Hong Kong Med J 2017 Apr;23(2):177–90 | Epub 17 Mar 2017
DOI: 10.12809/hkmj166098
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Best practices to prevent transmission and
control outbreaks of hand, foot, and mouth
disease in childcare facilities: a systematic review
Joyce HY Chan, MPH (HK)1;
CK Law, PhD1;
Esther Hamblion, PhD2;
H Fung, MHP (HK)1;
James Rudge3
1 The Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong, Shatin, Hong Kong
2 Independent Consultant Epidemiologist, London, United Kingdom
3 Communicable Diseases Policy Research Group, Department of Global
Health and Development, London School of Hygiene and Tropical
Medicine, Bangkok Office, Thailand
Corresponding author: Dr Joyce HY Chan (hychan@cuhk.edu.hk)
Abstract
Introduction: Hand, foot, and mouth disease
continues to cause seasonal epidemics in the Asia-Pacific Region. Since the current Enterovirus 71
vaccines do not provide cross-protection for all
Enterovirus species that cause hand, foot, and
mouth disease, there is an urgent need to identify
appropriate detection tools and best practice to
prevent its transmission and to effectively control its
outbreaks. This systematic review aimed to identify
characteristics of outbreak and assess the impact
and effectiveness of detection tools and public health
preventive measures to interrupt transmission. The
findings will be used to recommend policy on the
most effective responses and interventions in Hong
Kong to effectively minimise and contain the spread
of the disease within childcare facilities.
Methods: We searched the following databases
for primary studies written in Chinese or English:
MEDLINE, EMBASE, Global Health, WHO Western
Pacific Region Index Medicus database, China
National Knowledge Infrastructure Databases,
and Chinese Scientific Journals Database. Studies
conducted during or retrospective to outbreaks of
hand, foot, and mouth disease caused by Enterovirus
71 from 1980 to 2012 within childcare facilities and
with a study population of 0 to 6 years old were
included.
Results: Sixteen studies conducted on outbreaks in
China showed that hand, foot, and mouth disease
spread rapidly within the facility, with an outbreak
length of 4 to 46 days, especially in those with
delayed notification (after 24 hours) of clustered
outbreak (with five or more cases discovered within
the facility) to the local Center for Disease Control
and Prevention and delayed implementation of a
control response. The number of classes affected
ranged from 1 to 13, and the attack rate for children
ranged from 0.97% to 28.18%.
Conclusions: Communication between key
stakeholders about outbreak confirmation, risk
assessment, and surveillance should be improved.
Effective communication facilitates timely
notification (within 24 hours) of clustered outbreaks
to a local Center for Disease Control and Prevention.
Timely implementation of a control response is
effective in minimising incidence and length of an
outbreak in childcare facilities. The government
should provide incentives for childcare facilities to
train infection control specialists who can serve as
the first contact, knowledge, and communication
points, as well as facilitate exchange of information
and provision of support across stakeholders during
a communicable disease epidemic.
Introduction
Hand, foot, and mouth disease (HFMD) is an
increasing burden in the Western Pacific Region
including Australia, Brunei Darussalam, China,
Japan, Malaysia, Mongolia, the Republic of Korea,
Singapore, and Vietnam with 3 million cases and 400
deaths reported in 2014.1 It is a common infection in
children aged between 1 and 5 years during the hot
and humid season across East and South-East Asia.1 2 Transmission of HFMD is increased in crowded and
closed environments, such as kindergartens or child
daycare centres, where infectious droplets can easily
spread via sharing of objects or surfaces (fomites).3
In 2014, China accounted for nearly a third of
this burden with 2.6 million HFMD cases and 371
deaths.1 Hong Kong, a Special Administrative
Region (HKSAR) of China, has also been affected by
HFMD outbreaks. In 2015, there were around 700
institutional HFMD outbreaks affecting 4200 Hong
Kong people; 60% of which occurred in childcare
centres or kindergartens.4
Hand, foot, and mouth disease is caused by
viruses belonging to the Enterovirus genus, such
as Coxsackievirus A16 (CA16) and Enterovirus
71 (EV71). In the Western Pacific Region of the
World Health Organization (WHOWPR), infections
with high severity and complications are mainly
associated with EV71.5 6 7 Outbreaks of EV71 in the
region have been identified in 41% of HFMD cases,
81% of severe cases, and 93% of deaths, resulting in
higher rates of complications, neurological disease,
and fatalities compared with other causative agents
such as CA16.6 8 9 10 11 12 It is therefore important to
distinguish EV71 from other HFMD strains during
the outbreak.13
In 2011, following the emergence of HFMD
outbreaks throughout the Western Pacific Region,
the HKSAR Government announced EV71 infection
as one of the 47 statutorily notifiable communicable
diseases under the Prevention and Control of Disease
Ordinance (Central Notification Office).14 According
to HFMD outbreak management practices issued by
the WHO, the HKSAR Centre for Health Protection
(CHP) issued guidelines and letters to all childcare
facilities, specifically describing management plans
in the event of a suspected EV71 outbreak, including
steps and standard forms for notification of suspected
outbreaks. They also recommended that public
health control measures be implemented, namely
promoting personal and hand hygiene, regular body
checks, environmental disinfection, full or partial
closure of the facility, and sentinel surveillance.6 14 Despite efforts of the HKSAR Government and
health education through public media over the
years, institutional outbreaks of HFMD have
continued to occur within childcare facilities during
the summer and winter periods with approximately
780, 350, and 700 outbreaks in 2013, 2014, and 2015,
respectively.4 The outbreak size ranged from 2 to 56
persons (median, 4 persons).4 Among them, EV71
accounted for 19% of cases in 2014 but only 8% in
2015.4 No fatal case was reported in 2015, but 86%
of EV71 cases required hospitalisation and 11%
developed severe complications such as encephalitis,
meningitis, and cerebellitis albeit with no long-term
neurological consequences.4 The burden of
HFMD remains prevalent among young children in
institutional settings.15
Given the health and social impact in the
Western Pacific Region, China, Taiwan, and Singapore
started developing EV71 vaccines for children under
5 years old.16 Three vaccines that provided more than
90% protection against EV71-associated HFMD for
children under 5 years old were granted a license
from the China Food and Drug Administration in
2014.16 17 Nonetheless, despite their clinical efficacy, they could not provide cross-protection across other
Enterovirus species such as CA16, one of the main
agents responsible for HFMD outbreaks throughout
the Western Pacific Region.16 It remains a challenge
to control HFMD outbreaks at a community or
institutional level that are often caused by multiple
Enterovirus strains. Past studies suggested that early
implementation of outbreak management practices
can be effective in minimising HFMD spread.3 7 18 19
There has been no comprehensive systematic review
of evidence from HFMD outbreaks, however, to
elucidate the most important factors for outbreak
control.
