Hong
Kong Med J 2018 Feb;24(1):18–24 | Epub 5 Jan 2018
DOI: 10.12809/hkmj154764
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Implications of nipple discharge in Hong Kong Chinese
women
WM Kan, FCSHK, FHKAM (Surgery)1;
Clement Chen, FRCS, FHKAM (Surgery)2; Ava Kwong, FRCS, FHKAM
(Surgery)2
1 Department of Surgery, Queen Elizabeth
Hospital, Jordan, Hong Kong
2 Department of Surgery, Queen Mary
Hospital, Pokfulam, Hong Kong
Corresponding author: Dr Ava Kwong (avakwong@hku.hk)
Abstract
Introduction: There are no
recent data on nipple discharge and its association with malignancy in
Hong Kong Chinese women. This study reported our 5-year experience in
the management of patients with nipple discharge, and our experience of
mammography, ultrasonography, ductography, and nipple discharge cytology
in an attempt to determine their role in the management of nipple
discharge.
Methods: Women who attended our
Breast Clinic in a university-affiliated hospital in Hong Kong were
identified by retrospective review of clinical data from January 2007 to
December 2011. They were divided into benign and malignant subgroups.
Background clinical variables and investigative results were compared
between the two subgroups. We also reported the sensitivity,
specificity, and positive and negative predictive values of the
investigations that included mammography, ultrasonography, ductography,
and cytology.
Results: We identified 71 and 31
patients in the benign and malignant subgroups, respectively. The median
age at presentation for the benign subgroup was younger than that of the
malignant subgroup (48 vs 59 years; P=0.003). A higher proportion of
patients in the malignant subgroup than the benign subgroup presented
with blood-stained nipple discharge (87.1% vs 47.9%; P=0.002).
Mammography had a specificity of 98.4% and positive predictive value of
66.7%; ultrasonography had a specificity of 87.0% and negative
predictive value of 75.0%. Cytology and ductography were sensitive but
lacked specificity. Ductography had a negative predictive value of 100%
but a low positive predictive value (14.0%). Clinical variables
including age at presentation, duration of discharge, colour of
discharge, presence of an associated breast mass, and abnormal
sonographic findings were important in suggesting the underlying
pathology of nipple discharge. Multiple logistic regression showed that
blood-stained discharge and an associated breast mass were statistically
significantly more common in the malignant subgroup.
Conclusions: In patients with
non–blood-stained nipple discharge, a negative clinical breast
examination combined with negative imaging could reasonably infer a
benign underlying pathology.
New knowledge added by this study
- Blood-stained nipple discharge and an associated breast mass at presentation could suggest a higher chance of malignancy.
- A period of watchful waiting is a reasonable alternative to surgical intervention in patients with inferred benign pathology.
Introduction
Nipple discharge is a relatively uncommon complaint
in Hong Kong Chinese women. According to a study in 1997, nipple discharge
constituted 1.5% of all presenting complaints for women who attended a
breast clinic in Hong Kong.1 On the
contrary, nipple discharge accounted for up to 4% to 7% of all presenting
symptoms in other studies.2 3 This may be better explained by the unique Chinese
culture and help-seeking pattern rather than a true disease pattern. With
this understanding, any clinical survey will probably underestimate the
prevalence of nipple discharge in Chinese women. When patients approach
health care professionals because of nipple discharge, not only is it
important to differentiate malignant from benign causes of nipple
discharge, it is also a valuable opportunity to promote breast health
awareness.
Numerous studies have demonstrated the relationship
between breast cancer and nipple discharge, with malignancy reported in up
to 9.3% to 21% of all patients who present with nipple discharge.4 5 The most
challenging role of breast surgeons is to accurately identify these
patients. Notwithstanding, controversy persists about the value and
accuracy of individual investigative tools for nipple discharge.6
There are no recent data on nipple discharge and
its association with malignancy in Chinese women in Hong Kong. The primary
aim of this study was to report our recent experience in the management of
patients with nipple discharge in a single surgical centre. The secondary
aim was to report our experience of individual investigative tools in an
attempt to determine their role in the management of nipple discharge.
