Hong
Kong Med J 2017 Dec;23(6):609–15 | Epub 13 Oct 2017
DOI: 10.12809/hkmj166194
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Pathological outcome for Chinese patients with low-risk
prostate cancer eligible for active surveillance and undergoing radical
prostatectomy: comparison of six different active surveillance protocols
CF Tsang, MB, BS, FRCS (Edin); James HL Tsu, MB,
BS, FRCS (Edin); Terence CT Lai, MB, BS, FRCS (Edin); KW Wong, MB, ChB,
FRCS (Edin); Brian SH Ho, MB, BS, FRCS (Edin); Ada TL Ng, MB, BS, FRCS
(Edin); WK Ma, MB, ChB, FRCS (Edin); MK Yiu, MB, BS, FRCS (Edin)
Division of Urology, Department of Surgery, Queen
Mary Hospital, The University of Hong Kong, Pokfulam, Hong Kong
Corresponding author: Dr MK Yiu (pmkyiu@gmail.com)
Abstract
Introduction: Active
surveillance is one of the therapeutic options for the management of
patients with low-risk prostate cancer. This study compared the
performance of six different active surveillance protocols for prostate
cancer in the Chinese population.
Methods: Patients who underwent
radical prostatectomy for prostate cancer from January 1998 to December
2012 at a university teaching hospital in Hong Kong were reviewed. Six
active surveillance protocols were applied to the cohort. Statistical
analyses were performed to compare the probabilities of missing
unfavourable pathological outcome. The sensitivity and specificity of
each protocol in identifying low-risk disease were compared.
Results: During the study
period, 287 patients were included in the cohort. Depending on different
active surveillance protocols used, extracapsular extension, seminal
vesicle invasion, pathological T3 disease, and upgrading of Gleason
score were present on final pathology in 3.3%-17.1%, 0%-3.3%,
3.3%-19.1%, and 20.6%-34.5% of the patients, respectively. The
University of Toronto protocol had a higher rate of extracapsular
extension at 17.1% and pathological T3 disease at 19.1% on final
pathology than the more stringent protocols from John Hopkins (3.3%
extracapsular extension, P=0.05 and 3.3% pathological T3 disease,
P=0.03) and Prostate Cancer Research International: Active Surveillance
(PRIAS; 8.0% pathological T3 disease, P=0.04). The Royal Marsden
protocol had a higher rate of upgrading of Gleason score at 34.5%
compared with the more stringent protocol of PRIAS at 20.6% (P=0.04).
The specificities in identifying localised disease and low-risk
histology among different active surveillance protocols were 59%-98% and
58%-94%, respectively. The John Hopkins active surveillance protocol had
the highest specificity in both selecting localised disease (98%) and
low-risk histology (94%).
Conclusions: Active surveillance
protocols based on prostate-specific antigen and Gleason score alone or
including Gleason score of 3+4 may miss high-risk disease and should be
used cautiously. The John Hopkins and PRIAS protocols are highly
specific in identifying localised disease and low-risk histology.
New knowledge added by this study
- Active surveillance protocols based on prostate-specific antigen (PSA) and Gleason score only may miss high-risk prostate cancer.
- Active surveillance protocols using PSA density as an inclusion criteria were highly specific in identifying localised disease and low-risk pathology.
- When adopting active surveillance in patients with prostate cancer, protocols with PSA density as an inclusion criteria are preferred.
