Hong
Kong Med J 2017 Dec;23(6):622–34 | Epub 24 Nov 2017
DOI: 10.12809/hkmj176308
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE CME
Polycystic ovary syndrome: a common reproductive
syndrome with long-term metabolic consequences
Tiffany TL Yau, FHKCP, FHKAM (Medicine)1;
Noel YH Ng, BSc, MRes1; LP Cheung, FRCOG, FHKAM (Obstetrics and
Gynaecology)2; Ronald CW Ma, FRCP, FHKAM (Medicine)1,3
1 Department of Medicine and
Therapeutics, The Chinese University of Hong Kong, Shatin, Hong Kong
2 Department of Obstetrics and
Gynaecology, The Chinese University of Hong Kong, Prince of Wales
Hospital, Shatin, Hong Kong
3 Hong Kong Institute of Diabetes and
Obesity, The Chinese University of Hong Kong, Shatin, Hong Kong
TT Yau and NY Ng have equal contribution in this
study.
Corresponding author: Prof Ronald CW Ma (rcwma@cuhk.edu.hk)
Abstract
Polycystic ovary syndrome is the most common
endocrine disorder among women of reproductive age. Although
traditionally viewed as a reproductive disorder, there is increasing
appreciation that it is associated with significantly increased risk of
cardiometabolic disorders. Women with polycystic ovary syndrome may
present to clinicians via a variety of different routes and symptoms.
Although the impact on reproduction predominates during the reproductive
years, the increased cardiometabolic problems are likely to become more
important at later stages of the life course. Women with polycystic
ovary syndrome have an approximately 2- to 5-fold increased risk of
dysglycaemia or type 2 diabetes, and hence regular screening with oral
glucose tolerance test is warranted. Although the diagnostic criteria
for polycystic ovary syndrome are still evolving and are undergoing
revision, the diagnosis is increasingly focused on the presence of
hyperandrogenism, with the significance of polycystic ovarian morphology
in the absence of associated hyperandrogenism or anovulation remaining
uncertain. The management of women with polycystic ovary syndrome should
focus on the specific needs of the individual, and may change according
to different stages of the life course. In view of the clinical
manifestations of the condition, there is recent debate about whether
the current name is misleading, and whether the condition should be
renamed as metabolic reproductive syndrome.
Introduction
Polycystic ovary syndrome (PCOS) was first
described by Stein and Leventhal in 1935,1
when they noted an association between the presence of bilateral
polycystic ovaries and signs of amenorrhoea, oligomenorrhoea, hirsutism,
and obesity. It is now recognised as one of the most common endocrine
disorders of women, affecting between 6% and 12% of women overall.2 A study in southern Chinese reported a prevalence of
2.2% among women of reproductive age.3
The prevalence can be as high as 70% to 80% in women with oligoamenorrhoea
and 60% to 70% in women with anovulatory infertility. Women with PCOS have
an increased risk of gynaecological, reproductive, medical and sleep
problems, and hence are at risk of increased morbidities across the life
course (Fig 1).4
Despite being a common condition, however, the presenting features of PCOS
are often not recognised, resulting in a delay in diagnosis. In a recent
international survey, approximately half of the women with PCOS consulted
three or more health care providers before the diagnosis was made, and
more than one third experienced a diagnostic delay in excess of 2 years.5
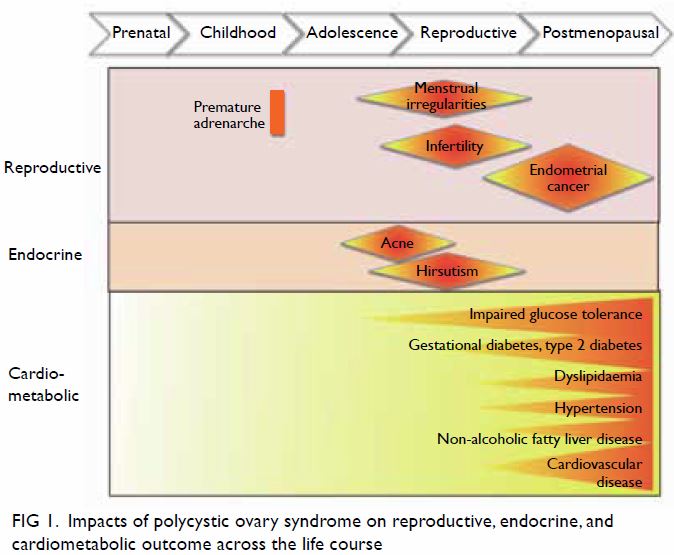
Figure 1. Impacts of polycystic ovary syndrome on reproductive, endocrine, and cardiometabolic outcome across the life course
Diagnosis of polycystic ovary syndrome: historical
aspects and evolution of diagnostic criteria
Polycystic ovary syndrome is considered to be a
heterogeneous disorder with multifactorial cause. The principal features
of PCOS include hyperandrogenism, oligomenorrhoea, and/or polycystic
ovaries. There have been several proposed diagnostic criteria for PCOS as
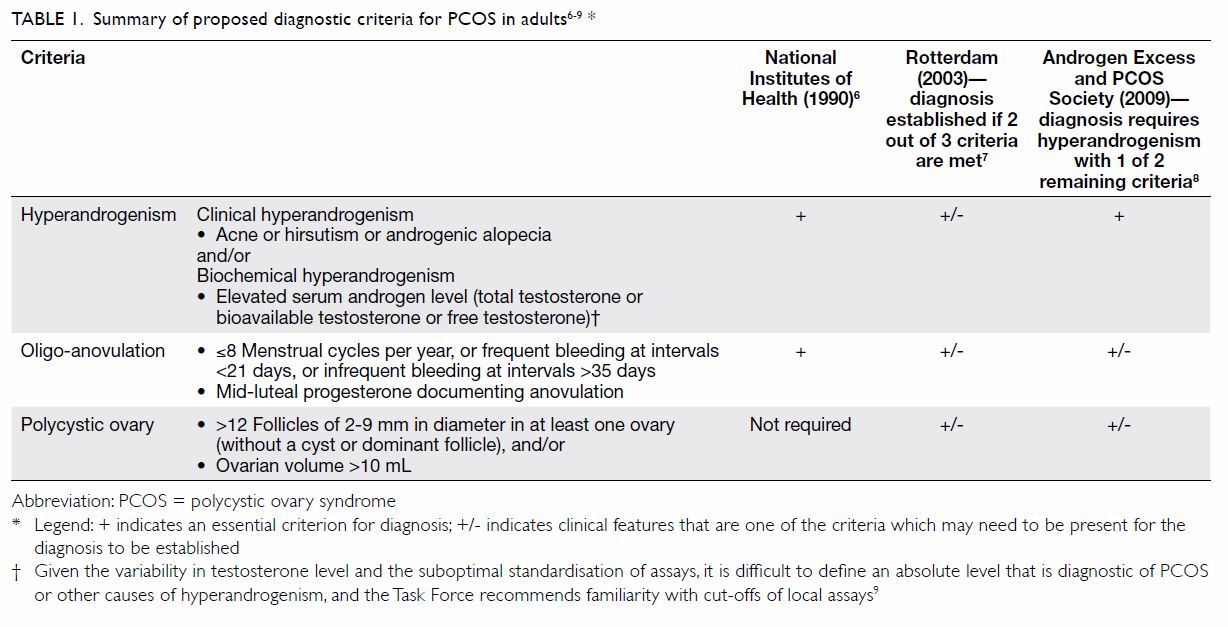
described in Table 1.6 7 8
9 All criteria require exclusion of
other disorders that may mimic the clinical features of PCOS, such as
thyroid dysfunction, hyperprolactinaemia, non-classic congenital adrenal
hyperplasia (CAH), and Cushing’s syndrome.