Methods
We retrospectively reviewed the clinical data of
patients who attended our Breast Clinic at the Queen Mary Hospital, a
university-affiliated hospital in Hong Kong, for nipple discharge from
January 2007 to December 2011. Potential subjects were identified when
diagnosis coding 611.79 (other signs and symptoms in breast) was entered
into our Clinical Management System, which is a territory-wide
computer-based medical record system designed for use in public hospitals,
and also from the prospective database of the Division of Breast Surgery,
The University of Hong Kong.
Data extraction and coding were performed by the
first author (WM Kan) and included duration of follow-up until December
2011, age at presentation, history of breast condition, and laterality and
duration of nipple discharge before first consultation. Clinical variables
included colour of nipple discharge, single- or multiple-duct discharge,
associated symptoms, mammographic and ultrasonographic imaging results, as
well as ductogram and cytology results. Pathology results were recorded
for patients who underwent surgery or biopsy.
In order to make a meaningful comparison, we
divided patients into malignant and benign subgroups. The malignant
subgroup was defined by malignant pathology on a surgically resected
specimen. The benign subgroup was defined by benign pathology of a
surgically resected or biopsy specimen, or clinical non-progression after
more than 2 years of follow-up. Patients who did not undergo surgery or
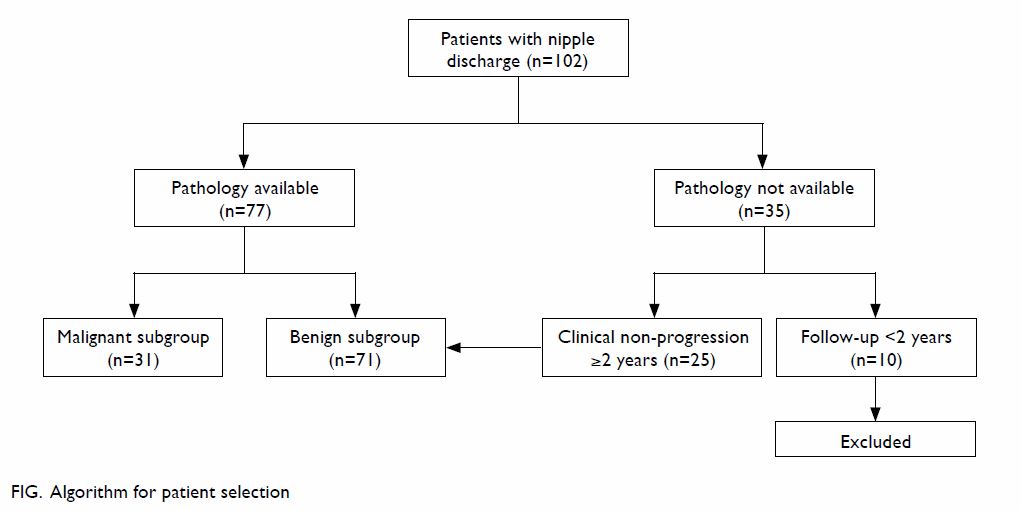
biopsy and who were followed up for less than 2 years were excluded (Fig).
In the first part of our study, we compared the
background clinical variables and investigative results between the two
subgroups. In the second part of our analysis, we reported the
sensitivity, specificity, positive predictive value, and negative
predictive value of individual investigative tools.
For the purpose of this analysis, we also
classified the results of clinical examination, mammography,
ultrasonography, and cytology as ‘test positive’ or ‘test negative’ for
underlying malignancy. Presence of a palpable breast mass (regardless of
mobility) was considered a positive result and no palpable breast mass a
negative result. For mammographic findings, microcalcifications were
considered a positive result. For ultrasonography, a detectable mass was
‘test positive’ for underlying malignancy; non-solitary dilated ducts,
cysts, and normal ultrasonogram were regarded as ‘test negative’. For
ductogram results, dilated ducts, irregularity, and the presence of ductal
filling defects were considered positive. For cytology, atypical,
suspicious, and malignant were considered ‘test positive’, and benign as
‘test negative’. This study was done in accordance with the principles
outlined in the Declaration of Helsinki.