Introduction
Prostate-specific antigen (PSA) plays a significant
role in the early detection of prostate cancer in current practice.1 2 It is,
however, a double-edged sword that leads to overdiagnosis, especially for
clinically insignificant prostate cancer.3
4 Curative treatments for low-risk
prostate cancer include radical prostatectomy and radiotherapy, both of
which are associated with significant morbidities.5 6 7 In recent years, the concept of active surveillance
(AS) has been adopted with the aim of monitoring clinically insignificant
prostate cancer until disease progression, at which point radical
prostatectomy or radiotherapy is considered. The ultimate objective is to
delay or avoid the morbidities associated with radical treatments without
compromising survival.8 9 10
Although AS is an established management option for
low-risk prostate cancer, different AS protocols have been adopted.11 12 13 14 15 16 17 The most commonly used include those from the
University of Toronto,11 Royal
Marsden,12 John Hopkins,13 14
University of California San Francisco (UCSF),15
Memorial Sloan Kettering Cancer Center (MSKCC),16
and Prostate Cancer Research International: Active Surveillance (PRIAS).17 Most AS protocols select
prostate cancer with a Gleason score of ≤6, PSA level of ≤10 ng/mL, and
clinical stage of ≤T2. Other parameters that are considered by some
protocols include PSA density, number of positive biopsy cores, and
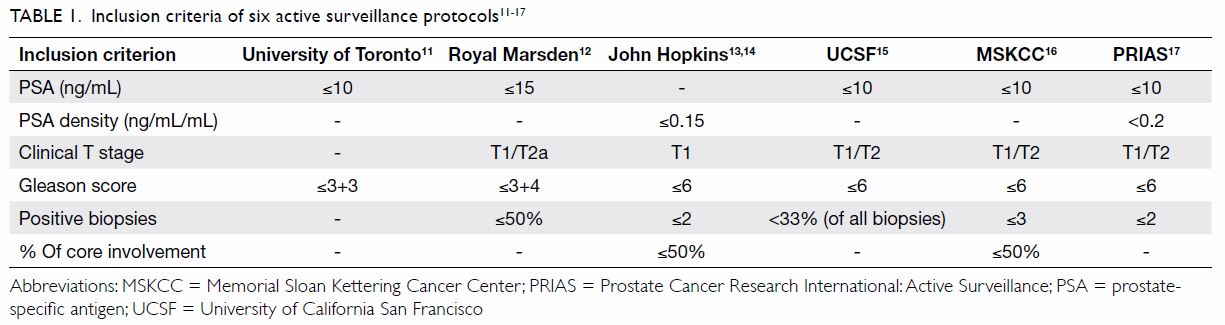
percentage of core involvement (Table 111 12 13
14 15
16 17).
Currently, there is no consensus regarding which AS
protocol we should adopt for our patients. In addition, direct comparisons
between different AS protocols are few. Before deciding to follow any
particular AS protocol, urologists and oncologists should be aware of
their individual strengths and limitations. Our study aimed to provide
some insight into this issue by performing a head-to-head comparison of
six AS protocols.
Methods
Patients who underwent radical prostatectomy for
prostate cancer from January 1998 to December 2012 at a university
teaching hospital in Hong Kong were reviewed. Indication for radical
prostatectomy was localised prostate cancer in patients with a life
expectancy exceeding 10 years. All patients underwent clinical assessment
including clinical T staging by digital rectal examination, serum PSA
level, and transrectal ultrasound-guided prostate biopsy. Sextant biopsies
were performed from 1998 to 2002, but changed to 10-core biopsies from
2002 to 2011 and subsequently 12-core biopsies thereafter. Preoperative
magnetic resonance imaging of the prostate was routinely performed from
2007. From 1998 to 2007, open or laparoscopic radical prostatectomies were
performed. After November 2007, all prostatectomies at our institution
were performed with the da Vinci robotic surgery system. Pathological
assessment of transrectal ultrasound-guided biopsy and radical
prostatectomy specimens was performed by a specialist pathologist in our
institution. All patients attended a follow-up visit with physical
examination 2 weeks after operation, and physical examination with serum
PSA level checked every 3 months for the first year, every 6 months for
the second year, and then annually thereafter. Data on patient
demographics, clinical T stage, serum PSA level, transrectal
ultrasound-guided biopsy results, and final pathology of radical
prostatectomy specimen were retrospectively retrieved by an independent
third party. Pathological assessment of the radical prostatectomy specimen
was performed by independent specialist pathologists.
In our current study, we compared six different AS
protocols, specifically from the University of Toronto,11 Royal Marsden,12
John Hopkins,13 14 UCSF,15
MSKCC,16 and PRIAS17 (Table 1). The six protocols were retrospectively
applied to our cohort and patients were stratified accordingly based on
clinical T stage, serum PSA level, PSA density, Gleason score on biopsy,
number of positive biopsy cores, and percentage of positive core
involvement. Data from the pathological assessment of radical
prostatectomy specimens including extracapsular extension, seminal vesicle
invasion, upgrading to pathological T3 disease, and upgrading of Gleason
score were analysed. The clinical data used in the AS protocols were those
available on diagnosis of prostate cancer and operations were performed
within 12 weeks of diagnosis.