The prevalence of PCOS depends on the diagnostic
criteria used to define the disorder. To date, the prevalence of PCOS has
been determined primarily using the National Institutes of Health 1990
criteria. A summary report from the National Institutes of Health
Evidence-based Methodology Workshop on PCOS in December 2012 concluded
that the Rotterdam criteria should be adopted for now because it is the
most inclusive.10 Using the
Rotterdam criteria, many patients can be diagnosed based on the history
and physical examination (eg a history of irregular menses, and clinical
signs of hyperandrogenism). The panel also suggested that the disorder
should be renamed to more adequately reflect the complex metabolic,
hypothalamic, pituitary, ovarian, and adrenal interactions that
characterise the syndrome. A previous local study by Lam et al11 comparing the different diagnostic criteria in Hong
Kong Chinese women concluded that the Rotterdam criteria are generally
applicable to our population. Nevertheless, recent discussion has centred
on the importance of hyperandrogenism, and emerging evidence suggests that
women with radiological evidence of polycystic ovaries, but no other
clinical features of PCOS, represent a population generally of lower risk
who are distinct from other women with PCOS who fulfil current diagnostic
criteria.12
Pathogenesis
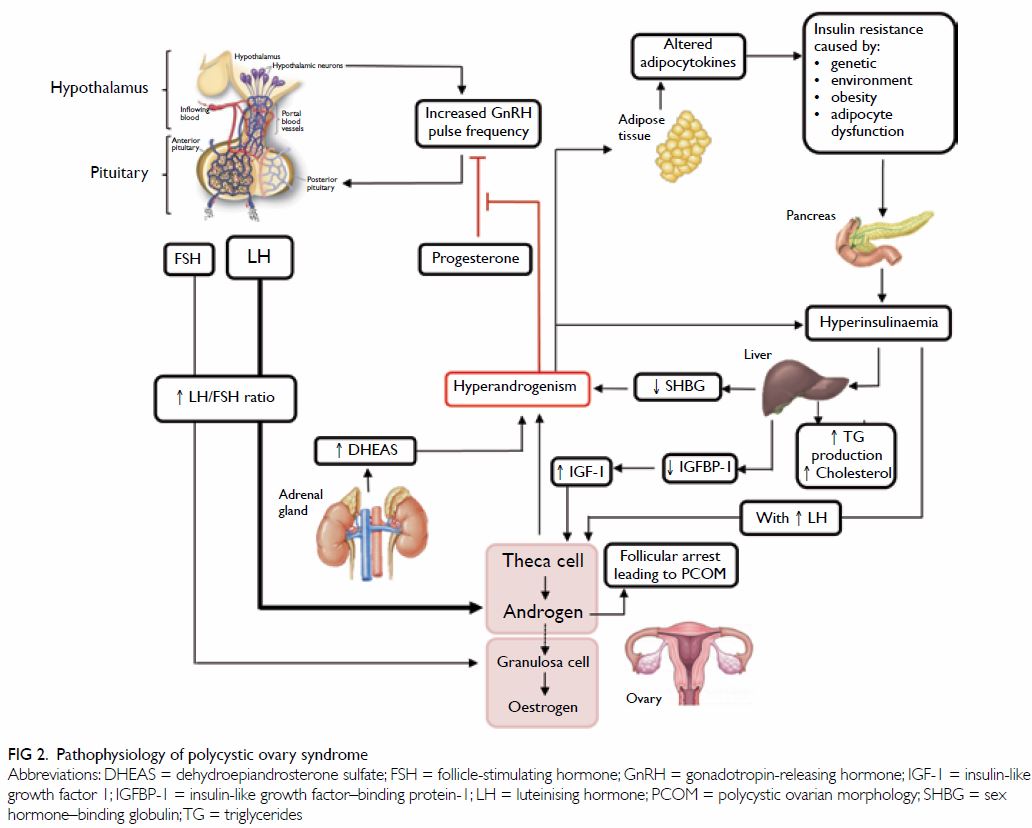
To date, the pathophysiology of PCOS remains
unclear; yet, substantial evidence suggests it is a multifactorial
condition, where interactions between endocrine, metabolic, genetic, and
environmental factors intrinsic to each other act in consonance towards a
common result (Fig 2).13 14 Also, the heterogeneity of PCOS
further reinforces its multifactorial nature. Although familial
segregation of cases suggests a genetic component in this syndrome, most
of the susceptibility genes and single-nucleotide polymorphisms remain to
be discovered.15 16 Among its diverse phenotypes, hyperandrogenism and
ovarian dysfunction are recognised as the two main features of PCOS.6 16
Hyperandrogenism in PCOS is recognised as the excessive androgen
biosynthesis, use, and metabolism. When the ovaries are stimulated to
produce excessive amounts of androgen, an accumulation of numerous
follicles or cysts can be observed in the ovary. Insulin resistance is
also a major cause of hyperandrogenism in PCOS, through stimulating the
secretion of ovarian androgen and inhibiting hepatic sex hormone–binding
globulin (SHBG) production.17
Approximately 80% to 85% of women with clinical hyperandrogenism have
PCOS.8 18
Women with PCOS and hyperandrogenism may experience excess hair growth,
acne, and/or abnormal folliculogenesis. Three major pathophysiological
pathways have been described, but they are not mutually exclusive. They
are ovulatory dysfunction, disordered gonadotropin release, and insulin
resistance.
Disordered gonadotropin release and excess androgen
release
In PCOS, hypersecretion of luteinising hormone (LH)
can lead to an increase in androgen production by the ovarian thecal
cells. This is thought to be due to increased gonadotropin-releasing
hormone (GnRH) pulse frequency, resulting in increased frequency and
pulsatile secretion of LH, and increased levels of LH relative to
follicle-stimulating hormone (FSH) in the circulation.14 19 20 There also appears to be resistance to the negative
feedback by progesterone to the GnRH pulse generator, which is often
present by puberty. The increased LH/FSH ratio, along with some ovarian
resistance to FSH, results in excess production of androgens from thecal
cells in ovarian follicles, leading to impaired follicular development,
and reduced inhibition of the GnRH pulse generator by progesterone,
thereby setting up a vicious cycle that exacerbates the hypersecretion of
LH, and ovulatory dysfunction.14 19 20
Ovulatory dysfunction
Unlike the ovarian follicular development in
healthy women, in PCOS cases, follicle growth is disrupted due to ovarian
hyperandrogenism, hyperinsulinaemia from insulin resistance, and
intra-ovarian paracrine signalling. Hyperinsulinaemia further impairs
follicle growth by amplifying LH-stimulated and insulin-like growth factor
1 (IGF-1)–stimulated androgen production.21
22 23
24 Hyperinsulinaemia also elevates
serum free testosterone levels through decreased hepatic SHBG production,
and enhances serum IGF-1 bioactivity through suppression of IGF-binding
protein production.25 Insulin
excess also promotes premature follicle luteinisation through enhanced
FSH-induced granulosa cell differentiation, which arrests granulosa cell
proliferation and subsequent follicle growth.26
Finally, overproduction of anti-Müllerian hormone (AMH)27 28 29 by the granulosa cells of ovarian follicles in PCOS
appears to antagonise FSH action in small PCOS follicles.30 The relatively lower FSH levels contribute to
arrested follicular development in the ovary, leading to amenorrhoea,
anovulation, and polycystic morphology.8
16
Insulin resistance
In PCOS cases, there is an increased level of
bioavailable androgens that leads to increased insulin resistance in
peripheral tissues (mostly in the skeletal muscle).31 Insulin resistance causes compensatory
hyperinsulinaemia and might contribute to hyperandrogenism and
gonadotropin aberrations through several mechanisms. Insulin may act
directly in the hypothalamus, the pituitary or both and thereby contribute
to abnormal gonadotropin levels. By facilitating the stimulatory role of
LH, hyperinsulinaemia leads to further increase in ovarian androgen
production in theca calls.32 High
insulin can also serve as a co-factor to stimulate adrenocorticotropic
hormone–mediated androgen production in the adrenal glands.33 Moreover, an insulin-induced decrease in the
production of SHBG in the liver increases the amount of free bioavailable
androgens.34
Most women with PCOS, particularly those who are
overweight or obese, do in fact have insulin resistance and compensatory
hyperinsulinaemia,35 36 partly attributable to an intrinsic insulin
resistance mechanism.36 37 38
Using the homeostasis model assessment, 50% to 70%
of women with PCOS demonstrate insulin resistance. Using the gold standard
technique of euglycaemic hyperinsulinaemic clamp, it was found that PCOS
exhibits insulin resistance that is independent of obesity, and is present
even among lean patients with PCOS, but this is further exacerbated in the
presence of obesity.39 40
A stepwise increase in the prevalence of glucose
intolerance with increasing body mass index (BMI) has been described in
cross-sectional studies performed in women with this disorder.41 Although most women with PCOS have normal insulin
secretory responsiveness, studies have suggested that PCOS women,
particularly those with a family history of type 2 diabetes (T2D), have
impaired β-cell function or a subnormal disposition index (an index of
β-cell function that takes insulin resistance into account).16 42 43
Adipose dysfunction
Although the full molecular mechanisms underlying
insulin resistance in PCOS remain unclear, primary defects in
insulin-medicated glucose transport,44
GLUT4 production,45 and insulin or
adrenergic regulated lipolysis46
in adipocytes (and sometimes in myocytes and fibroblasts) have been
reported. Insulin resistance in PCOS contributes to the dysfunctional
adipogenesis to some degree from an impaired capacity of regional adipose
tissue storage to properly expand with increased dietary caloric intake.47 48
49 Adipose tissue secretes
numerous factors to regulate metabolic function, appetite, neural
activity, and digestion. This tissue is also heavily infiltrated by
macrophages, and a crosstalk exists between adipocytes, macrophages, and
pluripotent cells for complex paracrine interactions. It is known that
dysregulation of adipokine production, such as adiponectin, by
macrophage-secreted cytokines in PCOS facilitates the development of
insulin resistance.50 Other
adipokines including leptin, retinol-binding protein 4, and visfatin have
also been implicated.51 Improved
understanding of the underlying mechanisms that govern adipose tissue
dysfunction and insulin resistance in PCOS would be beneficial in the
identification of novel therapeutic targets for PCOS and other related
disorders.16
Intrauterine environment
In humans, rhesus monkeys and sheep, inappropriate
testosterone exposure during fetal life alters the developmental
trajectory of the female leading to PCOS-like phenotypes, such as
phenotypic masculinisation; reproductive, neuroendocrine, ovarian
disruptions; and hyperinsulinaemia.52
In a human study, it has been shown that there is an increased prevalence
of PCOS in women with classic CAH and congenital adrenal virilising
tumours.53 In one human study,
higher testosterone levels compared with those usually observed in normal
females were found in the umbilical vein of female infants born to mothers
with PCOS54; yet, another
prospective study that investigated the relationship between prenatal
androgen exposure and the development of PCOS in female adolescence did
not confirm any association between these variables.55
Excess fetal exposure to maternal androgens is
thought to contribute to induction of the PCOS phenotype in
offspring/children. Nonetheless, more clinical studies are needed to
confirm the role of intrauterine androgen exposure on human fetal
development.
