Hong Kong Med J 2017 Aug;23(4):387–94 | Epub 26 Jun 2017
DOI: 10.12809/hkmj166049
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Current management of pregnancy-associated
breast cancer
Harry HY Yu, FCSHK, FHKAM (Surgery)1; Polly SY Cheung, FCSHK, FHKAM (Surgery)2; Roland CY Leung, MB, ChB, MRCP (UK)3;
TN Leung, FHKCOG, FHKAM (Obstetrics and Gynaecology)4; WH Kwan, FHKCR, FHKAM (Radiology)5
1 Department of Surgery, Ruttonjee & Tang Shiu Kin Hospitals, Wanchai, Hong Kong
2 Breast Care Centre, Hong Kong Sanatorium & Hospital, Happy Valley, Hong Kong
3 Department of Medicine, Queen Mary Hospital, Pokfulam, Hong Kong
4 Obstetrics & Gynaecology Centre, Hong Kong Sanatorium & Hospital, Happy Valley, Hong Kong
5 Comprehensive Oncology Centre, Hong Kong Sanatorium & Hospital, Happy Valley, Hong Kong
Corresponding author: Dr Polly SY Cheung (pollyc@pca.hk)
Abstract
Pregnancy-associated breast cancer is the most
common malignancy during pregnancy with an
expected rise in incidence. The belief in the need for
termination of pregnancy and that chemotherapy
is contra-indicated during pregnancy is challenged
by recent evidence. Patients can consider breast-conserving
surgery and sentinel lymph node
biopsy with acceptably low fetal risk from radiation
exposure. A range of chemotherapeutics is possible
in the second trimester in terms of drug class and
frequency. Hormonal therapy and monoclonal
antibody therapy are contra-indicated during
pregnancy and lactation. Fetal outcome after in-utero
exposure to chemotherapy appears similar
to that in a non-pregnant population. Future
pregnancy, in most situations, does not appear to
be contra-indicated but a multidisciplinary and
patient-centred approach is recommended. Fertility preservation
techniques are also being developed with reported success and consequent pregnancies.
Introduction
Pregnancy-associated breast cancer (PABC) is
defined as the diagnosis of breast cancer during
pregnancy and those occurring within 1 year
postpartum.1 It is the most common malignancy
associated with pregnancy and the incidence
is expected to rise in high-income countries.2 3 4 Historically, this disease entity was thought to have
a worse prognosis when compared with patients
diagnosed with cancer in the non-pregnant state
and termination of pregnancy was common. Here,
we reviewed recent publications and summarised
the most recent information.
‘Dual’ effect of pregnancy
Generally it is thought that pregnancy is a protective
factor that lowers the risk of breast cancer
development. Nonetheless, studies have found that
pregnancy increases the risk of breast cancer initially
following delivery and has a protective effect after a
period of time.3 The period of increased risk has been
estimated to be between 10 and 15 years following
a first pregnancy. The later the first pregnancy, the
longer the duration of increased risk before the
protective effect.5 In a Norwegian study, women who
waited until aged over 35 years for their first child permanently increased their risk of breast cancer
compared with nulliparous women.6
Risk of developing breast cancer decreases
with multiple pregnancies, but the age at first birth
remains the dominant influence on risk.5 BRCA1 and
BRCA2 mutation carriers are not protected by early
pregnancy from malignancy,2 but they do not have
an increased risk of developing PABC compared
with non-carrier women.5
Epidemiology
Breast cancer is the most common malignancy to
occur during pregnancy, followed by cervical cancer,
melanoma, and haematological malignancy.7 In
the United Kingdom, 1.3 to 2.4 cases of breast cancer in women
were diagnosed per 10 000 live births.1 It accounted
for 3.3% to 10% of women diagnosed with premenopausal
breast cancer.2 5
Meta-analysis has shown that risk of death
is more than 40% in women with PABC compared
with those with non-PABC (pooled hazard
ratio=1.44; 95% confidence interval, 1.27-1.63), but
other epidemiological studies have shown no direct
relationship between pregnancy and mortality.8
Moreover, the prognosis of patients with breast
cancer during pregnancy is similar to that of non-pregnant women of the same age and clinical stage at
diagnosis.9
In fact, metastasis is a rare event during
pregnancy, with only case series reported in the
literature.10
Epidemiological data indicate that breast
cancer diagnosed during lactation exhibits the
most aggressive trait and elevation in cause-specific
death.11 Compared with patients in the non-pregnant
state or during pregnancy, patients diagnosed with
breast cancer within 1 year of delivery have a shorter
time to relapse12 and increased risk of metastasis and
death.6 The lactating microenvironment is a strong
driver of tumour progression. Lactating stromal
adipose cells express higher levels of inflammatory
cytokines that are highly angiogenic and growth
promoting, causing the tumour to be more
aggressive.11
Other factors proposed to be related to the
poorer outcome include the change in breast tissue
and the hormonal environment. There is a shorter
phase of involution, an inflammatory-like process
that has been suggested to have tumour-promoting
properties by affecting the microenvironment and
malignant potential of microtumours.8 A generally
low index of suspicion of cancer in reproductive-aged