Statistical analyses to compare the rate of not
diagnosing clinically significant prostate cancer—defined as extracapsular
extension, seminal vesicle invasion, upgrading to T3 disease, and
upgrading of Gleason score in the final prostatectomy specimens—were
performed. The sensitivity and specificity of each protocol in selecting
localised prostate cancer (defined as pathological stage <T3) and
histological low-risk disease (defined as no upgrading of Gleason score on
final pathology) were compared.
Statistical analysis was performed using the SPSS
(Windows version 20.0; IBM Corp, Armonk [NY], US). Independent sample t test
and Pearson Chi-squared test were used for continuous and categorical
variables, respectively. A P value of <0.05 was considered
statistically significant. This study was done in accordance with the
principles outlined in the Declaration of Helsinki.
Results
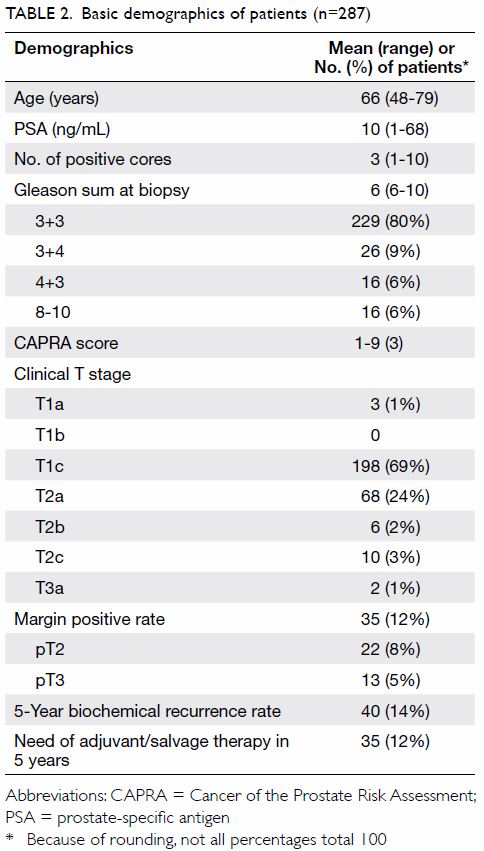
A total of 287 patients were included in the
cohort. The mean age was 66 years, mean serum PSA level was 10 ng/mL, mean
number of positive cores during biopsy was 3, and mean Gleason sum at
biopsy was 6. In the current cohort, 266 (93%) patients had clinical T1c
or T2a prostate cancer—198 (69%) had clinical T1c disease and 68 (24%) had
clinical T2a disease. Table 2 summarises the basic demographics of all
patients.
When the six AS protocols were applied to the
cohort, 30 to 152 patients were identified as low-risk; their mean serum
PSA level ranged from 5.3 ng/mL to 7.7 ng/mL, and mean PSA density ranged
from 0.12 ng/mL/mL to 0.25 ng/mL/mL. All six protocols had a mean biopsy
Gleason sum of 6. Table 3 summarises the clinical characteristics of