Recent insights from genetic studies
Polycystic ovary syndrome has a high heritability
of approximately 80%. Although a large number of candidate gene studies
have been conducted, no genetic variants have been found to be
consistently associated with PCOS. Recent hypothesis-free genome-wide
association studies using high-density genotyping arrays that
systematically investigate common variants across the genome have
identified several genetic loci to be significantly associated with PCOS (Table 2).56
These have shed light on the important role of the gonadotropin axis in
the pathogenesis of PCOS, as well as several other novel pathways,
including epidermal growth factor signalling. Interestingly, genetic
studies have revealed a significant overlap of findings when different
diagnostic criteria of PCOS have been applied, highlighting greater
homogeneity than previously appreciated.16
56 57
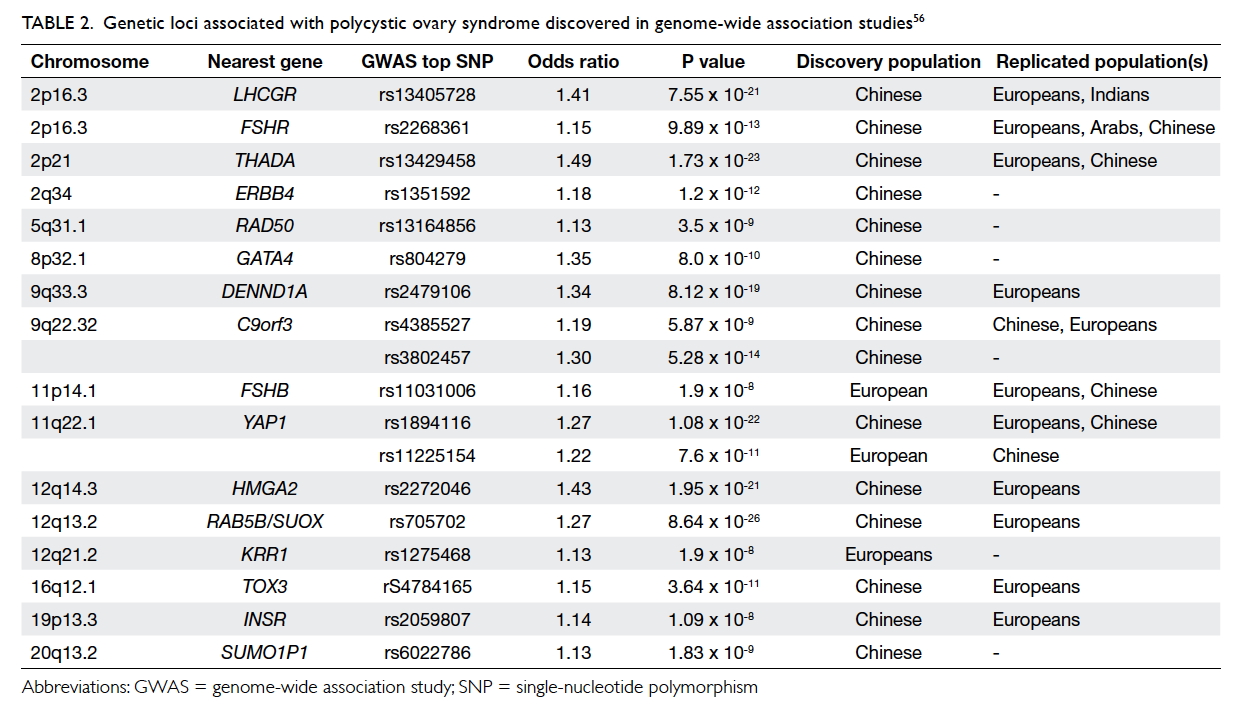
Table 2. Genetic loci associated with polycystic ovary syndrome discovered in genome-wide association studies
Clinical features and co-morbidities
Gynaecological and reproductive dysfunction
Menstrual dysfunction is common and is
characterised by oligomenorrhoea and, less often, amenorrhoea.
Nonetheless, menstrual problems are frequently neglected and anovulatory
infertility is frequently the initial complaint for which the patient
seeks medical advice. Women with PCOS have an increased risk of
miscarriage, gestational diabetes, pre-eclampsia, and preterm labour.58 59 60 A meta-analysis highlighted that the risks of
gestational diabetes, pregnancy-induced hypertension, and pre-eclampsia
are approximately 3-fold, whereas the risk for preterm labour is
approximately 2-fold among women with PCOS.58
The reasons for the adverse pregnancy outcomes are unclear, but
hypersecretion of LH, hyperandrogenaemia and hyperinsulinaemia have all
been postulated. Due to anovulatory dysfunction and consequent long-term
unopposed oestrogen stimulation, PCOS patients are at increased risk of
endometrial cancer.61 62 Nonetheless, there is currently no consensus to
support routine biopsy or ultrasound of the endometrium for endometrial
hyperplasia or cancer screening in asymptomatic women due its poor
diagnostic accuracy.63
Endocrine dysfunction
Women with PCOS have varying degrees and
manifestations of androgen excess. Clinical signs of hyperandrogenism
include acne, hirsutism, male-pattern hair loss, and/or elevated serum
androgen concentrations. Hirsutism is the most common symptom of
hyperandrogenism, affecting up to 70% of women with PCOS. It is commonly
noted on the upper lip, chin, periareolar area, in the mid-sternum, and
along the linea alba of the lower abdomen. There is substantial ethnic
variation in hirsutism where Asian women with PCOS have a lesser degree of
hirsutism.8 Signs of more severe
androgen excess—such as deepening of the voice, breast atrophy, and
clitoromegaly—occur rarely and suggest the possibility of ovarian
hyperthecosis or an androgen-secreting tumour.
Metabolic dysfunction and cardiovascular risks
Polycystic ovary syndrome is associated with
cardiovascular risk factors, including obesity, hypertension, glucose
intolerance, dyslipidaemia, and obstructive sleep apnoea.64 The high prevalence of metabolic disturbances and the
consequent increase in the long-term risk of T2D indicate that PCOS should
be considered a general health problem rather than just a reproductive
syndrome. Most investigators found that at least one half of PCOS women
are obese.8 The prevalence of
obesity in PCOS varies widely with the population studied, similar to the
wide variability in prevalence of obesity in the general population.