We performed a systematic review to
identify, collate, and review the current evidence
for the effectiveness of public health measures and
diagnostic tools to control and interrupt HFMD
transmission among young children in childcare
facilities. In this study, childcare facilities included
kindergartens, playgroups, nurseries, preschool
facilities, and child daycare centres. The evidence will
be used to inform practice and recommend policy
on the most effective responses and interventions in
the HKSAR to effectively minimise and contain the
spread of HFMD within childcare facilities.
Methods
Data sources and search strategies
We conducted electronic searches of the following
databases for primary studies written in Chinese
or English: MEDLINE (1946 to present), EMBASE
(1980 to 6 April 2012), and Global Health (1973 to
March 2012). Databases specific to South-East Asia
were also interrogated including the WHO Western
Pacific Region Index Medicus database and country-specific
databases within China—China National
Knowledge Infrastructure Databases (CNKI; www.cnki.net) [1989 to 2012] and Chinese Scientific
Journals Database (CSJD-VIP; www.cqvip.com)
[1990 to 2012]. We searched the database with the
medical subject headings or key words “hand foot
and mouth disease”, “child care facilities”, “prevention
and control”, “outbreak notification”, “molecular
diagnostic technique”, and “morbidity and mortality”.
Searches were supplemented by references identified
from reference lists of all included papers. To
ensure that no relevant studies were missed, the
following websites were also browsed: Centre for
Health Protection (www.chp.gov.hk/), World Health
Organization (www.who.int/), and WHO Western
Pacific Region (www.wpro.who.int/) for relevant
reports and grey literature.
Study selection
We included studies conducted during or
retrospective to HFMD outbreaks caused by EV71
from 1980 to 2012 within childcare facilities, with a
study population within the age range of 0 to 6 years.
Studies with a description of public health control
measures implemented and diagnostic methods
used to isolate EV71 from the HFMD outbreaks,
with findings supported by empirical data in text or
figures were included.
Identified studies were saved in endnote and
duplicate studies removed. The titles and abstracts
were screened for relevance and eligible full-text
papers obtained and reviewed. Studies without the
availability of full text were excluded as were those
that did not fulfil the inclusion criteria after full-text
review.
Data extraction
Two researchers extracted the data on outbreak
characteristics, methods for detection and
diagnosis of EV71, interventions applied, and
recommendations for dealing with future outbreaks.
Assessment of methodological quality was based
on study design, population settings, outcome,
interventions, strengths, weaknesses, and areas of
potential bias using the method recommended by
Critical Appraisal Skills Programme (CASP).20 Ratings
of ‘good’, ‘satisfactory’, and ‘poor’ were assigned
according to the number of criteria fulfilled. A
modified quality assessment tool for systematic
reviews of observational studies (QATSO) checklist21
was also developed for this review to assess external
validity, reporting bias, and confounding. This was
used to generate QATSO scores for each domain.
The studies were appraised according to objectives
to see if they provided sufficient evidence to measure
the impact and effectiveness of detection tools and
public health control measures during the HFMD
outbreak. Recommendations for dealing with future
outbreaks were extracted from the studies and
examined for relevance to the childcare setting in
Hong Kong.
Results
Search results
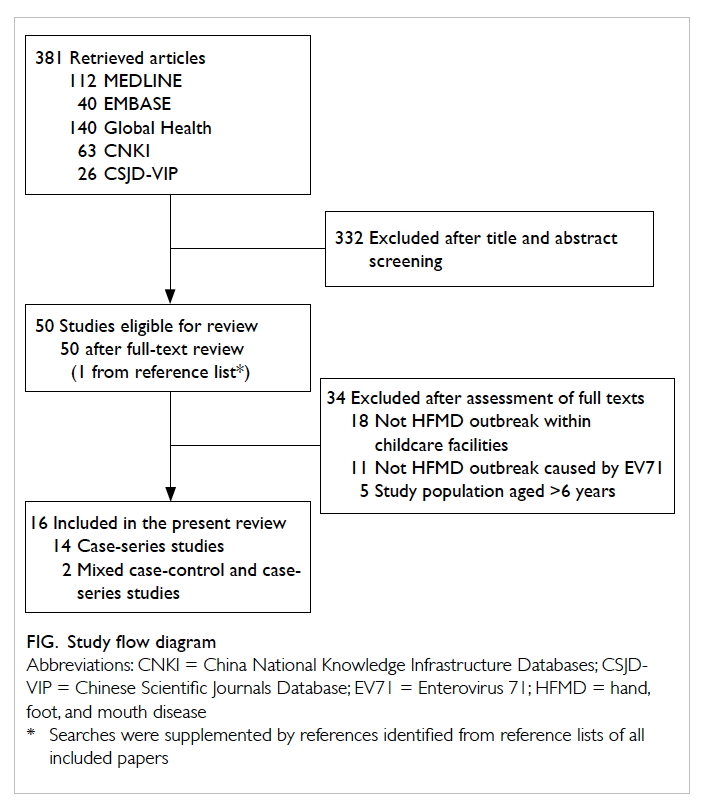
The Figure depicts the search and selection process
which identified 16 studies that met the inclusion
criteria. All studies were conducted on outbreaks
in China—14 were case-series studies (located in
Beijing City, Dafeng City, Dalian City, Jining City,
Laiwu City, Langfang City, Qianjiang City, Shanghai
City, Shenzhen City, Zhengzhou City), and two were
mixed case-control and case-series studies (located
in Shenzhen and Zhengzhou City). Of the 16 studies,
two investigated clustered outbreaks involving
multiple (7 and 61) kindergartens,22 23 and the other 14 studies each focused on an outbreak within a single
kindergarten.24 25 26 27 28 29 30 31 32 33 34 35 36 37 Of the included articles, 15 were
published by the Chinese Center for Disease Control
and Prevention (China CDC)22 23 24 25 26 27 28 29 30 31 32 33 34 36 37 and one by a
local hospital as an outbreak investigation report.35
All included articles were published in Chinese, with
some of them having an English abstract.