women and, in particular, pregnant women
has tended to delay diagnosis by approximately 1 to
2 months according to recent studies.8 13 A 1-month
delay in the treatment of a primary tumour increases
the risk of axillary metastases by 0.9% to 1.8%.14
Histopathology
As the group of affected women diagnosed
with malignancy generally represents a younger
population, PABC patients tend to have high-grade
tumours and display lymphovascular invasion.2 5 9
They also have a lower incidence of positive hormone
receptor status.3
Placental metastasis is rare but indicates poor
maternal prognosis. Pathological assessment after delivery is recommended in all cases.2
Diagnosis
Of breast masses that present to a breast clinic,
80% are benign although any mass that persists for
more than 2 to 4 weeks or that is associated with
skin changes raises clinical suspicion.2 15 Nipple
discharge and ‘milk rejection’ sign, ie refusal by
the infant to nurse from a diseased breast, can be
signs of underlying occult carcinoma, but are not
frequent.13 14
Ultrasonography (USG) is the first imaging
modality following clinical examination to assess
a discrete lump.1 Mammography (MMG) can also
supplement USG to assess extent of disease and
examine the contralateral breast. Accuracy of
MMG, however, is decreased in the pregnant state
because of increased water content and change in
the distribution of fat. Studies have shown a false-negative
rate as high as 25%2 with sensitivity of 63%
to 78%.14 Radiation exposure of the fetus from MMG
is minimal (0.001-0.01 mGy). The International
Commission on Radiological Protection (ICRP)
concluded in 2007 that there was no detrimental
effects of practical significance (threshold dose,
100 mGy).16 The use of magnetic resonance
imaging is controversial during pregnancy, due to
the challenges in discriminating malignant from
physiological hypervascularisation that occurs
during pregnancy.13 There are also no safety data
regarding the use of gadolinium in pregnancy,15 and
fetal abnormalities have been noted in rats exposed
to gadolinium.2
To complete triple assessment, core needle
or excision biopsy is the gold standard for tissue
diagnosis. Histological grade, receptor status, and
human epidermal growth factor receptor 2 (HER2)
information should be obtained, just as for non-pregnant
patients.1 2 Needle cytology shows good
sensitivity but with a higher risk of false-positive
results due to the presence of hyperproliferative cells
in mammary tissue during pregnancy and lactation.2 The risk of developing a subsequent lactating fistula
after biopsy is overestimated.15 Cabergoline can
reduce the risk of fistula or abscess formation by
suppressing lactation.2 Emptying the breast and the
use of ice packs and binding before biopsy might also
reduce the risk of fistula formation.13
Unless there is a strong clinical suspicion,
metastatic work-up is not mandatory.1 Chest X-ray
and USG of the liver are the main modalities used
to look for metastases. Fetal exposure from a chest
X-ray is about 0.1 mGy.13
Treatment
For those who develop breast cancer during
pregnancy, any treatment intervention during pregnancy shows a trend towards improved overall
survival compared with delaying evaluation and
treatment until after delivery—78.6% overall
survival for those who received treatment during
pregnancy compared with 44.7% for those patients
who did not.17 Management of postpartum breast
cancer is similar to that for non-pregnant patients.
Consideration of fetal well-being is a critical factor
when making treatment decisions for the pregnant
patient.
Surgery
Surgery is considered safe in all trimesters with
negligible risk to the fetus. Studies, including a large
review, concluded that surgery carries a similar
probability of miscarriage to the background risk of
spontaneous abortion, including first trimester.7 18
Mastectomy is the definitive procedure. There
is a trend for favouring breast-conserving surgery as
a suitable option for PABC patients, and this should
be discussed with the patient whenever possible.19
As epidemiological data show a higher
percentage of axillary metastases among PABC
patients, axillary dissection is generally offered.2 When the tumour is diagnosed at an early stage,
however, a considerable proportion of patients will
have node-negative disease and therefore might
benefit from sentinel lymph node biopsy (SLNB).
Such procedure remains controversial18 but some
studies have reported its success and safety.
The first issue to clarify in SLNB is fetal
radiation exposure relating to lymphoscintigraphy.
In Italy, Gentilini et al20 reviewed 26 young non-pregnant
women scheduled for lymphoscintigraphy
for SLNB. A single peritumoural injection of
99mTc-labelled human albumin colloid particles
in a volume of 0.2 mL was administered prior to
surgery. A thermoluminescent dosimeter was placed
around the abdomen (epigastrium, umbilicus, and
hypogastrium) to evaluate potential uptake by the
fetus in different trimesters. In 23 of 26 patients, all
absorbed dose measurements over the surface of the
abdomen at the supposed level of a fetus were lower
than the sensitivity of the dosimeter (<10 µGy). In
the remaining three patients, the absorbed doses
to the epigastrium, umbilicus, and hypogastrium
were 40-320, 120-150, and 30-40 µGy, respectively.