patients stratified according to different AS protocols.
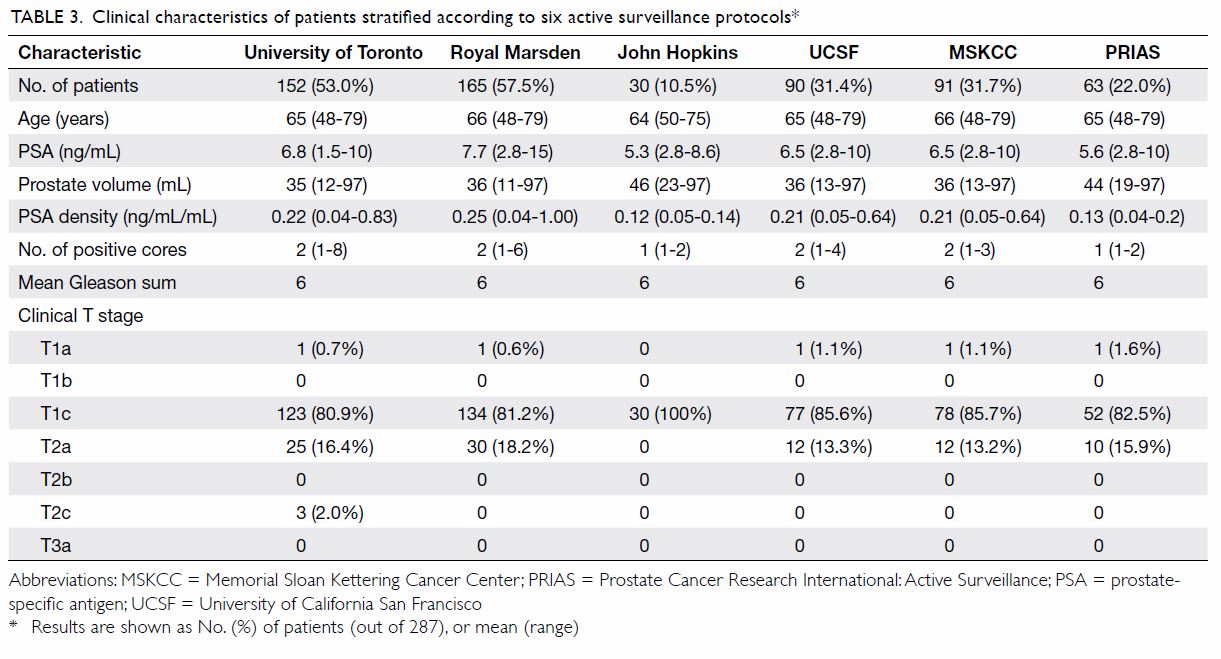
Table 3. Clinical characteristics of patients stratified according to six active surveillance protocols
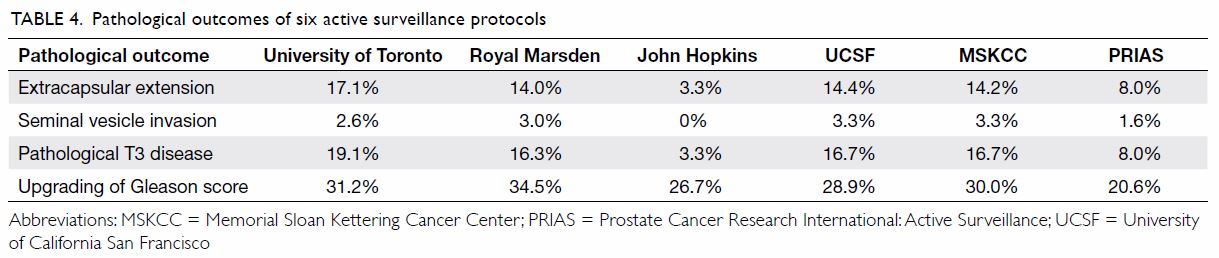
In the analyses of final pathological outcomes in
patients stratified into different AS protocols, extracapsular extension
rate varied from 3.3% to 17.1%. The incidence of seminal vesicle invasion
was low in all six protocols, ranging from 0% to 3.3%. The rate of
pathological T3 disease was lowest according to the John Hopkins criteria
(3.3%), while the University of Toronto criteria had the highest incidence
(19.1%). Regarding the upgrading of Gleason score in the radical
prostatectomy specimens, all six protocols had a relatively high rate
ranging from 20.6% to 34.5%. Table 4 summarises the pathological outcomes among
the six AS protocols.
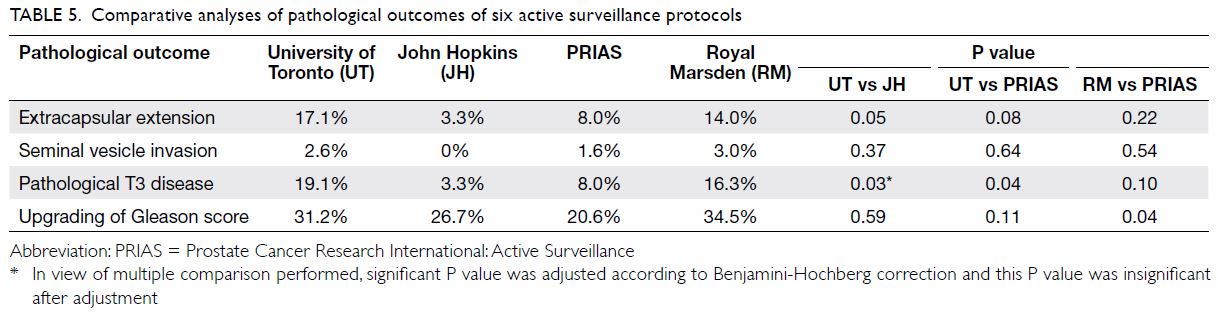
Comparative analyses of individual AS protocols
against each other were also performed (Table 5). The University of Toronto protocol had a
significantly higher rate of extracapsular extension at 17.1% and
pathological T3 disease at 19.1% when compared with the more stringent
protocol from John Hopkins (3.3% extracapsular extension, P=0.05 and 3.3%
pathological T3 disease, P=0.03) and PRIAS (8.0% pathological T3 disease,
P=0.04). In addition, the Royal Marsden protocol had a significantly
higher rate of upgrading of Gleason score at 34.5% when compared with the
more stringent protocol of PRIAS at 20.6% (P=0.04). There was no
significant difference in the incidence of seminal vesicle invasion
between the six protocols.
