DOI: 10.12809/hkmj164842
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Immunoglobulin G4–related disease masquerading as
tonsil carcinoma
TL Chow, FRCS (Edin), FHKAM (Surgery)1;
Nancy WF Yuen, FRCPath, FHKAM (Pathology)2; Wilson WY Kwan,
FRCS (Edin), FHKAM (Surgery)1; CY Choi, FRCS(Edin), FHKAM
(Surgery)1
1 Department of Surgery, United Christian Hospital, Kwun Tong, Hong Kong
2 Department of Anatomical Pathology, United Christian Hospital, Kwun Tong, Hong Kong
Corresponding author: Dr TL Chow (chowtl@ha.org.hk
/ tamlinc@yahoo.com)
Case report
An 85-year-old man presented with a history of
odynophagia since March 2015. He was a chronic smoker but did not drink
alcohol. He had a medical history of hypertension, diabetes mellitus, and
gout. He was first seen by us in April 2015. At the first presentation,
physical examination revealed an irregular 3-cm ulcerative mass arising
from the left tonsil (Fig 1). The rest of his oral cavity was otherwise
normal and there was no cervical lymphadenopathy. Carcinoma of the left
tonsil was suspected. Computed tomographic (CT) scan of the head and neck
disclosed a non-specific soft tissue thickening and mucosal contrast
enhancement at the left side of the oropharynx. Transoral punch biopsy of
the left tonsillar mass was performed. Histopathology revealed no evidence
of malignancy with heavy stromal infiltration by neutrophils, lymphocytes,
and plasma cells plus focal microabscess formation. No granuloma or fungal
elements were seen.
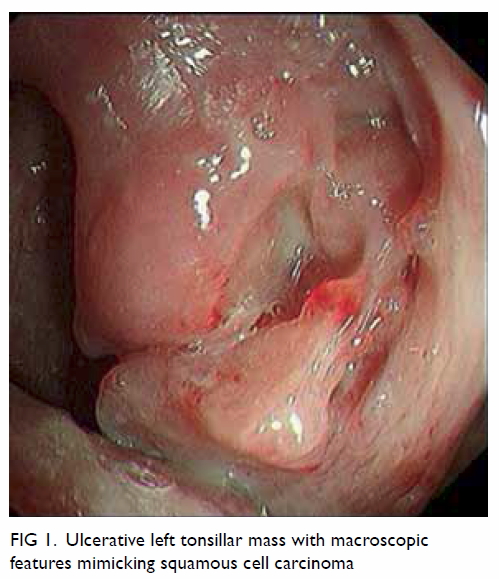
Figure 1. Ulcerative left tonsillar mass with macroscopic features mimicking squamous cell carcinoma
Subsequent upper gastrointestinal endoscopy was
performed and disclosed the left tonsillar mass with no other
abnormalities within the hypopharynx, oesophagus, or stomach. Because of
the progressive odynophagia, a nasogastric feeding tube was inserted; as
there was no improvement and the diagnosis was still elusive, left
tonsillectomy was performed on the same day and was uneventful.
Histopathological examination of the left tonsil
showed lymphoid tissue with preserved architecture present beneath the
stratified squamous epithelium. Hyperplastic lymphoid follicles with
germinal centre surrounded by mantle cells were present. Plasma cells
were seen at the interfollicular area. There were no malignant cells.
Immunostaining revealed more than 90% of plasma cells per high-power
field with an immunoglobulin (Ig) G4:IgG ratio of more than 40% (Fig
2). The final diagnosis was IgG4-related disease of the left
tonsil.
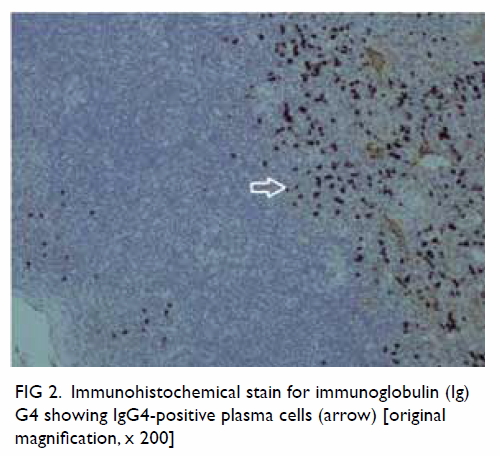
Figure 2. Immunohistochemical stain for immunoglobulin (Ig) G4 showing IgG4-positive plasma cells (arrow) [original magnification, x 200]
The patient refused workup with positron
emission tomography–CT scan but clinically there was no feature of
systemic IgG4-related disease so steroids were not prescribed. His
odynophagia gradually improved and he could tolerate oral feeding with
congee. The feeding nasogastric tube was removed. Unfortunately, the
patient had a depressive mood and general debility. He eventually died
of pneumonia in September 2015.