Statistical analysis
R version 3.0.2 (the R Foundation) and the SPSS
(Windows version 14.0; SPSS Inc, Chicago [IL], United States) were used
for data analysis. To determine the differences between subgroups,
Wilcoxon rank sum test and Fisher’s exact test were used for numerical
data and categorical data, respectively. Multiple logistic regression was
performed to examine the odds ratios of the factors. Backward selection
through likelihood ratio test with removal of P value of 0.1 was conducted
for model selection. Variables in univariate analysis with a P value of
<0.1 were included in the full model. A P value of <0.05 was
considered statistically significant.
Results
Table 1 summarises the first part of our analysis.
We identified 102 patients who presented to our Breast Clinic during the
study period. They had either a tissue diagnosis or had been followed up
for longer than 2 years without tissue diagnosis. There were 31 and 71
patients in the malignant and benign subgroups, respectively.
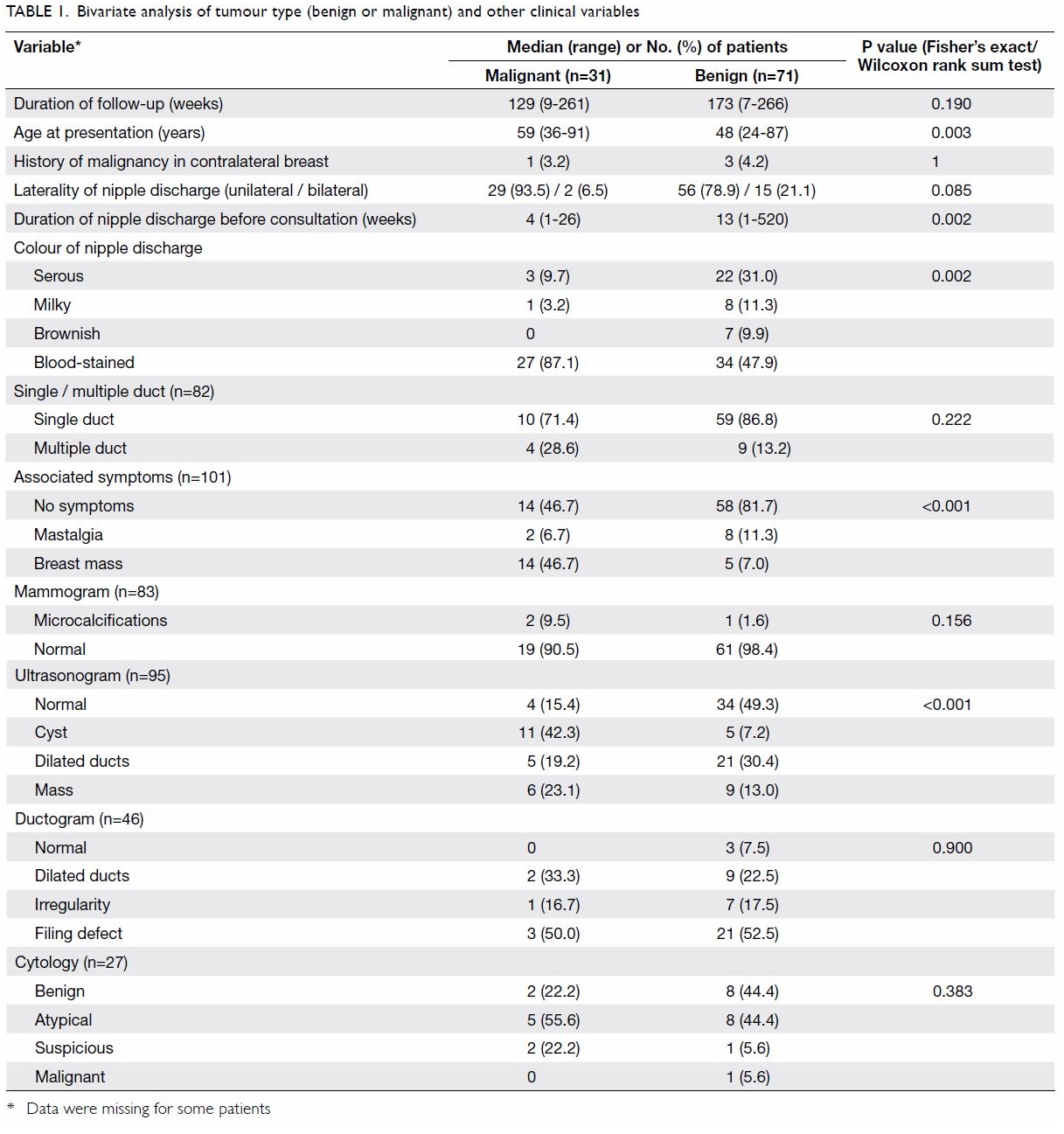
The median age at presentation of the benign
subgroup was significantly younger than that of the malignant subgroup (48
vs 59 years; P=0.003). The median interval between onset of nipple
discharge and first presentation was significantly longer in the benign
subgroup than in the malignant subgroup (13 vs 4 weeks; P=0.002).