In terms of the ability of each protocol to
identify pathological localised disease (defined as pathological stage
<T3) and histologically low-risk cancer (defined as no upgrading of
Gleason score), sensitivity varied from 13%-61% and 14-71%, respectively.
The John Hopkins criteria demonstrated highest specificity in identifying
pathological localised disease (98%) and histological low-risk cancer
(94%). Table 6 illustrates the sensitivity and specificity
of identifying localised and histological low-risk disease for the six AS
protocols.
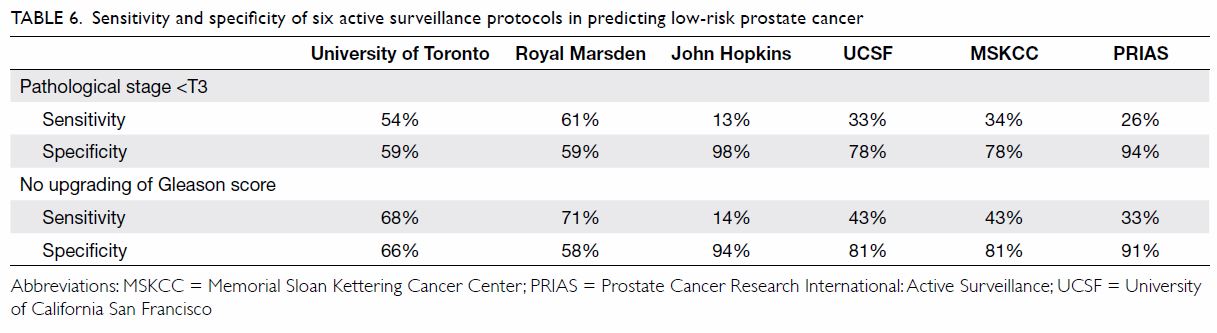
Table 6. Sensitivity and specificity of six active surveillance protocols in predicting low-risk prostate cancer
Discussion
Prostate cancer screening has always been a
controversial issue and evidence of improved survival is awaited.1 2 Nonetheless,
PSA screening has undoubtedly led to overdiagnosis of insignificant
prostate cancer.3 4 Active surveillance, with the purpose to delay or even
avoid radical treatments and their associated morbidities, plays an
important role in managing these patients. Unfortunately there are
different AS protocols with various inclusion criteria, and urologists and
oncologists may have difficulty deciding which protocol to adopt. The gold
standard to answer this question will be a prospective randomised trial to
compare overall survival following the application of different AS
protocols. This, however, will require decades to observe low-risk
prostate cancer patients before survival endpoints are reached. Our study
provides data on pathological outcomes when different AS protocols were
compared.
In our cohort, the proportion of patients eligible
for active surveillance varied widely from approximately 11% to 58%
according to different selection criteria (Table 3). Two recent series showed similar findings
of a large discrepancy in the proportion of patients eligible for
different AS protocols, varying from 16% to 63% and 28% to 69%.18 19 We
demonstrated that although all AS protocols aim to select low-risk
prostate cancer, the heterogeneity between them can be quite large.
Clinicians need to be vigilant before adopting any of the AS protocols for
their patients when further data from comparative analyses among different
protocols are unavailable. The proportion of patients who were eligible
for AS protocols in our study was lower than that in previous series.18 19 This may
be because some patients with localised prostate cancer were treated with
radiotherapy. The proportion of patients who can be selected in different
AS protocols will be affected by the proportion of patients who undergo
radiotherapy instead of surgery. In our centre, it is also possible that
low-risk patients were selected to undergo a non-operative approach.