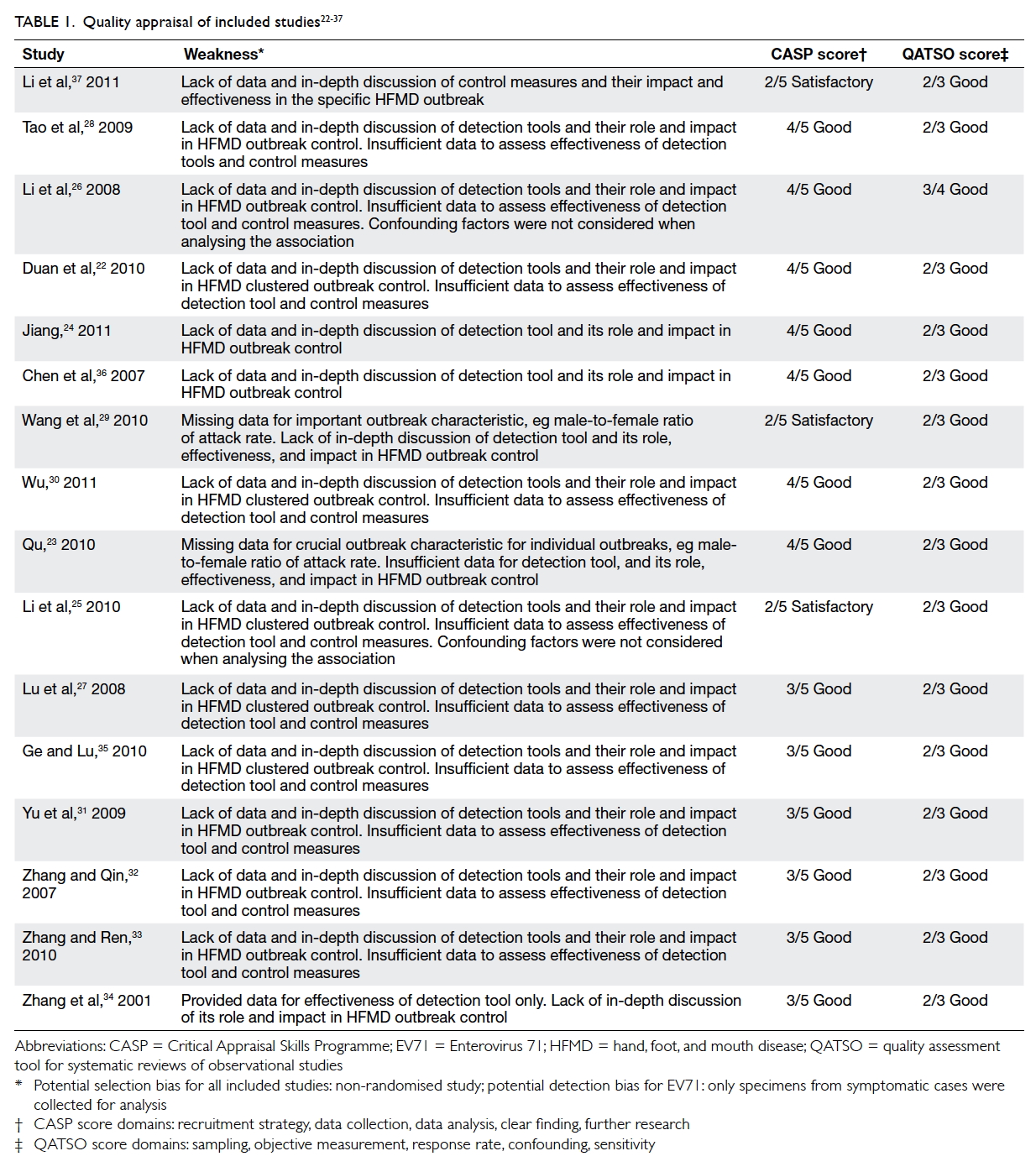
Quality appraisal of included studies
The methodological quality of included studies was
moderate to good based on the CASP assessment,
with three, six, and seven studies scoring 2, 3,
and 4 (out of 5), respectively. The most common
weaknesses across studies were insufficient data and
discussion of the role, impact, and effectiveness of
detection tools and control measures in controlling
HFMD outbreak (Table 122 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37).
The 14 descriptive case-series studies included
in this review can be classified as level IV evidence,
which is the lowest tier of the evidence hierarchy
proposed by the National Health and Medical
Research Council.38 The two studies that involved
a case-control design to identify risk factors of
HFMD can be classed as level III-2 evidence.38 There
was potential for selection bias as the participating
kindergartens were not randomly selected. Moreover,
there was likely detection bias as the majority of
specimens were collected only from symptomatic
children. Also, reporting bias is present due to
different versions (2008, 2009, or 2012 versions) of
the China CDC guidelines being applied and different
outcome definitions being used across studies. All
included studies scored good (QATSO score, 67%-100%) in the QATSO bias assessment. Most of the
included studies were outbreak investigation reports
and did not mention a sampling method. Laboratory
tests were used to measure the study objective for
the effectiveness of the detection tool. The response
rate of study participants was not applicable to the
epidemiological investigation (case series) reports.
It was only applicable in two case-control studies.
For HFMD cases, all studies used the anonymous
or surname only to describe HFMD cases to protect
their privacy. The sensitivity regarding HFMD-affected
cases, and kindergarten anonymity was
carefully considered and confidentiality maintained
even in risk communication among the public in
different regions of China.