Comparing the two subgroups, a larger proportion of
patients in the malignant subgroup presented with blood-stained discharge
(87.1% vs 47.9%; P=0.002) and had a breast mass at presentation (46.7% vs
7.0%; P<0.001). For the individual investigative modalities, with the
exception of ultrasonography, neither mammography, ductography nor
cytology showed any statistically significant difference between the
malignant and benign subgroups.
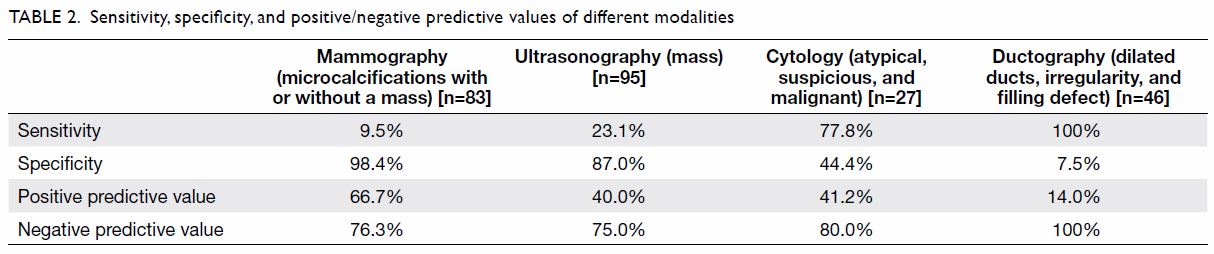
Table 2 summarises the second part of the study. We
calculated the sensitivity, specificity, and positive and negative
predictive values of mammographic, ultrasonographic, cytological, and
ductographic findings. There were 83, 95, 27, and 46 patients who
underwent mammography, ultrasonography, cytology, and ductography,
respectively. The positive and negative predictive values of cytology were
41.2% and 80.0%, respectively. Ductography had a sensitivity of 100%,
specificity of 7.5%, positive predictive value of 14.0%, and negative
predictive value of 100%.
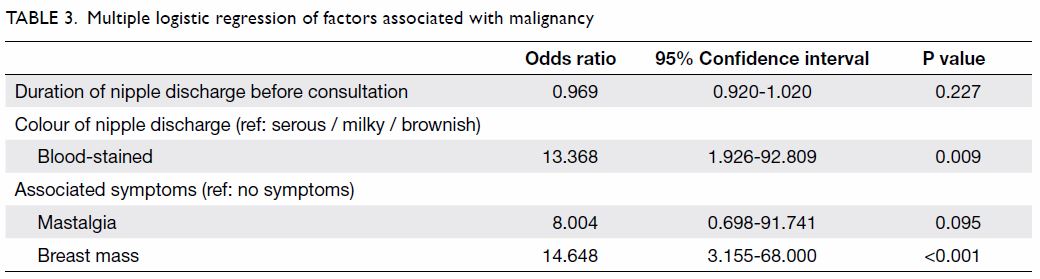
Multiple logistic regression analysis with backward
selection was performed. Covariates with a P value of <0.1 were
included in the full model (Table 1). By likelihood ratio test and removal of
variables with a P value of >0.1, duration of nipple discharge, colour
of nipple discharge, mastalgia, and associated mass remained in the final
model (Table 3).