Insulin resistance occurs in 60% to 80% of women
with PCOS, and 95% of obese women with PCOS. The risk of T2D is increased
in PCOS, particularly in women with a first-degree relative with T2D.65 In a systematic review, it was estimated that the
prevalence of impaired glucose tolerance and T2D was as high as 31% to
35%, and 7.5% to 20%, respectively, in women with PCOS by their fourth
decade, and the risks were significantly higher at all ages and all
weights even in young or lean subjects with PCOS.66
67 In Hong Kong, the prevalence of
T2D under 35 years old is 0.6% in the general population, but 7.5% in
women with PCOS.68
Dyslipidaemia is the most common metabolic
abnormality in PCOS. Most studies of women with PCOS have demonstrated low
high-density lipoprotein cholesterol and high triglyceride concentrations,
consistent with their insulin resistance, as well as an increase in
low-density lipoprotein cholesterol.69
70 71
Metabolic syndrome, characterised by a cluster of
cardiometabolic risk factors associated with insulin resistance, is a
disease with a large health impact as it confers a 5-fold increase in risk
of T2D and a 2-fold increase in risk of cardiovascular diseases.72 A cross-sectional study evaluated the cardiometabolic
risk factors in 295 Hong Kong Chinese women with PCOS with a mean age of
30 years.68 It found that the
prevalence of metabolic syndrome in this cohort was 24.9% despite their
relatively young age, a 5-fold increase in risk compared with women
without PCOS even after controlling for age and BMI.68 In another study involving 170 Asian women with PCOS,
metabolic syndrome as defined according to the International Diabetes
Federation criteria was present in 35.3% of the subjects.73
The prevalence of non-alcoholic fatty liver disease
(including non-alcoholic steatohepatitis), and obstructive sleep apnoea is
also increased in women with PCOS. Even after controlling for BMI, women
with PCOS are still 30 times more likely to have sleep-disordered
breathing and 9 times more likely than controls to have daytime
sleepiness.74 75
The presence of obesity, insulin resistance,
impaired glucose tolerance (or T2D), and dyslipidaemia may predispose
women with PCOS to coronary heart disease. An excess risk of coronary
heart disease or stroke in women with PCOS, however, is not well
established due to the lack of long-term prospective studies. Available
studies are mostly too small to detect differences in event rates, and
none have shown an evident increase in cardiovascular events.76 77 78 79
Therefore, the focus has been on risk factors of cardiovascular disease
although these may not necessarily equate with events or mortality.
Studies have found that women with PCOS have an increased carotid intima
media thickness and coronary artery calcification, the two major surrogate
markers for atherosclerotic cardiovascular disease.80 81 82 Serum concentrations of C-reactive protein, a
biochemical predictor of cardiovascular disease, also appear to be
commonly elevated in women with PCOS.83
Patient evaluation
Clinical features
The history-taking should include detailed inquiry
about growth and sexual development, menstrual pattern, reproductive
history, medical and drug history, symptoms of androgen excess,
co-existing cardiovascular risk factors such as tobacco and alcohol use,
and family history. Drug history is important as a history or current use
of sodium valproate has been shown to be associated with PCOS.
During the physical examination, it is essential to
search for signs of androgen excess (hirsutism, acne, androgenic alopecia)
and insulin resistance (acanthosis nigricans). Modified Ferriman-Gallwey
scoring is the method generally used to evaluate clinical hirsutism, but
is affected by subjective variability and cosmetic treatments. It has been
suggested that in East Asian patients, a lower cut-off of the modified
Ferriman-Gallwey score (of 3) should be used instead of the usual cut-off
of 8.16 19
As cosmetic hair removal is common in many Asian countries, evaluation of
hirsutism should always include enquiry about any previous hair-removal
procedures. Assessment of blood pressure, BMI, and waist circumference is
also essential. Features of virilisation, Cushing’s syndrome, and thyroid
dysfunction should also be looked for and excluded.19
Biochemical features
Laboratory measurements should include tests to
achieve the diagnosis, exclude other endocrine problems, and evaluate
cardiovascular risk factors. In someone with clinical signs of
hyperandrogenism, one could argue that biochemical testing is not
necessary according to current diagnostic criteria. Most expert groups,
however, suggest measuring total testosterone concentration in women who
present with hirsutism. Women with PCOS mostly have high-normal or
borderline elevated levels of testosterone.
Elevated total testosterone is the most direct
evidence for androgen excess, but it is important to note that most assays
are relatively inaccurate at the lower levels present in females, and use
of mass spectrometry–based assays of total testosterone are more accurate
and preferred.9 Measurement of free
testosterone is a more sensitive test, but commercially available free
testosterone assays are often unreliable. The free testosterone index,
calculated by total testosterone divided by SHBG, is considered more
reliable but is not routinely performed due to the high cost of measuring
SHBG.9 Serum LH and FSH levels
should be measured at the early follicular phase of the menstrual cycle.
Ovulatory assessment such as mid-luteal progesterone measurement is
sometimes required in patients seeking infertility treatment. In rare
instances where there are rapidly progressive features of
hyperandrogenism, virilising symptoms, or markedly elevated androgen
levels (such as a serum testosterone >5 nmol/L), additional
investigations to exclude an androgen-secreting tumour may be indicated,
including checking cortisol, dehydroepiandrosterone sulfate, and imaging
of the adrenal glands and ovaries. As mentioned earlier, AMH is implicated
in the pathogenesis of PCOS, and recent studies have highlighted its
potential utility in the diagnosis of women with PCOS,16 although no diagnostic cut-off value has been defined
yet due to the heterogeneity between the different AMH assay methods.
Blood tests to exclude other endocrine problems
include thyroid function tests, prolactin, or tests to exclude other
underlying causes of excess androgens, including 17-hydroxyprogesterone to
exclude late-onset CAH and the 1-mg overnight dexamethasone suppression
test to exclude Cushing’s syndrome. It is sometimes noted that women with
PCOS have mildly elevated prolactin.20
If the level of 17-hydroxyprogesterone is borderline elevated, a short
synacthen test with measurement of 17-hydroxyprogesterone may be indicated
to exclude late-onset CAH.19
Assessment of cardiovascular risk factors includes
an oral glucose tolerance test (OGTT) and fasting lipid profiles. Fasting
glucose, although more convenient, has been shown to underestimate
diabetes prevalence and cardiovascular risk when compared with OGTT,
particularly in obese subjects. Measuring fasting glucose alone is
therefore inadequate for the assessment of dysglycaemia in women with
PCOS.84 Patients with normal
glucose tolerance should be re-screened at least once every 2 years, or
more frequently if additional risk factors are identified. Patients with
impaired glucose tolerance should be screened annually for development of
T2D.
Ultrasound features
The use of ultrasound in the diagnosis of PCOS must
be tempered by an awareness of the broad spectrum of women with
ultrasonographic findings characteristic of polycystic ovaries.85 If the patient has both oligo-ovulation and
hyperandrogenism, a transvaginal ultrasound to document polycystic ovaries
is not necessary according to the Rotterdam criteria. In women who are
ready to conceive, ultrasound can be used to monitor and document
ovulation.
The ultrasound criteria in the diagnosis of PCOS
have evolved since the first ultrasound description of polycystic ovaries
in 1986.8 The Rotterdam criteria
described polycystic ovaries as the presence of ≥12 follicles in each
ovary measuring 2 to 9 mm in diameter and/or increased ovarian volume of
>10 mL. One ovary fulfilling this definition is sufficient to define
polycystic ovaries. More recently, it has been proposed that if newer
technology such as ultrasound machines with transducer frequency of ≥8 MHz
are available, then raising the follicle number per ovary to 25 for
diagnosing PCOS would be more specific.86
Ultrasonography is operator-dependent and requires
expertise. Transvaginal ultrasound is the method of choice, but is
practically difficult in patients without previous sexual experience.
Transrectal ultrasound examination is an alternative in women where
transvaginal scan is not possible. Transabdominal ultrasound has poorer
resolution, especially in obese subjects. Recent research suggests that
ultrasound might be useful to supplement the diagnosis in the event of
ovulatory disturbance without hyperandrogenism.12
86 87
Treatment approach
The management of women with polycystic ovary
varies according to the main symptoms and primary problem experienced by
the patient. The particular needs of the patient may change according to
different stages of the life course, from adolescence through to
reproductive age.88 Hence
management should involve a multidisciplinary approach involving
paediatricians, gynaecologists, endocrinologists, family physicians,
dietitians, clinical psychologists, and surgeons, as appropriate.84
Management of menstrual irregularity
Menstrual irregularity is one of the most common
presenting symptoms of patients with PCOS, and often reflects underlying
ovarian dysfunction and anovulation. Chronic anovulation and secondary
amenorrhoea can be associated with endometrial hyperplasia and increased
risk of endometrial carcinoma, along with other complications associated
with amenorrhoea including osteoporosis. Overweight women with PCOS should
be encouraged to lose weight; as low as a 5% reduction in body weight is
associated with improvement in amenorrhoea.88
89 90
Previous studies have highlighted that a lifestyle modification programme
is associated with improvement in menses, hirsutism, biochemical
hyperandrogenism, and insulin resistance.90
91
Progestagens can be administered both as a
diagnostic test to induce progesterone withdrawal, as well as to treat
amenorrhoea. Cyclical progestagens, preferably given 12 to 14 days per
month, can be used to ensure regular withdrawal bleeding to avoid
endometrial hyperplasia, and are associated with less-adverse
cardiometabolic effects than combined oestradiol-progestagen pills.