Discussion
Immunoglobulin G4–related disease is a
fibroinflammatory condition often associated with elevated serum IgG4
level.1 It is a multi-organ
entity that encompasses various conditions formerly considered to be
unrelated, single-organ diseases.2
It is notorious for its resemblance to malignant disease such as
carcinoma of the pancreas. It is a source of undue anxiety for both
patients and clinicians as the diagnosis may be difficult if the index
of clinical suspicion for IgG4-related disease is low. Systemic
symptoms (asthenia, weight loss, or fever) may occur in a minority of
patients. The disease can affect the pancreas (autoimmune pancreatitis
with abdominal pain), biliary tree (sclerosing cholangitis with
jaundice), salivary glands or lacrimal gland (parotid, submandibular
and lacrimal gland enlargement), lymph nodes, kidneys
(tubulointerstitial nephritis with renal failure), and retroperitoneum
(ureteric stricture).2
Measuring the serum IgG4 level is useful to
establish the diagnosis of this uncommon disease3 and may avoid unnecessary diagnostic surgery.
Although serum IgG4 level was initially thought to be a key diagnostic
feature of IgG4-related disease, more recent evidence has devalued the
significance of a raised level. The key to diagnosis is
immunohistochemical demonstration of tissue infiltration by
IgG4-bearing plasma cells and morphological evidence of
lymphoplasmacytic infiltrates, storiform fibrosis, and obliterative
phlebitis.2 Serum IgG4 assay
was not done as this investigation was not available at our hospital.
Nonetheless the diagnosis of IgG4-related disease of tonsil in this
patient was obvious based on the histopathological examination with
IgG4 immunostains.
Umehara et al4
proposed a set of criteria for the diagnosis of IgG4-related disease
designed to be used irrespective of the specific organ involvement.
The criteria are (1) serum IgG4 concentration of >135 mg/dL and (2)
>10 IgG-positive plasma cells demonstrated per high-power field, of
which >40% are IgG4-positive cells. Nevertheless the sensitivity is
low for the diagnosis of autoimmune pancreatitis based on these
criteria.
Of note, IgG4-related disease can manifest at
the head and neck region. Kuttner’s tumour of the submandibular gland
has been well reported and often masquerades as carcinoma.5 Nonetheless IgG4-related disease within the oral
cavity is very rare. To the best of our knowledge, only one case of
simultaneous IgG4-related lesions affecting the left border of the
tongue and right tonsil has been reported in the literature.6 This patient presented with an elevated and nodular
mass devoid of surface ulceration, associated with a generalised skin
rash. The oral and cutaneous lesions gradually resolved after therapy
with steroid.6 In contrast, our
patient had an ulcerative mass without any skin rash. Both cases were
initially suspected to be squamous cell carcinoma because of the
alarming clinical features.
Steroid remains the mainstay of treatment for
IgG4-related disease. It should be started early in order to prevent
tissue fibrosis and thus irreversible organ damage, except in cases of
asymptomatic disease of the submandibular gland or lymph node.7 Radiological abnormalities often vanish following
the commencement of steroid therapy. Unfortunately, disease relapse is
not uncommon after steroid therapy is tapered. There is no evidence to
support the effectiveness of adding conventional steroid-sparing agents
(eg azathioprine, methotrexate, or cyclophosphamide) to sustain
remission for IgG4-related disease.7
Although remission can be achieved in more than 80% of patients with
induction steroid therapy, some clinicians support the use of
maintenance steroid at 5 mg or 2.5 mg daily for a durable response.
However, disease may flare in a quarter of patients despite
maintenance therapy. Moreover, the optimal duration for maintenance
treatment is uncertain and the morbidity associated with steroid,
although in low dose, is not negligible. Fortunately, repeated
induction therapy with steroid for disease relapse is normally
effective.7
In summary, IgG4-related disease can involve
the tonsils and masquerade as tonsil carcinoma. Clinicians should
consider IgG4-related disease as one of the differential diagnoses for
a suspicious tonsillar mass. Early diagnosis of tonsillar mass with
biopsy is essential to exclude tonsil carcinoma and to allow prompt
steroid treatment for IgG4-related disease of tonsil which is curable.
References
1. Deshpande V, Zen Y, Chan JK, et al.
Consensus statement on the pathology of IgG4-related disease. Mod
Pathol 2012;25:1181-92. Crossref
2. Stone JH, Brito-Zerón P, Bosch X,
Ramos-Casals M. Diagnostic approach to the complexity of IgG4-related
disease. Mayo Clin Proc 2015;90:927-39. Crossref
3. Hamano H, Kawa S, Horiuchi A, et al.
High serum IgG4 concentrations in patients with sclerosing
pancreatitis. N Engl J Med 2001;344:732-8. Crossref
4. Umehara H, Okazaki K, Masaki Y, et
al. Comprehensive diagnostic criteria for IgG4-related disease
(IgG4-RD), 2011. Mod Rheumatol 2012;22:21-30. Crossref
5. Chow TL, Chan TT, Choi CY, Lam SH.
Kuttner’s tumour (chronic sclerosing sialadenitis) of the
submandibular gland: a clinical perspective. Hong Kong Med J
2008;14:46-9.
6. Khurram SA, Fernando M, Smith AT,
Hunter KD. IgG4-related sclerosing disease clinically mimicking oral
squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol
2013;115:e48-e51. Crossref
7. Khosroshahi A, Wallace ZS, Crowe JL,
et al. International consensus guidance statement on the management
and treatment of IgG4-related disease. Arthritis Rheumatol
2015;67:1688-99. Crossref