Compared with serous, milky and brownish discharge,
patients with blood-stained discharge had a significantly higher risk for
malignancy (odds ratio=13.368; 95% confidence interval, 1.926-92.809). In
addition, compared with patients having no symptoms, those with a breast
mass had a significantly higher risk for malignancy (odds ratio=14.648;
95% confidence interval, 3.155-68.000) [Table 3].
Discussion
A methodologically ideal study of nipple discharge
would require every patient to undergo the same investigations and also
surgery for final pathology. This, however, would be unethical. For
patients who opted for non-operative management of nipple discharge, our
retrospective study considered 2-year clinical non-progression a
reasonable surrogate for benign breast pathology.
Clinical variables
Women in the malignant subgroup were significantly
older at presentation than their benign counterparts. This was in
agreement with the fact that physiological nipple discharge is more common
in younger premenopausal women. Caution should be exercised in
postmenopausal women who present with nipple discharge and the possibility
of malignancy investigated before concluding a benign pathology.
With respect to the colour of nipple discharge,
underlying benign and malignant causes had a different pattern. Benign
pathology was more likely to be associated with non–blood-stained
discharge (n=37, 52.1%), whereas malignant pathology was more likely to be
associated with blood-stained discharge (n=27, 87.1%). This is not
pathognomonic but did reach statistical significance.
The differentiation between multiple-duct and
single-duct discharge showed no association with underlying pathology.
Mammography and ultrasonography
As shown in Table 2, mammography had a higher specificity of
98.4% and positive predictive value of 66.7% but a disappointingly low
sensitivity of 9.5%. Therefore, a normal mammogram did not confidently
exclude malignancy. On the other hand, breast ultrasonography had a
specificity and negative predictive value of 87.0% and 75.0%,
respectively. Mammography was routinely offered to patients who presented
with nipple discharge. Complementary breast ultrasonography was also
arranged, especially for younger Asian women with denser breasts on
mammography.7 In our experience,
complementary ultrasonography increases the overall sensitivity and
negative predictive value compared with mammography alone.
Nipple discharge cytology
Opinion is divided on the value of cytological
examination. While some studies report a complementary diagnostic value
and recommend its routine use,8 9 others report it has little such
value and advise against its routine use.10
Of the 102 patients, 36 had demonstrable nipple
discharge at consultation with a sample collected for examination. Of
these 36 specimens, only 27 showed a sufficient number of cells to make a
cytological diagnosis. Nonetheless, we attempted to analyse its accuracy.
The sensitivity and specificity of cytological examination were 77.8% and
44.4%, respectively. Its positive predictive value was disappointingly low
at 41.2% and its negative predictive value was 80.0%. The diagnostic value
of this investigation was limited as not every patient had demonstrable
nipple discharge and not every specimen contained adequate cells for
testing. Nonetheless, this investigation is minimally invasive so was
always performed if there was demonstrable nipple discharge, although it
rarely affected the clinical decision or plan of management.
Ductography
The value of ductography is debatable. While some
studies have validated the diagnostic value of preoperative ductography in
differentiating benign and malignant pathology,11
12 others doubt its value.13 Rather than differentiating benign and malignant
pathology, we used preoperative ductogram to aid in the localisation of
non-palpable lesions.14 15 The sensitivity was 100% whereas the specificity was
low at 7.5%, with a positive predictive value of 14.0% and a negative
predictive value of 100%.
Magnetic resonance imaging
Magnetic resonance imaging was not included in our
routine evaluation of patients with nipple discharge although we
acknowledge its value in the detection of carcinoma in these patients. It
has an exceptionally high sensitivity for both invasive and in-situ
carcinoma.16 Its routine use in
patients with a breast lesion is nonetheless limited by its relatively low
specificity of 72% (95% confidence interval, 67%-77%).17 The role of magnetic resonance imaging in patients
with nipple discharge has been extensively validated,18 19 20 21
suggesting that it may detect or exclude the presence of carcinoma with a
high degree of certainty. Magnetic resonance imaging may be considered
when all other available strategies are inconclusive.