Synthesis of evidence
Population
A majority of studies gave few or no details about
the childcare facility. Only eight studies, covering a
total of 14 outbreaks, reported whether they were
public or private facilities (four and 10 outbreaks,
respectively).22 23 24 27 28 29 30 35 Three outbreaks were reported in an urban area,28 29 32 and not specified for
the other outbreaks. In China, a city or district may
include counties, towns and villages, and encompass
both urban and rural areas. Therefore it was difficult
to distinguish the nature of the kindergarten when
it was not specified.39 The study population for
the individual outbreaks ranged from 102 to 889
children and for the clustered outbreaks in 7 and 61
kindergartens, the study sizes were 830 and 16 780
children, respectively (Table 222 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37).
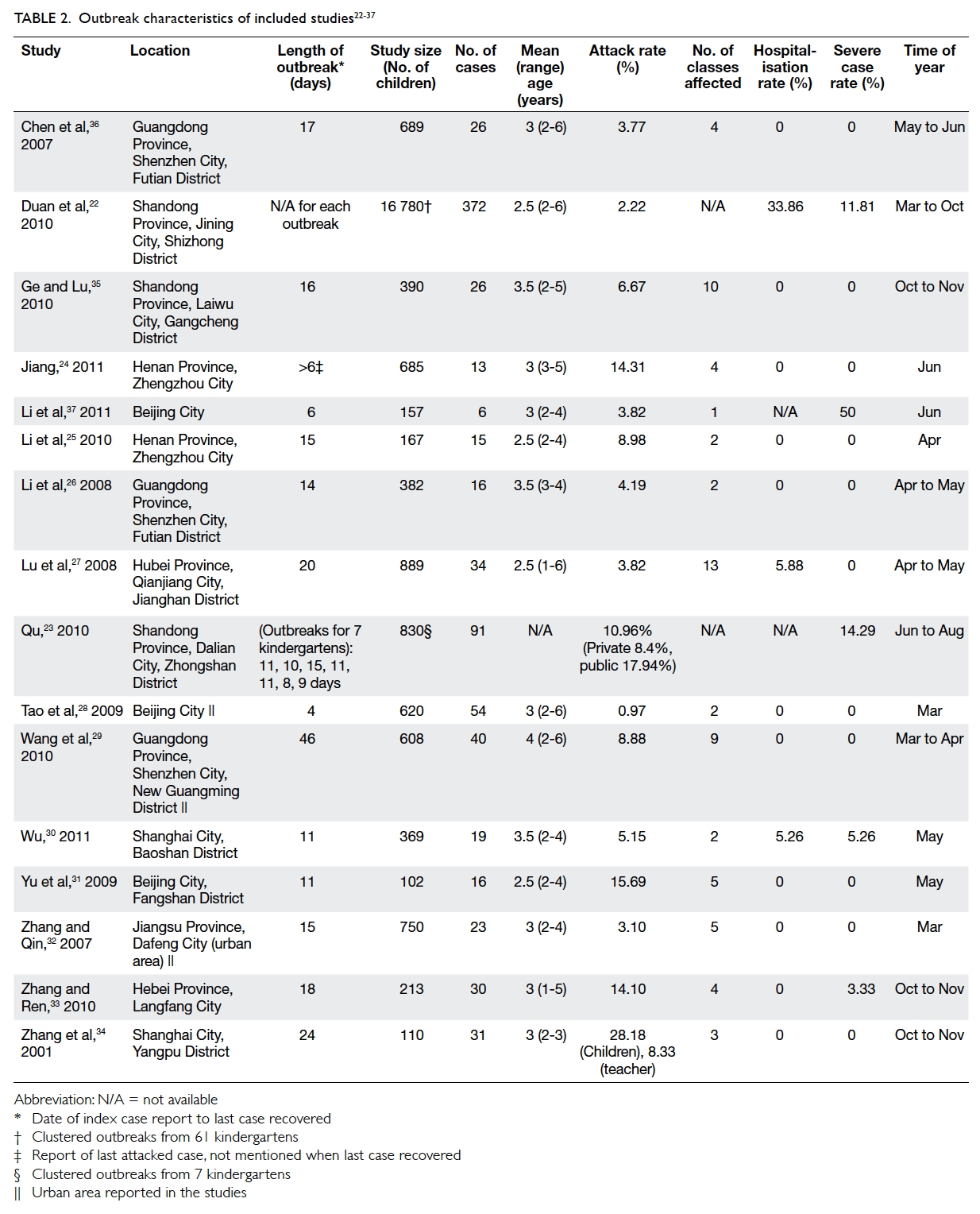
Table 2. Outbreak characteristics of included studies22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37
Characteristics of outbreaks
Of the included studies, 15 reported length of
outbreak, with a mean and median of 15 days, and
a range of 4 to 46 days. Minor variations were seen
in the definition of length of outbreak applied.
Of the 14 individual outbreaks, 11 (78.6%) were
reported to occur between March and June (Table 222 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37). The timing of the clusters of outbreaks that
were examined in two articles also included these
months. Different rates of HFMD were reported
across studies, with a mean attack rate of 8.4%
(range, 0.97%-28.18%), a mean severe case rate of
5.3% (range, 0%-50%), and a mean hospitalisation
rate of 2.8% (range, 0%-33.86%). The majority of
the sampled cases were symptomatic except for the
report of an asymptomatic healthy carrier in a study
by Wang et al.29 No deaths were reported in any of
the included studies.
Attack rate of HFMD was significantly higher
among children aged 2 to 3 years than those aged 4
to 6 years. Individual attack rates for those aged 2,
3, 4, 5, and 6 years were 2.4%, 2.95%, 1.15%, 0.72%,
and 0.39%, respectively (Chi squared=143.58) at
P<0.05 level.22 Li et al25 revealed that children who were admitted to kindergarten between 2 and 3 years
(odds ratio [OR]=7.0; 95% confidence interval [CI],
1.2-46; P=0.01) and had contact with symptomatic
cases (OR=6.75; 95% CI, 1.15-44; P=0.01) posed a
7-times higher risk for HFMD infection.