Periodic short courses of progestogen (2-3 monthly) are an alternative
option.
The use of the combined oral contraceptive (COC)
pill, with its beneficial effects on suppressing excess androgen and its
manifestations, has been a commonly used and convenient treatment for
amenorrhoea, with the added benefit of providing contraception. In women
with PCOS, COC formulations containing less androgenic progestagens are
preferred. Nonetheless, there has been some debate about whether the use
of COC may cause exacerbation of cardiometabolic risk.92 Contra-indications to use of a COC include heavy
smokers aged ≥35 years, those with hypertension or established cardiac
disease, and those with multiple cardiovascular risk factors.93 Nevertheless, current recommendations suggest that
this is a useful alternative, although clinicians should monitor for
changes in body weight, blood pressure, lipid profile as well as
dysglycaemia if patients are prescribed COC, especially if the patient is
overweight.
Management of hyperandrogenism
As highlighted earlier, administration of an
oestrogen-containing oral contraceptive has beneficial effects on
hyperandrogenism. Furthermore, the oral contraceptive pill containing the
anti-androgenic progestagen cyproterone acetate, administered in cyclical
doses, or drospirenone-containing COC might be beneficial for hirsutism.16 The use of
cyproterone-containing pills to alleviate hyperandrogenic symptoms should
ideally be limited to short-term use and discontinued 3 to 4 months after
symptom resolution due to higher thromboembolic risk than the first-line
COC pills.94 Other anti-androgens, such as finasteride, are sometimes used
in severe cases of hirsutism, although again patients need to ensure they
avoid conceiving whilst on anti-androgenic drugs.
In most circumstances, women may elect to use
cosmetic measures to treat the clinical manifestations of
hyperandrogenism. Different cosmetic approaches for hair removal—including
shaving, waxing, and electrolysis—have variable efficacy and duration of
effects. Laser therapy in the form of photoepilation represents a more
permanent solution but is also more costly.4
Other options for treatment of hirsutism due to hyperandrogenism include
use of topical eflornithine that may help reduce excess facial hair.
Management of anovulatory infertility
The presence of anovulatory infertility can be
investigated by measurement of progesterone in the mid-luteal phase of the
menstrual cycle (eg day 21 of a 28-day cycle, or day 28 of a 35-day
cycle), and can help to establish the presence of anovulation. The
monitoring of basal body temperature to confirm ovulation does not predict
ovulation reliably, and is no longer recommended.95
In patients with anovulatory infertility, clomiphene treatment is usually
considered the first-line treatment. Clomiphene should be started at a low
dose (eg 50 mg daily for 5 days per cycle) and gradually increased until
the lowest effective dose that achieves ovulation is reached, but this
requires close monitoring, especially for the potential side-effects of
multiple pregnancy and ovarian hyperstimulation. Treatment can be repeated
if unsuccessful, but the majority of patients who respond usually do so
within the first three cycles.4 96 The highest recommended dose for
clomiphene is 150 mg, and if the woman still does not respond, second-line
treatment should be considered.
Metformin has beneficial effects on anovulation. In
a systematic review and meta-analysis, metformin was found to be
associated with increased success at inducing ovulation.97 Doses vary in clinical trials from 1 g daily to
higher doses. In a multicentre randomised controlled trial, therapy-naїve
PCOS women who received metformin had a significantly lower live birth
rate than women who conceived through clomiphene alone, or were treated
with a combination of clomiphene and metformin.98
The use of metformin as a co-treatment with clomiphene has been shown to
improve ovulation in women with clomiphene-resistant PCOS.99 Metformin is in general stopped after successful
conception, although some advocate continued use during the first
trimester to reduce the risk of spontaneous miscarriage. This is still an
area of controversy, and the pros and cons of continuing metformin should
be carefully discussed. Metformin is known to cross the placenta but it
has also been shown to be a useful treatment for gestational diabetes.
Aromatase inhibitors reduce circulating oestrogen
levels, lead to a rise in pituitary FSH, and have previously shown
beneficial effects in a meta-analysis. Letrozole, an aromatase inhibitor,
was found to be superior to clomiphene in achieving live births in a
randomised clinical trial.100
Daily injections of exogenous gonadotropins,
including recombinant FSH or menopausal gonadotropin, have been found to
improve ovulation induction among women who did not respond to other
treatments. This treatment requires careful monitoring, and should be used
with a ‘chronic low-dose step-up’ approach as outlined by the ESHRE/ASRM
to avoid multiple pregnancies or ovarian hyperstimulation syndrome.96 101
Ovarian surgery/drilling/laparoscopic ovarian diathermy
Surgical procedures such as ovarian wedge resection
or ovarian drilling by diathermy or laser lead to a decreased number of
antral follicles, reduced ovarian androgen production, and improved
ovulation.16 Ovarian wedge
resection is no longer performed due to the higher extent of adhesion
formation and ovarian tissue damage. Ovarian drilling has been used as an
alternative to exogenous gonadotropins for treatment of anovulatory
infertility, with similar success rates. The main limitations include the
potential for formation of adhesions, and reduced ovarian reserve. In a
retrospective analysis of Chinese women with PCOS treated by laser
diathermy, spontaneous ovulation rates and cumulative pregnancy rates were
similar regardless of the presence or absence of metabolic syndrome.102
Assisted reproductive procedures
In women who fail second-line treatment such as
metformin, ovarian drilling or ovarian stimulation with gonadotropins,
third-line treatment such as intrauterine insemination or in-vitro
fertilisation can be considered.17
Management of cardiometabolic risk
Women with PCOS are at substantially increased
cardiometabolic risk, and therefore should undergo periodic evaluation of
associated risk factors.4 Overweight women with PCOS should undergo
comprehensive evaluation by a dietitian, and be encouraged to lose weight.
Weight loss of approximately 5% is already associated with improved
metabolic parameters as well as reproductive outcome. Even among women
with normal BMI, those with PCOS appear to have increased visceral
adiposity that contributes to the endogenous insulin resistance, and is
correlated with metabolic parameters, fatty liver as well as carotid
intimal-medial thickness.103
In addition to lifestyle measures, women should be
screened for glucose intolerance by an OGTT. Screening using fasting
glucose alone is inadequate in this high-risk population.4 Presence of impaired glucose tolerance may warrant
treatment with metformin given the multiple metabolic and reproductive
benefits, regardless of whether there is clinical evidence of insulin
resistance. Overt diabetes should be treated using an appropriate
combination of dietary treatment, metformin, other oral glucose-lowering
agents, and in some cases, insulin. The choice of agent should depend on
the underlying pathophysiology (eg whether obesity is present), but also
take into account the fertility wishes and plans of the patient. Metformin
in combination with lifestyle intervention has been found to be associated
with greater reduction in BMI compared with lifestyle intervention alone.104 Several studies have
demonstrated the efficacy of thiazolidinediones in improving metabolic
parameters as well as menses and hyperandrogenism in women with PCOS. Due
to possible adverse effects, however, this class of agent is currently not
recommended for treatment of insulin resistance among women with PCOS.4
Treatment of hypertension likewise should take into
account the fertility wishes of the patient. Screening for other secondary
causes of young-onset hypertension may be necessary, especially if
atypical features such as proteinuria are present. Preferred
anti-hypertensive agents in women contemplating pregnancy would be the
older agents such as methyldopa. It is notable that women with
pre-existing hypertension are more likely to develop hypertension-related
complications during pregnancy, and therefore require more strict
surveillance during pregnancy. Hyperlipidaemia can be managed using
dietary measures, and in some cases, lipid-lowering agents such as HMG CoA
(3-hydroxy-3-methylglutaryl coenzyme A) reductase inhibitors. If there are
plans for pregnancy, drug treatment with lipid-lowering treatment should
be withheld.
Psychological distress, anxiety, and depression are
common among women with PCOS, and may be linked to some of the skin
complications such as hirsutism and acne or presence of menstrual and
fertility problems; all these impact on psychological well-being.