Another study estimated the maximal absorbed dose
of radiation from SNLB by 99mTc sulfur colloid to be
4.3 mGy.18
Fetal exposure of >100 to 200 mGy is associated
with central nervous system problems. A radiation
dose that exceeds 100 mGy can result in reduced
intelligence quotient. With a dose of 10 mGy, the risk
of leukaemia and cancer is 1.4%, ie a 40% increase
over the normal incidence. An absorbed dose of
20 µGy is comparable with 1 to 2 days of natural
background radiation, 1/5000th of the threshold dose for malformation or other adverse effects.21 Of
note, reports of the ICRP have shown that the most
common procedures in diagnostic nuclear medicine
rarely represent an indication for termination of
pregnancy, and that pregnancy should not be a reason
to avoid diagnostic nuclear medicine studies.22
Gentilini et al21 then reported the use of SLNB
in pregnant patients from 2001 to 2007. During
the period, 12 of 45 patients diagnosed with breast
cancer during pregnancy were clinically node-negative
and all underwent SLNB. Lymphatic
mapping was performed by 99mTc-radiolabelled
colloid lymphoscintigraphy alone with mean activity
of 10 MBq (about 1 µGy/MBq) for the first eight
patients and 3-4 MBq for the later four. Hot spots
were identified in all patients (10 with 1 hot spot,
and two with 2 hot spots) and the mean number of
excised sentinel lymph nodes (SLNs) was two (range,
1-4). Ten patients had confirmed negative SLNs and
were spared axillary dissection. One patient had
micrometastasis in one of the four nodes and elected
not to undergo any further axillary surgery. Another
patient had a confirmed metastatic SLN intra-operatively
and underwent axillary clearance at the
same operation (3 of 24 positive nodes). No overt
axillary lymph node reappeared for patients with
negative SLN. Of the 12 pregnancies, 11 babies were
born with a normal weight and no malformation
after an uncomplicated pregnancy. One baby
underwent surgery at 3 months of age for cardiac
failure due to ventricular septal defect. This had
been suspected at the 21st week of gestation prior to
lymphoscintigraphy at the 26th week.
Two other studies also reported the use
of SLNB with 99mTc colloid and/or blue dye
(isosulfan or methylene) for pregnant patients.19 21
All 35 patients who underwent SLNB had successful
mapping and surgery was performed without
complications; 33 of 35 infants were healthy at
delivery. One patient found herself pregnant on
the day of her scheduled operation and decided to
terminate her pregnancy in the first trimester after
the breast surgery in order to start chemotherapy.
Another child was born with a cleft palate to a mother
who was a smoker with a history of methadone use.
Axillary staging provides important prognostic
information and allows better local control but does
not always influence the type of adjuvant therapy.
With an acceptably low false-negative rate and the
same capacity to detect nodal metastasis, SLNB has
a lower morbidity compared with axillary lymph
node dissection (lymphoedema, 5.3% vs 11.8%).21 23
The decrease in surgical morbidity might result in a
shorter postoperative recovery, potentially allowing
an earlier start for adjuvant chemotherapy. In young
premenopausal patients, such treatment can be
directed towards hormone insensitive tumours
and might improve outcome.20 Proponents of SLNB also point out the possibility of performing
breast-conserving surgery and SLNB under local
anaesthesia to further reduce the risk of preterm
labour and spontaneous miscarriage.20
There are, however, specific issues to be
solved regarding SLNB use during pregnancy.