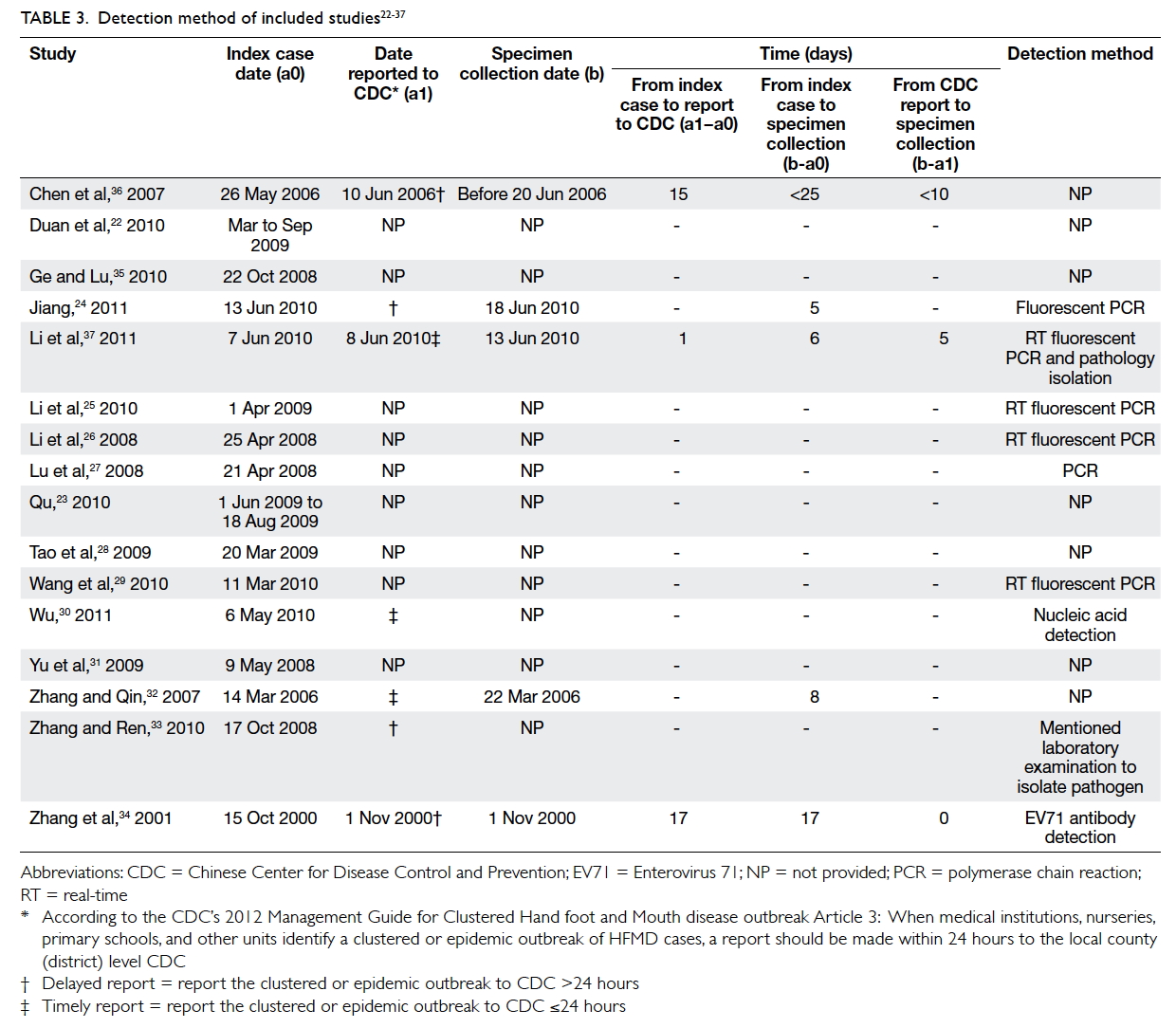
A majority of studies did not provide sufficient
data for assessment of delay between index case,
notification of CDC, and specimen collection (Table 322 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37). Across the three studies in which data were
available, the time between index case and reporting
to CDC varied widely, from just a single day in one
study to over 2 weeks in the other two studies.34 36 37
The “timely” or “delayed” description summarised
in Table 3 is based on study text that reflected the China CDC guidelines.24 30 32 33 Across the five studies for which data were available, the mean time
between index case and specimen collection was 12
days (range, 5-25 days).24 32 34 36 37
Specimen collection
A total of 315 specimens of five different types were
collected across the studies. Of these, 56.8% were
stool samples, 14.0% blood samples, 12.7% anal
swabs, 8.6% throat swabs, 0.6% oral rinse samples,
and 7.3% unspecified. As shown in Table 3, one study
collected samples from a selection of symptomatic
and asymptomatic cases (method of selection not
reported),29 three studies collected samples from all
symptomatic cases,28 36 37 and other studies collected
specimens from a sample of symptomatic cases
(method of selection was not reported across these
studies).22 23 24 25 26 27 30 31 32 33 34 35
Detection and diagnosis
All included studies reported the use of laboratory
diagnostic tools to detect EV71 strains as the
aetiological agent for HFMD (Table 3), although
only seven studies specified the approach, with
six using polymerase chain reaction (PCR)–based
detection,24 25 26 27 29 37 and two using immunodiagnosis.30 34
The EV71 strain was detected in all studies, with a
mean detection rate of 56.7% (range, 7.6%-100%),
and CA16 strain was detected in two studies,22 28 with a mean detection rate of 1.9% (range, 0%-11.76%).
The majority of the studies collected specimens and
tested a selection of symptomatic cases only. Wang et al29 reported that 8.9% were symptomatic and 7.6% cases were asymptomatic after testing a selection of
symptomatic and asymptomatic cases.
Control measures
All studies provided some description of the public
health measures applied during the outbreak. These
measures could be referenced to the “Management
guide for clustered and epidemic hand, foot and
mouth disease outbreak” shown in Table 422 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 and Appendix.40 The most frequently implemented public
health control measures included environmental
disinfection (all 16 studies) and facility closure (14
studies), with the latter usually last for 2 weeks (range,
6-30 days). Other commonly reported measures were
promotion of personal or hand hygiene (9 studies),
isolation of symptomatic cases (15 studies), body
check (11 studies), and health education (14 studies).