When the six protocols were compared after
stratifying patients according to different AS criteria, the University of
Toronto protocol had a significantly higher rate of extracapsular
extension at 17.1% and pathological T3 disease at 19.1% than the John
Hopkins protocol (3.3% extracapsular extension, P=0.05 and 3.3%
pathological T3 disease, P=0.03) and PRIAS criteria (8.0% pathological T3
disease, P=0.04) [Table 5]. This observation can be explained by the
difference in stringency of the two protocols. The University of Toronto
criteria selected patients by two factors only: PSA of <10 ng/mL and
Gleason score of ≤6; PSA density, number of positive biopsy cores, and
percentage of core involvement were not considered. On the contrary, the
John Hopkins criteria applied very strict criteria: a PSA density of 0.15
ng/mL/mL. In addition, only patients with T1 disease with at most two
positive cores during biopsy and no more than 50% involvement of each core
were selected (Table 1). Contrary to our findings, El Hajj et al19 found no significant difference
in the rate of extracapsular extension, upgrading of Gleason score, or
unfavourable disease when they compared the University of Toronto protocol
with the John Hopkins protocol. The difference can be explained by the
high rate of extracapsular extension (15%) and unfavourable disease (46%)
within the John Hopkins criteria in their series, compared with 3%
extracapsular extension and 3% pathological T3 disease in our cohort. This
also implies that disease heterogeneity among different populations may
influence the choice and results of different AS protocols.
In our study, analyses of final pathology revealed
that the Royal Marsden protocol had a significantly higher rate of
upgrading of Gleason score at 34.5% compared with the PRIAS criteria at
20.6% (P=0.04; Table 5). This result can be explained by the
less-stringent selection criteria of the Royal Marsden protocol. First, it
is the only protocol that allowed a Gleason score of 3+4 to be selected.
Second, PSA level up to 15 ng/mL was permitted. These factors will
invariably result in the inclusion of a proportion of patients with
higher-risk disease. In the study by El Hajj et al,19 the Royal Marsden protocol were compared with the
John Hopkins protocol and significantly more unfavourable disease was
observed in the Royal Marsden group. Klotz et al11
also demonstrated that inclusion of Gleason score of 4 on biopsy into AS
was a risk factor in predicting definitive treatment during active
surveillance. These findings illustrate that active surveillance in
patients with Gleason score of 3+4 is likely to miss higher-risk disease.
It should be used cautiously and preferably not in young patients who are
otherwise fit for radical treatments.
We have shown that less pathological T3 disease and
Gleason score upgrading were present in the more-stringent John Hopkins
and PRIAS protocols compared with the less stringent University of Toronto
and Royal Marsden criteria. Nonetheless their sensitivity in identifying
low-risk disease may be compromised by the more stringent selection
criteria. More low-risk disease may therefore be excluded from
surveillance by these stringent criteria. We addressed this issue in the
last part of our analyses. The sensitivity and specificity in identifying
localised disease (pathological stage <T3) and low-risk histology (no
upgrading of Gleason score) among different AS protocols were compared (Table 6). The most stringent protocols of the John
Hopkins and PRIAS had the highest specificity when selecting localised
disease (94%-98%) and low-risk histology (91%-94%). However, inclusion of
less pathological T3 disease and Gleason score upgrading by the more
stringent protocols of John Hopkins and PRIAS should be cautious because
it will, inevitably, be at the expense of low-risk patients who is
excluded from AS and may receive unnecessary aggressive treatments. A
recent study by Iremashvili et al18
showed that the PRIAS criteria had a better balance of sensitivity and
specificity compared with the UCSF and MSKCC criteria. From our point of
view, we tend to place more emphasis on high specificity since low
specificity will include patients with high-risk tumours into active
surveillance and thus patient survival may be jeopardised.
The present study had several limitations. First,
the number of biopsy cores was not consistent throughout the study period.
A proportion of patients had six-core biopsies in the early period of the
cohort versus the current more recent standard of 10-12–core biopsies.
Second, the sample size was relatively small due to the low incidence of
prostate cancer in our population. Third, the tumour volume in
prostatectomy specimens that might predict low-risk prostate cancer was
not assessed. Lastly, the final prostatectomy pathology in this study was
from patients who were operated on soon after diagnosis and not after a
period of post-diagnosis surveillance. As a note of caution, it would be
expected that the final pathology would show even worse pathological
features if the patients were put on AS and operated on later. This should
be noted when interpreting the results of the current study and
counselling patients.