Microdochectomy
Emerging evidence suggests that neither clinical
variables nor preoperative investigations reliably distinguish benign and
malignant pathology so duct excision should be offered to every patient
with nipple discharge.22 23 24 25 26 We
offered microdochectomy to patients with no palpable breast lesion based
on two indications: clinical or radiological suspicion, or a patient’s
wish to stop nipple discharge by surgery. It is likely that offering
microdochectomy to all patients with nipple discharge would result in
overtreatment as the final pathology was benign in most cases. In
patients with negative clinical examination and negative imaging findings,
a period of watchful waiting with regular follow-up is a reasonable
alternative to surgical intervention.
The association of blood-stained discharge with
malignancy is controversial. Morrogh et al24
reported that haemorrhagic discharge did not indicate malignancy or high
risk, and non-haemorrhagic discharge did not exclude malignancy. In our
study, we showed that blood-stained discharge was associated with
malignancy but was not pathognomonic.
On the other hand, presence of an associated breast
mass was a significant finding. This may be because it is the most common
presenting symptom of breast cancer, and its incidence rises with age.
Limitations
Our study had several limitations. First, as data
collection was retrospective, there might have been inconsistent or
incomplete recording of clinical findings. Study subjects might not be
representative and some data for importable variables might have been
missing. No blinding during information extraction or coding could be
achieved as it was performed by the first author. Second, the small sample
size limited the power of our study although this could in part be due to
the relatively conservative culture and help-seeking pattern of Hong Kong
Chinese women. The unequal arm size also limited the interpretation of
statistical significance of comparisons. Third, our assumption of 2-year
clinical non-progression as benign pathology might have underestimated the
true incidence of malignancy in our group of patients. Lastly, the small
number of adequate cytology specimens limited meaningful analysis of this
investigation. As the sample taken for cytology is usually small, it will
affect the sensitivity.
Conclusions
Clinical variables including age at presentation,
duration and colour of discharge, presence of an associated breast mass,
and abnormal sonographic findings were important in suggesting the
underlying pathology of nipple discharge. Only blood-stained nipple
discharge and an associated breast mass remained in the multiple logistic
regression model and were statistically significant. In patients with
non–blood-stained nipple discharge, as well as a negative clinical breast
examination and imaging, we may infer an underlying benign pathology.
Further prospective studies with a larger sample size are advocated.
Declaration
All authors have disclosed no conflicts of
interest.
Acknowledgements
The authors would like to thank Mr Wing-pan Luk and
Mr Ling-hiu Fung, Medical Physics & Research Department, Hong Kong
Sanatorium & Hospital, Hong Kong for their statistical contribution to
this paper.
References
1. Cheung KL, Alagaratnam TT. A review of
nipple discharge in Chinese women. J R Coll Surg Edinb 1997;42:179-81.
2. Murphy IG, Dillon MF, Doherty AO, et al.
Analysis of patients with false negative mammography and symptomatic
breast carcinoma. J Surg Oncol 2007;96:457-63. Crossref
3. Vargas HI, Vargas MP, Eldrageely K,
Gonzalez KD, Khalkhali I. Outcomes of clinical and surgical assessment of
women with pathological nipple discharge. Am Surg 2006;72:124-8.
4. Murad TM, Contesso G, Mouriesse H.
Nipple discharge from the breast. Ann Surg 1982;195:259-64. Crossref
5. King TA, Carter KM, Bolton JS, Fuhrman
GM. A simple approach to nipple discharge. Am Surg 2000;66:960-6.
6. Jain A, Crawford S, Larkin A, Quinlan R,
Rahman RL. Management of nipple discharge: technology chasing application.