Various approaches are summarised in Table 4.
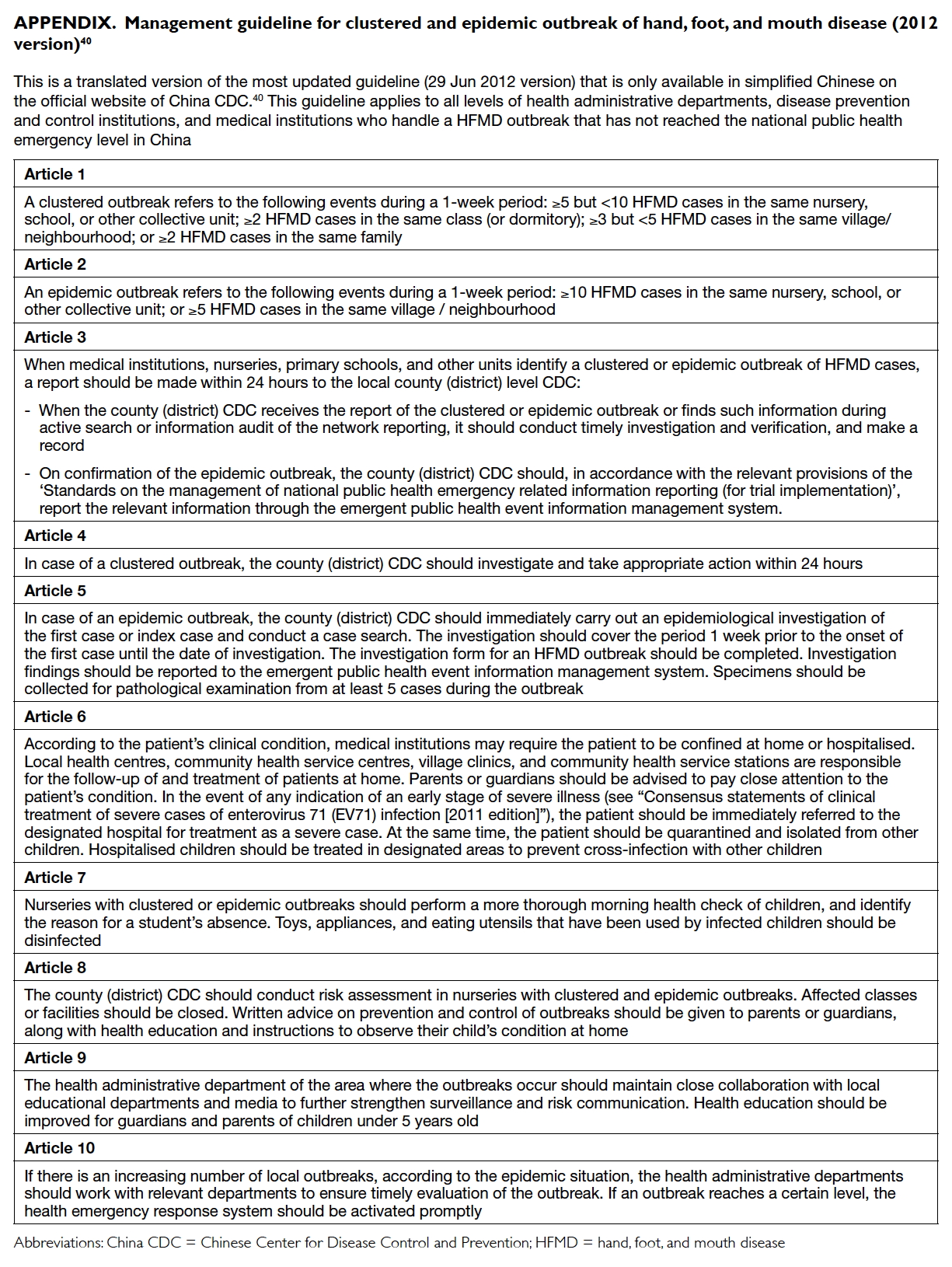
Appendix. Management guideline for clustered and epidemic outbreak of hand, foot, and mouth disease (2012 version)40
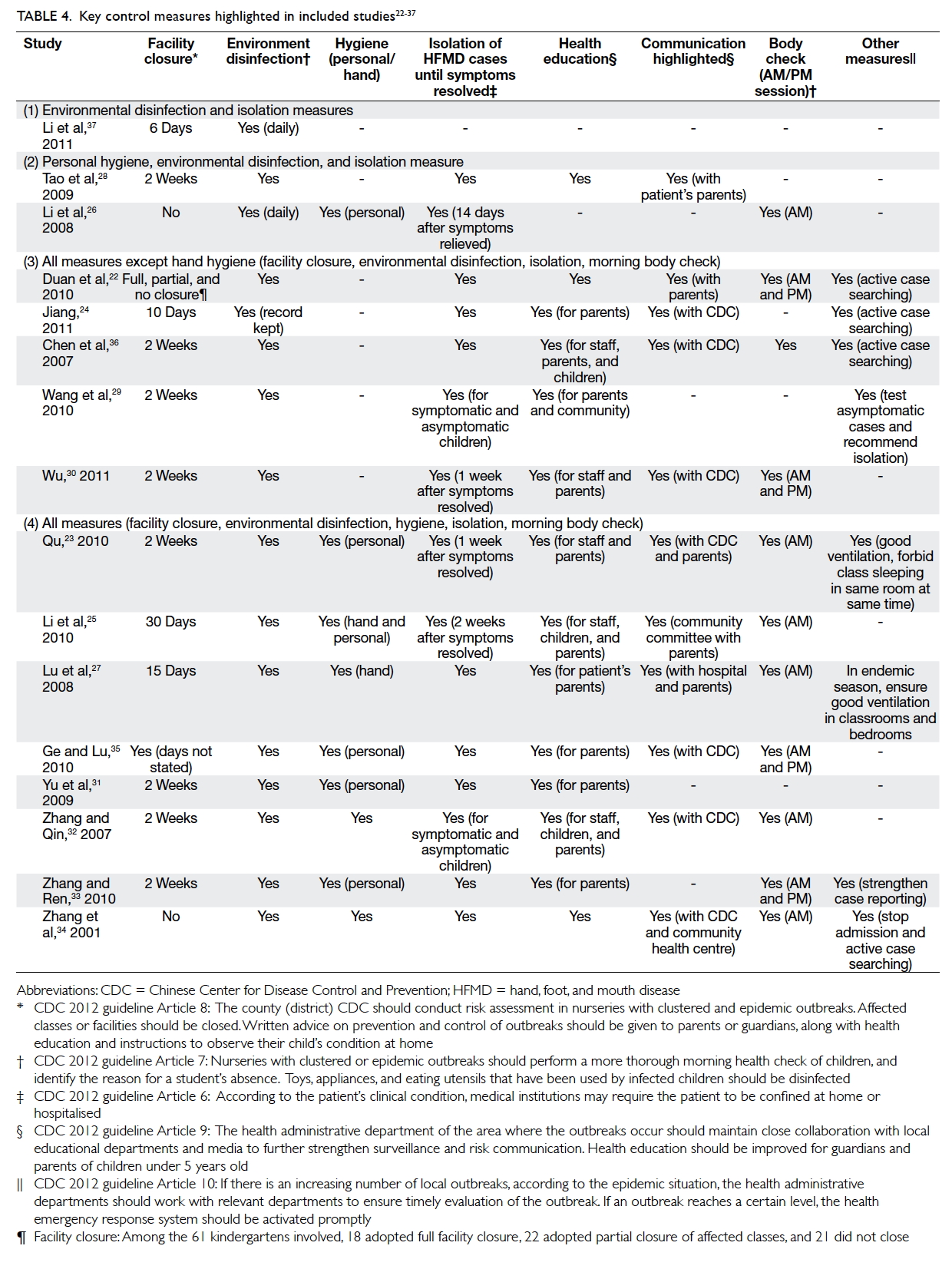
Table 4. Key control measures highlighted in included studies22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37
The impact of control interventions on outbreaks
Given the limited number of included studies with
frequently missing information and the wide range
of reported contexts and control measures, it is
difficult to make any meaningful comparison of
outbreak characteristics (eg attack rate and duration)
and the use and timeliness of specific interventions
across studies. It is, however, worth noting that the
mean attack rate for the three studies with timely
notification to the CDC was 4.0% (range, 3.1%-5.2%).30 32 37 The mean attack rate for the four studies
with delayed outbreak report to the CDC was 15.1%
(range, 3.8%-28.2%).24 33 34 36
In addition, the study by Jiang24 identified
deliberate misreporting of patient names and
kindergartens for symptomatic cases at hospitals
or clinics during HFMD check-up. This was because
isolation measures for symptomatic cases could
stigmatise a child and their family within their
community.
Discussion
Main findings of this study
The purpose of this review was to investigate the
role, impact, and effectiveness of detection methods
and public health control measures within childcare
centres for children aged ≤6 years. All the
included studies applied a list of management
practices advised by the China CDC after
epidemiological investigation and risk assessment.
Of these studies, central to HFMD outbreak
investigation was the effectiveness of identification,
reporting, and response with the implementation of
appropriate control measures to minimise its spread
and incidence. In this review, similar outbreak
progression and population characteristics were
evident across studies.
According to the China CDC, a clustered
outbreak is defined as two or more HFMD cases in
the same class or five or more cases within the whole
facility.40 Timely notification of a clustered outbreak
within 24 hours to the China CDC was crucial to
shortening length of outbreak and attack rate.40
Length of outbreak with timely notification (≤24
hours) could be shortened to 6 days with a lower
attack rate and number of classroom units affected
within the facility37 compared with late notification