In conclusion, there is a wide range of variation
in the selection criteria of different AS protocols. Active surveillance
protocols based on PSA and Gleason score alone or including Gleason score
of 3+4 may miss higher-risk disease and should be applied cautiously. The
more stringent criteria of John Hopkins protocol and the PRIAS protocol
were highly specific in identifying localised disease and low-risk
histology.
Declaration
All authors have disclosed no conflicts of interest
References
1. Schröder FH, Hugosson J, Roobol MJ, et
al. Screening and prostate-cancer mortality in a randomized European
study. N Engl J Med 2009;360:1320-8. Crossref
2. Andriole GL, Crawford ED, Grubb RL 3rd,
et al. Mortality results from a randomized prostate-cancer screening
trial. N Engl J Med 2009;360:1310-9. Crossref
3. Ploussard G, Epstein JI, Montironi R, et
al. The contemporary concept of significant versus insignificant prostate
cancer. Eur Urol 2011;60:291-303. Crossref
4. Etzioni R, Penson DF, Legler JM, et al.
Overdiagnosis due to prostate-specific antigen screening: lessons from
U.S. prostate cancer incidence trends. J Natl Cancer Inst
2002;94:981-90. Crossref
5. Novara G, Ficarra V, Rosen RC, et al.
Systematic review and meta-analysis of perioperative outcomes and
complications after robot-assisted radical prostatectomy. Eur Urol
2012;62:431-52. Crossref
6. Boorjian SA, Eastham JA, Graefen M, et
al. A critical analysis of the long-term impact of radical prostatectomy
on cancer control and function outcomes. Eur Urol 2012;61:664-75. Crossref
7. Zaorsky NG, Harrison AS, Trabulsi EJ, et
al. Evolution of advanced technologies in prostate cancer radiotherapy.
Nat Rev Urol 2013;10:565-79. Crossref
8. Bastian PJ, Carter BH, Bjartell A, et
al. Insignificant prostate cancer and active surveillance: from definition
to clinical implications. Eur Urol 2009;55:1321-30. Crossref
9. Cooperberg MR, Carroll PR, Klotz L.
Active surveillance for prostate cancer: progress and promise. J Clin
Oncol 2011;29:3669-76. Crossref
10. Dall’Era MA, Albertsen PC, Bangma C,
et al. Active surveillance for prostate cancer: a systematic review of the
literature. Eur Urol 2012;62:976-83. Crossref
11. Klotz L, Zhang L, Lam A, Nam R,
Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large,
active surveillance cohort with localized prostate cancer. J Clin Oncol
2010;28:126-31. Crossref
12. van As NJ, Norman AR, Thomas K, et al.
Predicting the probability of deferred radical treatment for localised
prostate cancer managed by active surveillance. Eur Urol
2008;54:1297-305. Crossref
13. Carter HB, Kettermann A, Warlick C, et
al. Expectant management of prostate cancer with curative intent: an
update of the Johns Hopkins experience. J Urol 2007;178:2359-64. Crossref
14. Tosoian JJ, Trock BJ, Landis P, et al.
Active surveillance program for prostate cancer: an update of the Johns
Hopkins experience. J Clin Oncol 2011;29:2185-90. Crossref
15. Dall’Era MA, Konety BR, Cowan JE, et
al. Active surveillance for the management of prostate cancer in a
contemporary cohort. Cancer 2008;112:2664-70. Crossref
16. Berglund RK, Masterson TA, Vora KC,
Eggener SE, Eastham JA, Guillonneau BD. Pathological upgrading and up
staging with immediate repeat biopsy in patients eligible for active
surveillance. J Urol 2008;180:1964-7. Crossref
17. van den Bergh RC, Roemeling S, Roobol
MJ, Roobol W, Schröder FH, Bangma CH. Prospective validation of active
surveillance in prostate cancer: the PRIAS study. Eur Urol
2007;52:1560-3. Crossref
18. Iremashvili V, Pelaez L, Manoharan M,
Jorda M, Rosenberg DL, Soloway MS. Pathologic prostate cancer
characteristics in patients eligible for active surveillance: a
head-to-head comparison of contemporary protocols. Eur Urol
2012;62:462-8. Crossref
19. El Hajj A, Ploussard G, de la Taille
A, et al. Patient selection and pathological outcomes using currently
available active surveillance criteria. BJU Int 2013;112:471-7. Crossref