, Chen JY, et al.
Evaluating pleural ADA, ADA2, IFN-γ and IGRA for diagnosing tuberculous
pleurisy. J Infect 2013;67:294-302. Crossref
17. Li M, Wang H, Wang X, Huang J, Wang J,
Xi X. Diagnostic accuracy of tumor necrosis factor-alpha,
interferon-gamma, interleukin-10 and adenosine deaminase 2 in differential
diagnosis between tuberculous pleural effusion and malignant pleural
effusion. J Cardiothorac Surg 2014;9:118. Crossref
18. Sharma SK, Banga A. Pleural fluid
interferon-gamma and adenosine deaminase levels in tuberculosis pleural
effusion: a cost-effectiveness analysis. J Clin Lab Anal 2005;19:40-6. Crossref
19. Ungerer JP, Oosthuizen HM, Retief JH,
Bissbort SH. Significance of adenosine deaminase activity and its
isoenzymes in tuberculous effusions. Chest 1994;106:33-7. Crossref
20. Andreasyan NA, Hairapetian HL,
Sargisova YG, Mardanyan SS, Badalyan LT, Khanoyan AS. Activity of
adenosine deaminase and its isoforms in pleural fluid in tuberculous
pleuritis. Med Sci Monit 2002;8:CR708-12.
21. Yildiz PB, Yazar EE, Gorgun D, Secik
F, Cakir G. Predictive role of adenosine deaminase for differential
diagnosis of tuberculosis and malignant pleural effusion in Turkey. Asian
Pac J Cancer Prev 2011;12:419-23.
22. Kawamatawong T, Panompong K,
Kiatboonsri S, Khupulsup K. The appropriate cutoff level of pleural fluid
adenosine deaminase activity by diazyme commercial kit for diagnosis
pleural tuberculosis in Ramathibodi hospital. Chest 2008;134(Suppl
2):p55001. Crossref
23. Valdés L, San José E, Alvarez D, Valle
JM. Adenosine deaminase (ADA) isoenzyme analysis in pleural effusions:
diagnostic role, and relevance to the origin of increased ADA in
tuberculous pleurisy. Eur Respir J 1996;9:747-51. Crossref
24. Strankinga WF, Nauta JJ, Straub JP,
Stam J. Adenosine deaminase activity in tuberculous pleural effusions: a
diagnostic test. Tubercle 1987;68:137-40. Crossref
25. Porcel JM, Esquerda A, Bielsa S.
Diagnostic performance of adenosine deaminase activity in pleural fluid: a
singlecenter experience with over 2100 consecutive patients. Eur J Intern
Med 2010;21:419-23. Crossref
26. Ogata Y, Aoe K, Hiraki A, et al. Is
adenosine deaminase in pleural fluid a useful marker for differentiating
tuberculosis from lung cancer or mesothelioma in Japan, a country with
intermediate incidence of tuberculosis? Acta Med Okayama 2011;65:259-63.
27. Light RW, Macgregor MI, Luchsinger PC,
Ball WC Jr. Pleural effusions: the diagnostic separation of transudates
and exudates. Ann Intern Med 1972;77:507-13. Crossref
28. Light RW. Parapneumonic effusions and
empyema. Proc Am Thorac Soc 2006;3:75-80. Crossref
29. Davies HE, Davies RJ, Davies CW, BTS
Pleural Disease Guideline Group. Management of pleural infection in
adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax
2010;65 Suppl 2:ii41-53. Crossref
30. Antunes G, Neville E, Duffy J, Ali N,
Pleural Diseases Group, Standards of Care Committee, British Thoracic
Society. BTS guidelines for the management of malignant pleural effusions.
Thorax 2003;58 Suppl 2:ii29-38. Crossref
31. Feres MC, Martino MC, Maldijian S,
Batista F, Gabriel Júnior A, Tufik S. Laboratorial validation of an
automated assay for the determination of adenosine deaminase activity in
pleural fluid and cerebrospinal fluid [in English, Portuguese]. J Bras
Pneumol 2008;34:1033-9. Crossref
32. Wu YH, Zhao GW, Wang XF, Wang MS.
Pleural effusion adenosine deaminase is not accurate in diagnosis of
pediatric tuberculous pleural effusion: a retrospective study. Eur Rev Med
Pharmacol Sci 2015;19:1706-10.
33. Neves DD, Dias RM, Cunha AJ.
Predictive model for the diagnosis of tuberculous pleural effusion. Braz J
Infect Dis 2007;11:83-8. Crossref
34. Chang KC, Leung CC, Yew WW, Tam CM.
Standard anti-tuberculosis treatment and hepatotoxicity: do dosing
schedules matter? Eur Respir J 2007;29:347-51. Crossref
35. Chang KC, Leung CC, Yew WW, Lau TY,
Tam CM. Hepatotoxicity of pyrazinamide: cohort and case-control analyses.
Am J Respir Crit Care Med 2008;177:1391-6. Crossref