(>24 hours) of over 2 weeks.33 34 36
Effective communication, environmental
disinfection, and sanitation instructed by the facility
and control supervision from the local CDC may
be another reason for the disparity in attack rates
and length of outbreak between these facilities.
Outbreak control response showed a similar trend
to length of outbreak suggesting its influence on
main outbreak characteristics (Tables 2 and 4). The China CDC 2012 prevention and control guideline40
advised a 24-hour outbreak report, epidemiological
investigation, and prevention enforcement such as
isolation of symptomatic cases (infected children
must stay home until symptoms resolve), improved
regular body checks, environmental disinfection,
and facility closure following risk assessment.
Asymptomatic healthy carriers (7.62%) could
serve as a source of a HFMD outbreak, according
to laboratory results from both symptomatic
and asymptomatic cases.29 Early isolation of
symptomatic individuals was the most effective
means of containing the spread of HFMD.6 These
measures were employed in all studies immediately
after identification of the first case. In two studies,
affected children were only allowed to return
following medical proof of recovery.24 26 Full or partial facility closure was recommended by the China CDC
where there was failure to contain spread within
affected classes using isolation and environmental
disinfection measures.41 Compared with facility or
class closure, the socio-economic impact of isolating
symptomatic cases and environmental disinfection
on parents and kindergartens is likely to be lower.
The majority of the existing research focuses
on the virology and immunology of epidemic strains.
It is known that EV71 and CA16 have prolonged
survival on a contaminated object or environment
surfaces for up to 3 days with 90% relative humidity.42
Environmental disinfection of classrooms, common
areas, or commonly shared objects is therefore
likely to play an important role in interrupting
HFMD transmission. No studies recommended
a specific cleaning method or reagent for EV71
inactivation. A non-enveloped virus like EV71 is
resistant to common disinfectants, eg 70% alcohol
and 1% quaternary ammonium compounds.43
Kadurugamuwa and Shaheen44 recommended the
use of “sodium hypochlorite at a concentration of
3120 ppm for 5 minutes” for effective cleaning to
reduce the viral load of EV71 or CA16 and minimise
the risk of environmental spread during HFMD
outbreaks.