Isosulfan blue and methylene blue are Food and
Drug Administration pregnancy category C drugs,
with unknown potential risks for teratogenicity.23
Anaphylaxis has been reported with the use of
blue dye.18 24 25 Adverse outcomes associated with
methylene blue dye include intestinal atresia and
fetal demise.26
Fetal radiation exposure and successful
mapping of lymphoscintigraphy depend on the
administered activity and size of radiocolloid. To
minimise unnecessary radiation exposure, pregnant
patients should avoid contact with other patients
receiving nuclear medicine therapy, eg by scheduling
pregnant patients as the first procedure of the day,
and keeping them in a single-bed room. Reducing
the time between lymphoscintigraphy and surgery
might further reduce the dose of administered
radiocolloids.20
The latest European Society for Medical
Oncology (ESMO) guidelines do not discourage
SLNB in pregnant breast cancer patients in centres
where SLNB is routinely practised in the non-pregnant
setting, but discourages the use of blue
dye.27 Despite weak evidence, however, the latest
American Society of Clinical Oncology guidelines are
still against performing SLNB during pregnancy.28
Immediate reconstruction is not recommended
and should be delayed to avoid prolonged
anaesthesia and to allow optimal symmetrical breast
reconstruction after delivery.1
Systemic chemotherapy
Indications for chemotherapy are the same as for
non-pregnant breast cancer patients. When the
indication for chemotherapy is clear, it should not
be delayed due to the potential detrimental effect
on maternal outcome, with the exception of breast
cancer diagnosed during the first trimester.29 Adjuvant chemotherapy should be started within 3
weeks of surgery in patients with hormone-negative
tumours.2 29
Most chemotherapeutic agents are of low
molecular weight, highly lipid soluble, and loosely
protein bound. This facilitates transplacental transfer
from the mother to the fetus.7 Teratogenicity is
directly related to timing and dosage delivered. The
most vulnerable period for fetal malformation and
spontaneous abortion is 10 days to 8 weeks after
conception, ie the period of organogenesis (17%).2 29 Throughout the first trimester, chemotherapy is still
considered contra-indicated due to concerns about
adverse events associated with ocular formation, genitals, the haematopoietic system, and the central
nervous system before the 14th week.1
Chemotherapy is regarded as safe during the
second trimester.1 However, administration during
the second and third trimesters is associated with
increased risk of intrauterine growth restriction and
low birth rate, which may be related to both tumour
burden and/or the aggressive nature of the tumour,
as well as the toxicity of chemotherapy.29 Changes
that occur during pregnancy, such as generation of a
third space (fetal-placental amniotic fluid), increased
volume distribution, and changes in the metabolism
and elimination of drugs, may determine different
toxicity patterns that may also indirectly affect the
fetus.15 General chemotherapy risks include preterm
delivery, low birth weight, transient tachypnoea of
the newborn, and transient neonatal leukopenia.2
The reported fetal malformation risk following
chemotherapy during the second and third trimesters
is 3.8%, no higher than that in the general population.18
Incidence of preterm delivery for chemotherapy-exposed
gestational breast cancer is 5% to 8%.29 Most
children showed normal neurological development
after exposure to chemotherapy in utero, although
behavioural and emotional issues need further
clarification and follow-up.29 30 For PABC patients,
chemotherapy-induced gonadotoxicity may cause
permanent amenorrhoea with complete loss of germ
cells, transient amenorrhoea, menstrual irregularity,
and subfertility, but this depends on the dose, agent,
and patient age.1
The German Breast Group reported a
multicentre study that included 197 patients who
received chemotherapy during pregnancy (a total
of 447 patients in 8 years).4 Overall, 50% of breast
cancer patients delivered preterm, compared with
10% to 15% of the general population. Delivery
before the 37th week of gestation is associated with
a higher chance of side-effects, malformations, or
newborn complications. Low birth weight is affected
by chemotherapy exposure after adjustment for
gestational age, but not by number of chemotherapy
cycles (P=0.018). Adverse events were more common
in those who received chemotherapy in utero than
in those who were not exposed (31 [15%] of 203 vs
7 [4%] of 170 infants; P=0.00045). The proportion of
malformations in the study was no different to that
for the general population (approximately 9%). Two
fetal deaths were reported and both were exposed to
chemotherapy and delivered prematurely. Neither
was thought to be related to treatment—one was
related to diagnosis of trisomy 18 and the other died
of necrotising enterocolitis after delivery at the 31st
week with weight of 1895 g. The study concluded that
although chemotherapy exposure in utero resulted
in lower birth weight and more complications, the
differences were not clinically significant and most
likely related to premature delivery.
Optimum use of cytotoxic drugs in pregnant
patients remains undefined, particularly regarding
drug selection, dosing, and dose density.31
Assessment of treatment effectiveness in pregnant
patients is complex. Calculation of chemotherapy
dose is uncertain for the pregnant state. Physiological
changes in pregnancy can also greatly affect drug
disposition.