Clinicians need to have a high level of awareness and screen for these
symptoms when appropriate and offer the necessary referrals for
psychological support. Sleep-disordered breathing including obstructive
sleep apnoea is also common, impacts sleep quality, and can exacerbate
both mood problems as well as cardiometabolic risk. It should be screened
for and managed accordingly. In those with marked obesity, bariatric
surgery is an option to address obesity and associated metabolic
abnormalities. Interestingly, a systematic review including 13 primary
studies found that the incidence of PCOS was reduced from 45.6% to 7.1%
after bariatric surgery.105
The screening and management of metabolic
abnormalities is particularly relevant in those women with PCOS who are
planning a pregnancy or undergoing fertility treatment. Women with PCOS
are at increased risk of different complications including gestational
diabetes and pre-eclampsia. Undiagnosed gestational diabetes/maternal
hyperglycaemia or poorly controlled blood pressure all contribute to
poorer pregnancy outcome among women with PCOS. Optimal management before
pregnancy and intrapartum can help to minimise the risk of these pregnancy
complications.
Conclusions
Polycystic ovary syndrome is a multi-faceted
syndrome that is becoming increasingly recognised, and is an important
contributor to multiple medical and reproductive problems. As illustrated
in this review, given the multiple reproductive and metabolic
complications associated with PCOS, patients may seek medical attention
via a variety of different channels, and may present to clinicians through
different disciplines. Clinicians therefore need to recognise the
multi-faceted nature of this complex disorder and be aware of the
associated complications. Diagnostic criteria are still evolving, although
currently the Rotterdam criteria remain the most widely accepted. Given
the burden of metabolic complications associated with the disorder, there
has been much recent discussion regarding the potential need to rename the
syndrome to better highlight its metabolic consequences, in addition to
the known reproductive features. The long-term risks of the different
complications are still not clearly defined, given the scarcity of
well-conducted prospective studies. These limitations in our current
knowledge highlight the need to follow-up this group of high-risk women.
Acknowledgement
RCW Ma acknowledges support from the Research
Grants Council General Research Fund (Ref. 14110415).
Declaration
All authors have disclosed no conflicts of
interest.
References
1. Stein IF, Leventhal ML. Amenorrhea
associated with bilateral polycystic ovaries. Am J Obstet Gynecol
1935;29:181-91. Crossref
2. March WA, Moore VM, Willson KJ, Phillips
DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a
community sample assessed under contrasting diagnostic criteria. Hum
Reprod 2010;25:544-51. Crossref
3. Chen X, Yang D, Mo Y, Li L, Chen Y,
Huang Y. Prevalence of polycystic ovary syndrome in unselected women from
southern China. Eur J Obstet Gynecol Reprod Biol 2008;139:59-64. Crossref
4. Legro RS, Arslanian SA, Ehrmann DA, et
al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine
Society clinical practice guideline. J Clin Endocrinol Metab
2013;98:4565-92. Crossref
5. Gibson-Helm M, Teede H, Dunaif A, Dokras
A. Delayed diagnosis and a lack of information associated with
dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol
Metab 2017;102:604-12.
6. Zawadski J, Dunaif A. Diagnostic
criteria for polycystic ovary syndrome: towards a rational approach. In:
Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic ovary
syndrome. Boston: Blackwell Scientific Publications; 1992: 377-84.
7. Rotterdam ESHRE/ASRM-Sponsored PCOS
Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria
and long-term health risks related to polycystic ovary syndrome. Fertil
Steril 2004;81:19-25. Crossref
8. Azziz R, Carmina E, Dewailly D, et al.
The Androgen Excess and PCOS Society criteria for the polycystic ovary
syndrome: the complete task force report. Fertil Steril 2009;91:456-88. Crossref
9. Rosner W, Auchus RJ, Azziz R, Sluss PM,
Raff H. Position statement: utility, limitations, and pitfalls in
measuring testosterone: an Endocrine Society position statement. J Clin
Endocrinol Metab 2007;92:405-13. Crossref
10. Evidence-based Methodology Workshop on
Polycystic Ovary Syndrome (PCOS). December 3-5, 2012. Available from:
https://www.nichd.nih.gov/about/meetings/2012/Pages/120512.aspx. Accessed
10 Jul 2017.
11. Lam PM, Ma RC, Cheung LP, Chow CC,
Chan JC, Haines CJ. Polycystic ovarian syndrome in Hong Kong Chinese
women: patient characteristics and diagnostic criteria. Hong Kong Med J
2005;11:336-41.
12. Boyle JA, Teede HJ. PCOS: Refining
diagnostic features in PCOS to optimize health outcomes. Nat Rev
Endocrinol 2016;12:630-1. Crossref
13. Rojas J, Chávez M, Olivar L, et al.
Polycystic ovary syndrome, insulin resistance, and obesity: navigating the
pathophysiologic labyrinth. Int J Reprod Med 2014;2014:719050. Crossref
14. Cheung LP. Polycystic ovary syndrome:
not only a gynaecological disease. J Paediatr Obstet Gynaecol
2008(May/Jun):125-31.
15. Diamanti-Kandarakis E, Piperi C.
Genetics of polycystic ovary syndrome: searching for the way out of the
labyrinth. Hum Reprod Update 2005;11:631-43. Crossref
16. Azziz R, Carmina E, Chen Z, et al.
Polycystic ovary syndrome. Nat Rev Dis Primers 2016;2:16057. Crossref
17. Goodarzi MO, Dumesic DA, Chazenbalk G,
Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis.
Nat Rev Endocrinol 2011;7:219-31. Crossref
18. Azziz R, Carmina E, Dewailly D, et al.
Positions statement: criteria for defining polycystic ovary syndrome as a
predominantly hyperandrogenic syndrome: an Androgen Excess Society
guideline. J Clin Endocrinol Metab 2006;91:4237-45. Crossref
19. McCartney CR, Marshall JC. Clinical
practice. Polycystic ovary syndrome. N Engl J Med 2016;375:54-64. Crossref
20. Franks S. Polycystic ovary syndrome. N
Engl J Med 1995;333:853-61. Crossref
21. Agarwal SK, Judd HL, Magoffin DA. A
mechanism for the suppression of estrogen production in polycystic ovary
syndrome. J Clin Endocrinol Metab 1996;81:3686-91. Crossref
22. Jakimiuk AJ, Weitsman SR, Magoffin DA.
5alpha-reductase activity in women with polycystic ovary syndrome. J Clin
Endocrinol Metab 1999;84:2414-8. Crossref
23. Moghetti P, Castello R, Negri C, et
al. Metformin effects on clinical features, endocrine and metabolic
profiles, and insulin sensitivity in polycystic ovary syndrome: a
randomized, double-blind, placebo-controlled 6-month trial, followed by
open, long-term clinical evaluation. J Clin Endocrinol Metab
2000;85:139-46. Crossref
24. Bergh C, Carlsson B, Olsson JH,
Selleskog U, Hillensjö T. Regulation of androgen production in cultured
human thecal cells by insulin-like growth factor I and insulin. Fertil
Steril 1993;59:323-31. Crossref
25. Balen AH, Conway G, Homburg R, Legro
R. Polycystic ovary syndrome: A guide to clinical management. UK: Taylor
& Francis; 2006.
26. Franks S, Gilling-Smith C, Watson H,
Willis D. Insulin action in the normal and polycystic ovary. Endocrinol
Metab Clin North Am 1999;28:361-78. Crossref
27. Knight PG, Glister C. Local roles of
TGF-beta superfamily members in the control of ovarian follicle
development. Anim Reprod Sci 2003;78:165-83. Crossref
28. Seifer DB, MacLaughlin DT, Cuckle HS.
Serum mullerian-inhibiting substance in Down’s syndrome pregnancies. Hum
Reprod 2007;22:1017-20. Crossref
29. Visser JA, de Jong FH, Laven JS,
Themmen AP. Anti-Müllerian hormone: a new marker for ovarian function.
Reproduction 2006;131:1-9. Crossref
30. Pellatt L, Hanna L, Brincat M, et al.
Granulosa cell production of anti-Müllerian hormone is increased in
polycystic ovaries. J Clin Endocrinol Metab 2007;92:240-5. Crossref
31. Dunaif A. Insulin resistance and the
polycystic ovary syndrome: mechanism and implications for pathogenesis.