Breast J 2010;16:451-2. Crossref
7. Kwong A, Cheung PS, Wong AY, et al. The
acceptance and feasibility of breast cancer screening in the East. Breast
2008;17:42-50. Crossref
8. Pritt B, Pang Y, Kellogg M, St. John T,
Elhosseiny A. Diagnostic value of nipple cytology: study of 466 cases.
Cancer 2004;102:233-8. Crossref
9. Kalu ON, Chow C, Wheeler A, Kong C,
Wapnir I. The diagnostic value of nipple discharge cytology: breast
imaging complements predictive value of nipple discharge cytology. J Surg
Oncol 2012;106:381-5. Crossref
10. Kooistra BW, Wauters C, van de Ven S,
Strobbe L. The diagnostic value of nipple discharge cytology in 618
consecutive patients. Eur J Surg Oncol 2009;35:573-7. Crossref
11. Hou MF, Huang TJ, Liu GC. The
diagnostic value of galactography in patients with nipple discharge. Clin
Imaging 2001;25:75-81. Crossref
12. Hou MF, Huang CJ, Huang YS, et al.
Evaluation of galactography for nipple discharge. Clin Imaging
1998;22:89-94. Crossref
13. Dawes LG, Bowen C, Venta LA, Morrow M.
Ductography for nipple discharge: no replacement for ductal excision.
Surgery 1998;124:685-91. Crossref
14. Peters J, Thalhammer A, Jacobi V, Vogl
TJ. Galactography: an important and highly effective procedure. Eur Radiol
2003;13:1744-7. Crossref
15. Lamont JP, Dultz RP, Kuhn JA, Grant
MD, Jones RC. Galactography in patients with nipple discharge. Proc (Bayl
Univ Med Cent) 2000;13:214-6. Crossref
16. Heywang-Koebrunner SH. Diagnosis of
breast cancer with MR—review after 1250 patients. Electromedica
1993;61:43-52.
17. Peters NH, Borel Rinkes IH, Zuithoff
NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the
diagnosis of breast lesions. Radiology 2008;246:116-24. Crossref
18. Orel SG, Dougherty CS, Reynolds C,
Czerniecki BJ, Siegelman ES, Schnall MD. MR imaging in patients with
nipple discharge: initial experience. Radiology 2000;216:248-54. Crossref
19. Nakahara H, Namba K, Watanabe R, et
al. A comparison of MR imaging, galactography and ultrasonography in
patients with nipple discharge. Breast Cancer 2003;10:320-9. Crossref
20. Hirose M, Otsuki N, Hayano D, et al.
Multi-volume fusion imaging of MR ductography and MR mammography for
patients with nipple discharge. Magn Reson Med Sci 2006;5:105-12. Crossref
21. Ballesio L, Maggi C, Savelli S, et al.
Role of breast magnetic resonance imaging (MRI) in patients with
unilateral nipple discharge: preliminary study [in English, Italian].
Radiol Med 2008;113:249-64. Crossref
22. Adepoju LJ, Chun J, El-Tamer M,
Ditkoff BA, Schnabel F, Joseph KA. The value of clinical characteristics
and breast-imaging studies in predicting a histopathologic diagnosis of
cancer or high-risk lesion in patients with spontaneous nipple discharge.
Am J Surg 2005;190:644-6. Crossref
23. Lanitis S, Filippakis G, Thomas J,
Christofides T, Al Mufti R, Hadjiminas DJ. Microdochectomy for single-duct
pathologic nipple discharge and normal or benign imaging and cytology.
Breast 2008;17:309-13. Crossref
24. Morrogh M, Park A, Elkin EB, King TA.
Lessons learned from 416 cases of nipple discharge of the breast. Am J
Surg 2010;200:73-80. Crossref
25. Alcock C, Layer GT. Predicting occult
malignancy in nipple discharge. ANZ J Surg 2010;80:646-9. Crossref
26. Foulkes RE, Heard G, Boyce T, Skyrme
R, Holland PA, Gateley CA. Duct excision is still necessary to rule out
breast cancer in patients presenting with spontaneous bloodstained nipple
discharge. Int J Breast Cancer 2011;2011:495315. Crossref