Anthracycline-based regimens (eg
epirubicin and doxorubicin) are the most widely
used chemotherapeutic agents as they have a
favourable safety profile when administered during
pregnancy,29 although no particular preference is
given for one regimen over another.27 Assessment
of maternal cardiac function by echocardiogram is
recommended if an anthracycline-based regimen
is to be administered.14 Studies are yet to show an
increased risk of fetal cardiotoxicity secondary to
in-utero exposure.27 Common side-effects include
neutropenia, oral ulcers, anaphylaxis, constipation,
tachycardia, and cellulitis. Fetal side-effects include
low birth weight (7.6%) and birth defects (3.8%).18 Risk of congenital malformations is similar to that
of patients not receiving chemotherapy. Long-term
follow-up of children indicates that there are no
sequelae associated with growth and maturation.9
Taxanes appear to be another alternative,
but have not been as extensively studied as
anthracyclines and most studies used small sample
sizes.18 A meta-analysis showed that the addition of
taxanes to anthracycline-based regimens resulted
in a statistically significant reduction in the risk
of relapse (relative reduction, 17%) and death
(relative reduction, 15%) for high-risk early breast
cancer patients. Disease-free survival benefit was
independent of oestrogen-receptor expression,
degree of nodal involvement, and type of taxane
used.32 Taxanes are substrates of the P-glycoprotein
that is highly expressed on the maternal compartment
of the placenta. P-glycoprotein protects the fetus
against xenobiotics and might therefore reduce
transplacental transfer of taxanes.29 Data for baboon
and human models showed that taxanes are hardly
detectable in the fetus,4 and a recent overview of 50
breast cancer patients treated with taxanes showed that
completely healthy neonate was born in a majority of
cases.33 In a review of 40 pregnant women prescribed
taxanes, there were no reports of intrauterine death
or congenital malformations other than one infant
with pyloric stenosis.34 Another retrospective cohort
study of 12 patients with breast cancer and four with
ovarian cancer who were exposed to taxane-based
chemotherapy during pregnancy reported a mean
gestational age at delivery of 36.9 weeks and mean
birth weight of 2452 g (interquartile range, 2155-2619 g). One baby was diagnosed with hypertrophic
pyloric stenosis at 4 weeks and underwent surgery
at 6 weeks. One of a set of twins born in this study had hyperbilirubinaemia and
jaundice, and was later diagnosed with Asperger’s syndrome and
Tourette’s syndrome, while his twin exposed to the same chemotherapy is
developmentally normal and excelled at school.32
Taxanes are metabolised by cytochrome P450
that increases by 50% to 100% during the third
trimester, possibly resulting in a shorter half-life
and higher clearance that could result in a reduced
toxicity profile during pregnancy.29 Nonetheless
this lowered serum concentration is a concern for
drug efficacy during pregnancy.14 There are no data
to analyse different taxanes for pharmacokinetics,
toxicity profile, or efficacy due to small sample size.
The latest ESMO guidelines also endorsed the use of
taxanes during pregnancy in cases where “they are
clinically indicated or the use of anthracyclines is
contraindicated”.27
A dose-dense chemotherapy regimen for
pregnant patients is another topic of heated
debate. Chemotherapy cycles were administered
every 1 to 2 weeks compared with 3-weekly cycles
for conventional therapy. One study compared
10 patients who received doxorubicin and
cyclophosphamide dose-dense therapy during
gestation with 99 patients receiving conventional
chemotherapy after the first trimester, and reported
completion of chemotherapy for all patients (98%
in conventional group). They had similar delivery
outcomes, risk of congenital anomalies, incidence,
and time to recurrence, and maternal overall
survival at 3.5 years.35 Proponents suggest that dose-dense
chemotherapy in pregnancy may allow faster
completion of chemotherapy, sufficient time for
maternal recovery for delivery,35 closer pregnancy
monitoring, and better toxicity profile, and no need
for high-dose steroid premedication or prophylactic
use of granulocyte-colony stimulating factor.27
Chemotherapy is advised to be withheld 3
weeks before delivery or after 35 weeks of gestation
to minimise the risk of sepsis and haemorrhage
in the mother and newborn.1 2 It allows time for
fetal drug excretion via the placenta, especially
for preterm babies who have a limited ability to
metabolise drugs through an immature liver and
kidneys. Chemotherapy can resume after adequate
recovery from delivery.29 Breastfeeding is contra-indicated
during the treatment period but can
resume 3 to 4 weeks after the last administered dose
of chemotherapy.2
Radiotherapy
Radiotherapy is contra-indicated until after delivery
unless it is used for life-saving issues or to preserve
organ function, eg spinal cord compression.1 If
radiotherapy is indicated during the pregnant state,
fetal shielding should be considered or the option of
elective early delivery discussed. Substituting whole-breast radiotherapy with partial-breast treatment
is another alternative.2 Excess cancer risk to a fetus
receiving radiation is 6.57 cases per 10 000 children
per rad (0.01 Gy) per year. The typical external beam
radiation dose to the breast ranges from 45 to 60 Gy,
and may result in a fetal radiation exposure of 3.9
to 15 rad in the first trimester and up to 200 rad in
the late third trimester.14 Other risks of radiotherapy
include miscarriage, teratogenicity, microcephaly,
fetal growth restriction, and induction of childhood
malignancy and haematological disorders.
Adjuvant radiotherapy is not considered an
urgent procedure and should be postponed until
after delivery.27 Delaying treatment after 12 weeks,
however, can increase the likelihood of axillary
metastases by 0.028% to 0.057% per day2 and a
delay over 6 months can increase the risk of local
recurrence.27
Hormonal therapy
Tamoxifen is not used until after delivery.1 It is
associated with oculo-auriculo-vertebral dysplasia
(Goldenhar’s syndrome) and ambiguous genitalia.2 18 Because of unknown transmission of the drug in
milk, it is also contra-indicated in breastfeeding.1
Long-term effects of the drug on female offspring
are unknown.14
Monoclonal antibody
Trastuzumab is contra-indicated during pregnancy
due to reported adverse fetal outcomes.1 Meta-analysis
showed the main adverse event to be
oligohydramnios (61.1%), with the incidence
increasing with duration of treatment.18 Alteration
of amniotic fluid volume is mostly attributed to the
effect of trastuzumab on the fetal kidney, where the
HER2 receptor is highly expressed.29 Nonetheless, if
the fetus was exposed to the drug exclusively during
the first trimester, all children were completely
healthy at birth: transplacental transport of immunoglobulin G is very low early in pregnancy
and increases gradually during the second trimester
to reach concentrations similar to the mother by the
end of gestation.29 Oligohydramnios is reversible if
the drug is stopped, with good outcome observed in
the majority of cases.