Endocr Rev 1997;18:774-800. Crossref
32. Diamanti-Kandarakis E, Argyrakopoulou
G, Economou F, Kandaraki E, Koutsilieris M. Defects in insulin signaling
pathways in ovarian steroidogenesis and other tissues in polycystic ovary
syndrome (PCOS). J Steroid Biochem Mol Biol 2008;109:242-6. Crossref
33. Moghetti P, Castello R, Negri C, et
al. Insulin infusion amplifies 17 alpha-hydroxycorticosteroid
intermediates response to adrenocorticotropin in hyperandrogenic women:
apparent relative impairment of 17,20-lyase activity. J Clin Endocrinol
Metab 1996;81:881-6. Crossref
34. Yki-Järvinen H, Mäkimattila S,
Utriainen T, Rutanen EM. Portal insulin concentrations rather than insulin
sensitivity regulate serum sex hormone-binding globulin and insulin-like
growth factor binding protein 1 in vivo. J Clin Endocrinol Metab
1995;80:3227-32. Crossref
35. Poretsky L, Cataldo NA, Rosenwaks Z,
Giudice LC. The insulin-related ovarian regulatory system in health and
disease. Endocr Rev 1999;20:535-82. Crossref
36. Gambineri A, Patton L, Altieri P, et
al. Polycystic ovary syndrome is a risk factor for type 2 diabetes:
results from a long-term prospective study. Diabetes 2012;61:2369-74. Crossref
37. Bremer AA, Miller WL. The serine
phosphorylation hypothesis of polycystic ovary syndrome: a unifying
mechanism for hyperandrogenemia and insulin resistance. Fertil Steril
2008;89:1039-48. Crossref
38. Corbould A, Kim YB, Youngren JF, et
al. Insulin resistance in the skeletal muscle of women with PCOS involves
intrinsic and acquired defects in insulin signaling. Am J Physiol
Endocrinol Metab 2005;288:E1047-54. Crossref
39. Dunaif A, Segal KR, Futterweit W,
Dobrjansky A. Profound peripheral insulin resistance, independent of
obesity, in polycystic ovary syndrome. Diabetes 1989;38:1165-74. Crossref
40. Stepto NK, Cassar S, Joham AE, et al.
Women with polycystic ovary syndrome have intrinsic insulin resistance on
euglycaemic-hyperinsulaemic clamp. Hum Reprod 2013;28:777-84. Crossref
41. Legro RS. Diabetes prevalence and risk
factors in polycystic ovary syndrome. Obstet Gynecol Clin North Am
2001;28:99-109. Crossref
42. Dunaif A, Finegood DT. Beta-cell
dysfunction independent of obesity and glucose intolerance in the
polycystic ovary syndrome. J Clin Endocrinol Metab 1996;81:942-7. Crossref
43. Ehrmann DA, Kasza K, Azziz R, Legro
RS, Ghazzi MN; PCOS/Troglitazone Study Group. Effects of race and family
history of type 2 diabetes on metabolic status of women with polycystic
ovary syndrome. J Clin Endocrinol Metab 2005;90:66-71. Crossref
44. Dunaif A, Wu X, Lee A,
Diamanti-Kandarakis E. Defects in insulin receptor signaling in vivo in
the polycystic ovary syndrome (PCOS). Am J Physiol Endocrinol Metab
2001;281:E392-9.
45. Ciaraldi TP, Aroda V, Mudaliar S,
Chang RJ, Henry RR. Polycystic ovary syndrome is associated with
tissue-specific differences in insulin resistance. J Clin Endocrinol Metab
2009;94:157-63. Crossref
46. Ciaraldi TP. Molecular defects of
insulin action in the polycystic ovary syndrome: possible tissue
specificity. J Pediatr Endocrinol Metab 2000;13 Suppl 5:1291-3.
47. Ibáñez L, Sebastiani G, Diaz M,
Gómez-Roig MD, Lopez-Bermejo A, de Zegher F. Low body adiposity and high
leptinemia in breast-fed infants born small-for-gestational-age. J Pediatr
2010;156:145-7. Crossref
48. Virtue S, Vidal-Puig A. It’s not how
fat you are, it’s what you do with it that counts. PLoS Biol 2008;6:e237.
Crossref
49. Virtue S, Vidal-Puig A. Adipose tissue
expandability, lipotoxicity and the metabolic syndrome—an allostatic
perspective. Biochim Biophys Acta 2010;1801:338-49. Crossref
50. Chazenbalk G, Trivax BS, Yildiz BO, et
al. Regulation of adiponectin secretion by adipocytes in the polycystic
ovary syndrome: role of tumor necrosis factor-{alpha}. J Clin Endocrinol
Metab 2010;95:935-42. Crossref
51. Barber TM, Franks S. Adipocyte biology
in polycystic ovary syndrome. Mol Cell Endocrinol 2013;373:68-76. Crossref
52. Padmanabhan V, Manikkam M, Recabarren
S, Foster D. Prenatal testosterone excess programs reproductive and
metabolic dysfunction in the female. Mol Cell Endocrinol 2006;246:165-74.
Crossref
53. Dumesic DA, Schramm RD, Abbott DH.
Early origins of polycystic ovary syndrome. Reprod Fertil Dev
2005;17:349-60. Crossref
54. Legro RS, Bentley-Lewis R, Driscoll D,
Wang SC, Dunaif A. Insulin resistance in the sisters of women with
polycystic ovary syndrome: association with hyperandrogenemia rather than
menstrual irregularity. J Clin Endocrinol Metab 2002;87:2128-33. Crossref
55. Hickey M, Sloboda DM, Atkinson HC, et
al. The relationship between maternal and umbilical cord androgen levels
and polycystic ovary syndrome in adolescence: a prospective cohort study.
J Clin Endocrinol Metab 2009;94:3714-20. Crossref
56. Dumesic DA, Oberfield SE,
Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement
on the diagnostic criteria, epidemiology, pathophysiology, and molecular
genetics of polycystic ovary syndrome. Endocr Rev 2015;36:487-525. Crossref
57. Hayes MG, Urbanek M, Ehrmann DA, et
al. Genome-wide association of polycystic ovary syndrome implicates
alterations in gonadotropin secretion in European ancestry populations.
Nat Commun 2015;6:7502. Crossref
58. Boomsma CM, Eijkemans MJ, Hughes EG,
Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in
women with polycystic ovary syndrome. Hum Reprod Update 2006;12:673-83. Crossref
59. Weerakiet S, Srisombut C, Rojanasakul
A, Panburana P, Thakkinstian A, Herabutya Y. Prevalence of gestational
diabetes mellitus and pregnancy outcomes in Asian women with polycystic
ovary syndrome. Gynecol Endocrinol 2004;19:134-40. Crossref
60. Qin JZ, Pang LH, Li MJ, Fan XJ, Huang
RD, Chen HY. Obstetric complications in women with polycystic ovary
syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol
2013;11:56. Crossref
61. Chittenden BG, Fullerton G, Maheshwari
A, Bhattacharya S. Polycystic ovary syndrome and the risk of
gynaecological cancer: a systematic review. Reprod Biomed Online
2009;19:398-405. Crossref
62. Haoula Z, Salman M, Atiomo W.
Evaluating the association between endometrial cancer and polycystic ovary
syndrome. Hum Reprod 2012;27:1327-31. Crossref
63. Timmermans A, Opmeer BC, Khan KS, et
al. Endometrial thickness measurement for detecting endometrial cancer in
women with postmenopausal bleeding: a systematic review and meta-analysis.
Obstet Gynecol 2010;116:160-7. Crossref
64. Sartor BM, Dickey RP. Polycystic
ovarian syndrome and the metabolic syndrome. Am J Med Sci 2005;330:336-42.
Crossref
65. Colilla S, Cox NJ, Ehrmann DA.
Heritability of insulin secretion and insulin action in women with
polycystic ovary syndrome and their first degree relatives. J Clin
Endocrinol Metab 2001;86:2027-31. Crossref
66. Legro RS, Gnatuk CL, Kunselman AR,
Dunaif A. Changes in glucose tolerance over time in women with polycystic
ovary syndrome: a controlled study. J Clin Endocrinol Metab
2005;90:3236-42. Crossref
67. Boudreaux MY, Talbott EO, Kip KE,
Brooks MM, Witchel SF. Risk of T2DM and impaired fasting glucose among
PCOS subjects: results of an 8-year follow-up. Curr Diab Rep 2006;6:77-83.
Crossref
68. Cheung LP, Ma RC, Lam PM, et al.
Cardiovascular risks and metabolic syndrome in Hong Kong Chinese women
with polycystic ovary syndrome. Hum Reprod 2008;23:1431-8. Crossref
69. Berneis K, Rizzo M, Lazzarini V,
Fruzzetti F, Carmina E. Atherogenic lipoprotein phenotype and low-density
lipoproteins size and subclasses in women with polycystic ovary syndrome.