Another risk of trastuzumab for the fetus is
renal failure,9 but there are no reports of serious
fetal cardiac effects.14 Breastfeeding is also contra-indicated
due to unknown transmission of the drug
in milk. The drug is not associated with impaired
fertility.1
Supportive agents
Serotonin antagonists and dexamethasone are
the preferred antiemetics.1 Granulocyte-colony
stimulating factor is recommended to minimise
potential maternal and fetal problems associated
with neutropenia1 and erythropoietin has been
safely administered in pregnant patients.18
In-utero exposure to bisphosphonates has been
shown to increase the risk of fetal skeletal anomalies
and result in hypocalcaemia that may affect uterine
contraction. It is suggested that these drugs be
administered after delivery whenever possible.29
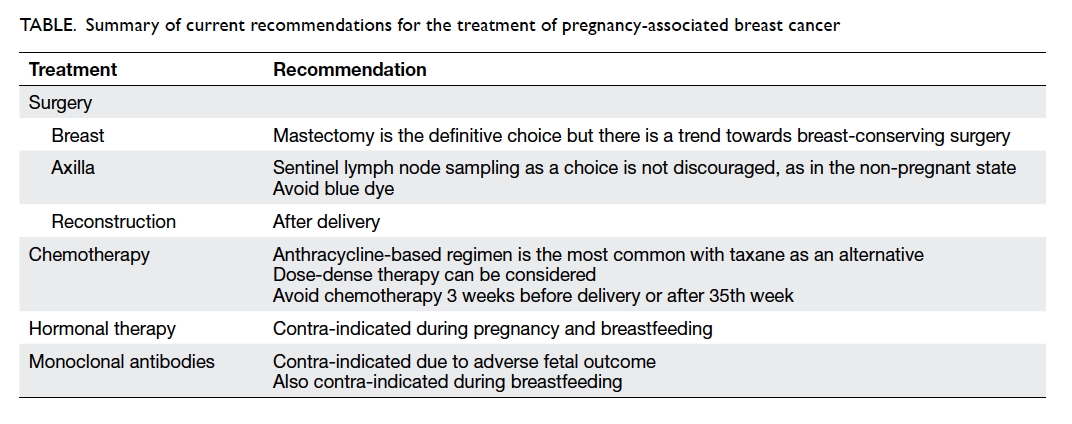
A summary of current recommendations
regarding treatment of PABC is shown in the Table.
Antenatal care
There is no evidence of a need for additional
antenatal care, but it is standard practice to establish
fetal well-being by USG before any treatment.2 15 Serial fetal growth assessment should be performed
every 3 to 4 weeks, or prior to each chemotherapy
cycle. Other forms of assessment of fetal well-being
may be beneficial, such as umbilical artery Doppler
to assess the status of the placenta, Doppler of the
fetal middle cerebral artery to exclude fetal anaemia,
and serial fetal echocardiograms (when potential
cardiotoxic drugs like anthracyclines are being used). Assessment of amniotic fluid volume is also
necessary because it can decrease reversibly with the
use of some drugs.15
Unless there is a clear oncological or obstetric
indication, delivery should be delayed until after the
37th week. Morbidity and mortality in newborns
are directly related to gestational age at delivery.
Infants born in late preterm (34th to 35th week)
have increased morbidities including perinatal
death, transient tachypnoea, respiratory distress,
hypoglycaemia, pulmonary hypertension, as well as
long-term cognitive and behavioural morbidities.7
Vaginal delivery is preferred because it is less
likely to delay initiation of chemotherapy due to lower
morbidity.29 Caesarean delivery should be reserved
for the usual obstetric indications.2 Deep venous
thrombosis prophylaxis should be considered, as
pregnancy and malignancy are both risk factors for
venous thromboembolism.