J Clin Endocrinol Metab 2007;92:186-9. Crossref
70. Phelan N, O’Connor A, Kyaw-Tun T, et
al. Lipoprotein subclass patterns in women with polycystic ovary syndrome
(PCOS) compared with equally insulin-resistant women without PCOS. J Clin
Endocrinol Metab 2010;95:3933-9. Crossref
71. Valkenburg O, Steegers-Theunissen RP,
Smedts HP, et al. A more atherogenic serum lipoprotein profile is present
in women with polycystic ovary syndrome: a case-control study. J Clin
Endocrinol Metab 2008;93:470-6. Crossref
72. Isomaa B, Almgren P, Tuomi T, et al.
Cardiovascular morbidity and mortality associated with the metabolic
syndrome. Diabetes Care 2001;24:683-9. Crossref
73. Weerakiet S, Bunnag P,
Phakdeekitcharoen B, et al. Prevalence of the metabolic syndrome in Asian
women with polycystic ovary syndrome: using the International Diabetes
Federation criteria. Gynecol Endocrinol 2007;23:153-60. Crossref
74. Vgontzas AN, Legro RS, Bixler EO,
Grayev A, Kales A, Chrousos GP. Polycystic ovary syndrome is associated
with obstructive sleep apnea and daytime sleepiness: role of insulin
resistance. J Clin Endocrinol Metab 2001;86:517-20. Crossref
75. Gopal M, Duntley S, Uhles M, Attarian
H. The role of obesity in the increased prevalence of obstructive sleep
apnea syndrome in patients with polycystic ovarian syndrome. Sleep Med
2002;3:401-4. Crossref
76. Wild S, Pierpoint T, McKeigue P,
Jacobs H. Cardiovascular disease in women with polycystic ovary syndrome
at long-term follow-up: a retrospective cohort study. Clin Endocrinol
(Oxf) 2000;52:595-600. Crossref
77. Lo JC, Feigenbaum SL, Yang J, Pressman
AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular risk profile
of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab
2006;91:1357-63. Crossref
78. Cibula D, Cifkova R, Fanta M, Poledne
R, Zivny J, Skibova J. Increased risk of non-insulin dependent diabetes
mellitus, arterial hypertension and coronary artery disease in
perimenopausal women with a history of the polycystic ovary syndrome. Hum
Reprod 2000;15:785-9. Crossref
79. Schmidt J, Landin-Wilhelmsen K,
Brännström M, Dahlgren E. Cardiovascular disease and risk factors in PCOS
women of postmenopausal age: a 21-year controlled follow-up study. J Clin
Endocrinol Metab 2011;96:3794-803. Crossref
80. Shroff R, Kerchner A, Maifeld M, Van
Beek EJ, Jagasia D, Dokras A. Young obese women with polycystic ovary
syndrome have evidence of early coronary atherosclerosis. J Clin
Endocrinol Metab 2007;92:4609-14. Crossref
81. Luque-Ramirez M, Mendieta-Azcona C,
Alvarez-Blasco F, Escobar-Morreale HF. Androgen excess is associated with
the increased carotid intima-media thickness observed in young women with
polycystic ovary syndrome. Hum Reprod 2007;22:3197-203. Crossref
82. Talbott EO, Zborowski JV, Rager JR,
Boudreaux MY, Edmundowicz DA, Guzick DS. Evidence for an association
between metabolic cardiovascular syndrome and coronary and aortic
calcification among women with polycystic ovary syndrome. J Clin
Endocrinol Metab 2004;89:5454-61. Crossref
83. Boulman N, Levy Y, Leiba R, et al.
Increased C-reactive protein levels in the polycystic ovary syndrome: a
marker of cardiovascular disease. J Clin Endocrinol Metab 2004;89:2160-5.
Crossref
84. Fauser BC, Tarlatzis BC, Rebar RW, et
al. Consensus on women’s health aspects of polycystic ovary syndrome
(PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop
Group. Fertil Steril 2012;97:28-38.e25. Crossref
85. Johnstone EB, Rosen MP, Neril R, et
al. The polycystic ovary post-rotterdam: a common, age-dependent finding
in ovulatory women without metabolic significance. J Clin Endocrinol Metab
2010;95:4965-72. Crossref
86. Dewailly D, Lujan ME, Carmina E, et
al. Definition and significance of polycystic ovarian morphology: a task
force report from the Androgen Excess and Polycystic Ovary Syndrome
Society. Hum Reprod Update 2014;20:334-52. Crossref
87. Quinn MM, Kao CN, Ahmad A, et al.
Raising threshold for diagnosis of polycystic ovary syndrome excludes
population of patients with metabolic risk. Fertil Steril
2016;106:1244-51. Crossref
88. Conway G, Dewailly D,
Diamanti-Kandarakis E, et al. The polycystic ovary syndrome: a position
statement from the European Society of Endocrinology. Eur J Endocrinol
2014;171:P1-29. Crossref
89. Moran LJ, Noakes M, Clifton PM,
Tomlinson L, Galletly C, Norman RJ. Dietary composition in restoring
reproductive and metabolic physiology in overweight women with polycystic
ovary syndrome. J Clin Endocrinol Metab 2003;88:812-9. Crossref
90. Moran LJ, Hutchison SK, Norman RJ,
Teede HJ. Lifestyle changes in women with polycystic ovary syndrome.
Cochrane Database Syst Rev 2011;(7):CD007506. Crossref
91. Pasquali R, Gambineri A, Pagotto U.
The impact of obesity on reproduction in women with polycystic ovary
syndrome. BJOG 2006;113:1148-59. Crossref
92. Diamanti-Kandarakis E, Baillargeon JP,
Iuorno MJ, Jakubowicz DJ, Nestler JE. A modern medical quandary:
polycystic ovary syndrome, insulin resistance, and oral contraceptive
pills. J Clin Endocrinol Metab 2003;88:1927-32. Crossref
93. World Health Organization. Medical
eligibility criteria for contraceptive use. 2015. Available from:
http://apps.who.int/iris/bitstream/10665/181468/1/9789241549158_eng.pdf.
Accessed 10 Jul 2017.
94. The Faculty of Sexual and Reproductive
Healthcare of the Royal College of Obstetricians and Gynaecologists
(FSRH). FSRH Statement: Venous thromboembolism (VTE) and hormonal
contraception Nov 2014. Available from:
https://www.fsrh.org/standards-and-guidance/documents/fsrhstatementvteandhormonalcontraception-november/.
Accessed 10 Jul 2017.
95. National Institute for Health and Care
Excellence. Fertility problems: assessment and treatment. 20 Feb 2013.
Available from: https://www.nice.org.uk/guidance/cg156. Accessed 10 Jul
2017.
96. Jayasena CN, Franks S. The management
of patients with polycystic ovary syndrome. Nat Rev Endocrinol
2014;10:624-36. Crossref
97. Lord JM, Flight IH, Norman RJ.
Metformin in polycystic ovary syndrome: systematic review and
meta-analysis. BMJ 2003;327:951-3. Crossref
98. Legro RS, Barnhart HX, Schlaff WD, et
al. Clomiphene, metformin, or both for infertility in the polycystic ovary
syndrome. N Engl J Med 2007;356:551-66. Crossref
99. Moll E, van der Veen F, van Wely M.
The role of metformin in polycystic ovary syndrome: a systematic review.
Hum Reprod Update 2007;13:527-37. Crossref
100. Legro RS, Brzyski RG, Diamond MP, et
al. Letrozole versus clomiphene for infertility in the polycystic ovary
syndrome. N Engl J Med 2014;371:119-29. Crossref
101. Thessaloniki ESHRE/ASRM-Sponsored
PCOS Consensus Workshop Group. Consensus on infertility treatment related
to polycystic ovary syndrome. Hum Reprod 2008;23:462-77. Crossref
102. Kong GW, Cheung LP, Lok IH. Effects
of laparoscopic ovarian drilling in treating infertile anovulatory
polycystic ovarian syndrome patients with and without metabolic syndrome.
Hong Kong Med J 2011;17:5-10.
103. Ma RC, Liu KH, Lam PM, et al.
Sonographic measurement of mesenteric fat predicts presence of fatty liver
among subjects with polycystic ovary syndrome. J Clin Endocrinol Metab
2011;96:799-807. Crossref
104. Naderpoor N, Shorakae S, de Courten
B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in
polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod
Update 2015;21:560-74. Crossref
105. Skubleny D, Switzer NJ, Gill RS, et
al. The impact of bariatric surgery on polycystic ovary syndrome: a
systematic review and meta-analysis. Obes Surg 2016;26:169-76. Crossref