Termination of pregnancy
There is no evidence to suggest that termination of
pregnancy improves prognosis.2 10 Once pregnancy
has occurred, induction of abortion has no impact
on maternal prognosis and is therefore strongly
discouraged for such purposes.27 If maternal
outcomes are not negatively impacted by the
pregnancy itself, continuation of pregnancy seems not
only reasonable, but recommended.7 Nevertheless,
in case of advance disease stage (stage III or IV)
or for high-grade or aggressive primary tumours
diagnosed in the early first trimester, termination
of pregnancy may be considered (teratogenic risk of
chemotherapy during the first trimester).2 15
Future pregnancy
There is evidence that pregnancy after breast cancer
does not lead to increased risk of recurrence and
may even improve survival, although these findings
could be due to the ‘healthy mother effect’.5 14 Large
matched multicentre retrospective studies including
more than 1000 patients confirmed that pregnancy
after oestrogen receptor (ER)–positive breast cancer
was not detrimental, at least during the first 5 years
following pregnancy.27 The latest ESMO guidelines
also “do not discourage pregnancy following breast
cancer diagnosis irrespective of the ER status”.27
Nonetheless, the chance of subsequent
pregnancy is nearly 70% lower when compared
with the general population, probably secondary to
frequent treatment with gonadotoxic chemotherapy,
prolonged treatment periods with tamoxifen in
patients with hormone sensitive disease, and also
a general misconception that pregnancy could
stimulate cancer recurrence given that it is a
hormonally driven disease.27 The chance of recovery
of menses is higher for patients under 40 years of age and the use of taxane-based chemotherapy.5
“Consult before conceive”—a multidisciplinary
approach is recommended before planning a
pregnancy. Anecdotal evidence suggests a 2-year wait
after treatment and a 5-year wait for recurrent stage
I and II disease.2 Patients with metastatic disease are
advised against pregnancy due to their limited life expectancy
and possible compromised treatment of
disease.1 Interruption of full-course tamoxifen may
have detrimental effects on breast cancer outcome.
If, however, a woman is willing to accept the risk,
interruption after 2 to 3 years of tamoxifen may be
considered to allow pregnancy. Tamoxifen should
be stopped for 3 months before trying to conceive.
Latest ESMO guidelines “strongly encourage the
resumption of tamoxifen following delivery”.1 27 It is
also advised to continue active contraception up to 3
to 6 months following the last administered dose of
anti-cancer therapy.27
Embryo or oocyte cryopreservation is the
main method to preserve female fertility.36 Ovarian
stimulation is carried out before commencing
chemotherapy, but may result in relative delay in
oncological treatment and increase serum oestradiol
levels. This may be of concern in hormone-driven
tumours like breast cancer. Laparoscopic ovarian
tissue sampling and freezing before treatment
are considered experimental. When needed, re-implantation
of ovarian tissue in the pelvis after
thawing may be a unique option for young girls with
cancer. Over 60 pregnancies have been reported.37
Conclusion
The prognosis of PABC is similar to that of breast
cancer in the non-pregnant state. Treatment should
commence after diagnosis is established. Surgical
treatment options are expanding and extensive data
show that chemotherapy during pregnancy is safe
and more options for treatment are now available.
Whenever possible, the aim should be to carry the
fetus to term. Future pregnancy is generally not
contra-indicated.
References
1. RCOG Green-top guideline No. 12. Pregnancy and
breast cancer. UK Royal College of Obstetricians and
Gynaecologists; 2011.
2. Padmagirison R, Gajjar K, Spencer C. Management
of breast cancer during pregnancy. Obstet Gynecol
2010;12:186-92. Crossref
3. Genin AS, Lesieur B, Gligorov J, Antoine M, Selleret L,
Rouzier R. Pregnancy-associated breast cancers: do they
differ from other breast cancers in young women? Breast
2012;21:550-5. Crossref
4. Lobiol S, Han SN, von Minchkitz G, et al. Treatment of
breast cancer during pregnancy: an observational study.
Lancet Oncol 2012;13:887-96. Crossref
5. Bell RJ, Fradkin P, Parathithasan N, Robinson PJ, Schwarz
M, Davis SR. Pregnancy-associated breast cancer and pregnancy following treatment for breast cancer, in a
cohort of women from Victoria, Australia, with a first
diagnosis of invasive breast cancer. Breast 2013;22:980-5. Crossref
6. Borges VF, Schedin PJ. Pregnancy-associated breast
cancer: an entity needing refinement of the definition.
Cancer 2012;118:3226-8. Crossref
7. Walton JR, Prasad MR. Obstetric and neonatal outcomes
of cancer treated during pregnancy. Clin Obstet Gynecol
2011;54:567-73. Crossref
8. Johansson AL, Andersson TM, Hsieh CC, et al. Stage
at diagnosis and mortality in women with pregnancy-associated
breast cancer (PABC). Breast Cancer Res Treat
2013;139:183-92. Crossref
9. Córdoba O, Llurba E, Saura C, et al. Multidisciplinary
approach to breast cancer diagnosed during pregnancy:
maternal and neonatal outcomes. Breast 2013;22:515-9. Crossref
10. Azim HA Jr, Peccatori FA. Treatment of metastatic
breast cancer during pregnancy: we need to talk! Breast
2008;17:426-8. Crossref
11. McCready J, Arendt LM, Glover E, et al. Pregnancy-associated
breast cancers are driven by differences in
adipose stromal cells present during lactation. Breast
Cancer Research 2014;16:R2. Crossref
12. Halaska MJ, Pentheroudakis G, Strnad P, et al. Presentation,
management and outcome of 32 patients with pregnancy-associated
breast cancer: a matched controlled study.
Breast J 2009;15:461-7. Crossref
13. Rovera F, Frattini F, Coglitore A, et al. Breast cancer in
pregnancy. Breast J 2010;16 Suppl 1:S22-5. Crossref
14. Viswanathan S, Ramaswamy B. Pregnancy-associated
breast cancer. Clin Obstet Gynecol 2011;54:546-55. Crossref
15. Sánchez Martínez MC, Ruiz Simón A. Breast cancer during pregnancy. Breast Cancer Rest Treat 2010;123
Suppl 1:55-8. Crossref
16. International Commission on Radiological Protection. The 2007 recommendations of the International Commission on Radiological Protection. Ann ICRP 2007;37:1-332.
17. Beadle BM, Woodward WA, Middleton LP, et al. The
impact of pregnancy on breast cancer outcomes in women
≤35 years. Cancer 2009;115:1174-84. Crossref
18. Krishna I, Lindsay M. Breast cancer in pregnancy. Obstet
Gynecol Clin North Am 2013;40:559-71. Crossref
19. Khera SY, Kiluk JV, Hasson DM, et al. Pregnancy-associated
breast cancer patients can safely undergo lymphatic
mapping. Breast J 2008;14:250-4. Crossref
20. Gentilini O, Cremonesi M, Trifirò G, et al. Safety of sentinel
node biopsy in pregnant patients with breast cancer. Ann
Oncol 2004;15:1348-51. Crossref
21. Gentilini O, Cremonesi M, Toesca A, et al. Sentinel lymph
node biopsy in pregnant patients with breast cancer. Eur J
Nucl Med Mol Imaging 2010;37:78-93. Crossref
22. International Commission on Radiological Protection.
Pregnancy and medical radiation. Ann ICRP 2000;30:iii-viii,1-43.
23. Gropper AB, Calvillo KZ, Dominici L, et al. Sentinel lymph
node biopsy in pregnant women with breast cancer. Ann
Surg Oncol 2014;21:2506-11. Crossref
24. Karam A. Update on breast cancer surgery approaches.
Curr Opin Obstet Gynecol 2013;25:74-80. Crossref
25. te Velde EA, Sonke G, Rutgers EJ. Breast cancer and
pregnancy: diagnosis and treatment options. Breast Cancer
Online 2009;12:e10. Crossref
26. Pruthi S, Haakkenson C, Brost BC, et al. Pharmacokinetics
of methylene blue dye for lymphatic mapping in breast
cancer—implications for use in pregnancy. Am J Surg
2011;201:70-5. Crossref
27. Peccatori FA, Azim HA Jr, Orecchia R, et al. Cancer,
pregnancy and fertility: ESMO clinical practice guidelines
for diagnosis, treatment and follow-up. Ann Oncol 2013;24
Suppl 6:vi160-70. Crossref
28. Lyman GH, Temin S, Edge SB, et al. Sentinel lymph
node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice
guideline update. J Clin Oncol 2014;32:1365-83. Crossref
29. Azim HA Jr, Del Mastro L, Scargone G, Peccatori FA.
Treatment of breast cancer during pregnancy: regimen
selection, pregnancy monitoring and more… Breast
2011;20:1-6. Crossref
30. Cardonick E. Treatment of maternal cancer and fetal
development. Lancet Oncol 2012;13:218-20. Crossref
31. Cardonick E, Bhat A, Gilmandyar D, Somer R. Maternal
and fetal outcomes of taxane chemotherapy in breast and
ovarian cancer during pregnancy: case series and review of the
literature. Ann Oncol 2012;23:3016-23. Crossref
32. Mir O, Berveiller P. Increased evidence for use of
chemotherapy in pregnancy. Lancet Oncol 2012;13:852-4. Crossref
33. Zagouri F, Sergentanis TN, Chrysikos D, et al. Taxanes for
breast cancer during pregnancy: a systematic review. Clin
Breast Cancer 2013;13:16-23. Crossref
34. Mir O, Berveiller P, Goffinet F, et al. Taxanes for breast
cancer during pregnancy: a systematic review. Ann Oncol
2010;21:425-6. Crossref
35. Cardonick E, Gilmandyar D, Somer RA. Maternal and
neonatal outcomes of dose-dense chemotherapy for breast
cancer in pregnancy. Obstet Gynecol 2012;120:1267-72. Crossref
36. Angarita AM, Johnson CA, Fader AN, Christianson MS.
Fertility preservation: a key survivorship issue for young
women with cancer. Front Oncol 2016;6:102. Crossref
37. Donnez J, Dolmans MM. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide
expansion of the technique towards routine clinical
practice. J Assist Reprod Genet 2015;32:1167-70. Crossref