Real-time PCR was widely used for EV71 or
CA16 detection in past international outbreaks to
provide a cheaper and rapid confirmation of the
pathogen within 3 hours and with high accuracy,45 46 47
and is recommended by the WHOWPR Office.6
Nonetheless, a standardised evaluation system is
required to compare their reliability to identify the
pathogen in different outbreak settings.6
Applicability of the findings
This study reported results from the first systematic
review of institution-based public health prevention
of HFMD. To date, little is known about the impact
of various health prevention practices. The findings
from this review derive mainly from studies in China
which has a loose cross-border relationship with
HKSAR regarding climate pattern, culture, and
ethos.48 A series of political events has increased the
population mix in Hong Kong at a social, economic,
and cultural level since Hong Kong was returned to
China as a special administrative region in 1997.48 49 50
Under the ‘One Way Permit Scheme’, the influx of 150
immigrants daily from China to reside in Hong Kong
has increased the population mix across the border.50
The number of cross-border newborns increased
under the Basic Law Article 24 that states Chinese
citizens born in Hong Kong enjoyed the right of
abode regardless of their parents’ immigration status
until the enforcement of the “Zero quota” policy for
births to non-local pregnant women for delivery in
Hong Kong on 1 January 2013.49 51 By 2016, these newborns had become cross-border students who
accounted for 20% of the kindergarten population in
Hong Kong, a rise of 30% since 2011.52 53
The findings from this review are likely to be of
value and to inform policymakers to control HFMD
in Hong Kong childcare institutions. In Hong Kong,
one important difference is the private nature of
childcare facilities compared with public-operated
facilities.54 The kindergartens must bear the financial
cost of preventive resources within an already-limited
budget to operate the facility itself. Most cannot
afford to hire a public health specialist to coordinate
infection control measures from notification to
implementation of interventions within the facility.
On the contrary, most public kindergartens in China
received extra support, for example, a school doctor
on duty to communicate with the CDC and reinforce
these guidelines during a HFMD outbreak.25 26 32
Limitations of this study
There were some limitations in this review.
First, the aim and objective of some studies were
more biological and aetiological in nature and
less concerned with the focus on the review of
effectiveness and impact of intervention.23 37 Different versions of the China CDC prevention and
control guidelines were applied in different studies
to define outcome measures. Included studies were
heterogeneous and focused on different aspects of
public health.40 55 56
Second, insufficient data were provided to
assess the risk factors, effectiveness, and impact of
multiple strategic measures, either as a combined or
separate approach in outbreak detection and control.
Only two included studies interpreted and analysed
the OR for potential risk factors, where younger age,
direct contact with HFMD cases, and travelling on
the school bus would heighten the risk of HFMD
infection.25 26 In contrast, other studies proposed risk factors but did not further analyse them or provide
statistical evidence.22 23 24 27 28 29 30 31 32 33 34 35 36 37 57 58 For example, morning
body check and health education were described in
detail, but their effectiveness and impact were hardly
measured which is inappropriate in the context
of outbreak investigations (Table 4).22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 Dates for
implementation of control measures and notification
of outbreaks were often not reported, meaning that
delays between outbreak onset and response could
not be estimated in most studies. Most studies did
not quantify ‘effectiveness’ at different time points of
the detection process, nor discuss further the value
of these outbreak detection methods in managing a
HFMD outbreak.
Third, all included studies concerned children
in a kindergarten in various provinces of China. Apart
from public and private kindergartens in China,
there are few data about outbreak management
at child daycare centres or other paediatric care
facilities.3 59 Evidence available from other countries has derived mostly from community surveillance
or outbreaks among wider population age-groups,
without laboratory confirmation of EV71 as the
causative strain, and were therefore excluded from
this review.7 44 58 60 61 62 63 64 65
Recommendations for future outbreaks
Cost-effectiveness studies and mathematical modelling
Wang et al29 pointed out that 7.62% of asymptomatic
cases acted as carriers for transmission within
the kindergarten. Two studies mentioned the
contribution of asymptomatic cases to HFMD
transmission in a kindergarten, but no further tests
were conducted to confirm their presence.28 33 It is possible that the attack rate across studies could be
underestimated due to the presence of asymptomatic
cases. To investigate the incidence of asymptomatic
cases and their serological characteristics, a
prospective cohort study of EV71 immunity status
could be conducted using a neutralisation test
against EV71 to measure the herd EV71 immunity
level within the community before and at different
time intervals after an outbreak.
A compartment disease transmission model
suggested by Roy and Halder66 could be used to
explore the relationship in the modelled population
that may include EV71 susceptible cases, infective
symptomatic cases, infective asymptomatic cases,
and recovered cases at an initial time and the disease
transmission rate at any given time. The model could
reveal the impact of asymptomatic cases and the
epidemic potential at different time points of the
outbreak.
Furthermore, a cost-effectiveness study could
be conducted to identify a cheap, quick, accurate, and
user-friendly diagnostic tool that can be applied to a
large population to actively search for asymptomatic
cases, for example, a rapid antigen detection kit for
EV71.
To compare the effectiveness of different
public health measures, we could apply a spatial
deterministic epidemic model to all the kindergartens
located in each of the 18 districts in Hong Kong.67
It is possible to assess the effectiveness of basic
(eg personal health measure, health education)
and contingency public health control measures
(isolation, facility closure) implemented during the
epidemic.67 At the same time, the model can explore
the expected impact of an isolation or facility closure
policy depending on when it is implemented.67
To facilitate early warning and implementation
of precautions, Li et al37 recommended the use
of molecular epidemiological analysis of EV71
genotypes using the VP1 sequence database as
described in their study. The geographical and
evolution origin, transmission pattern, and current
prevalence strains could be detected by comparing
genetic variation between EV71 strains.37
Policy recommendations
At the moment, a standard guideline on prevention
of communicable diseases within childcare
facilities and two letters to kindergartens with a
hotline number to warn about the disease is the
only information issued by the CHP. The following
measures should be incorporated into future HFMD
prevention and control policy in Hong Kong.
(1) A clear definition of an outbreak should be developed for individual and clustered outbreaks. Currently, there is only a section for the definition of an outbreak of communicable diseases, and a brief definition provided in a letter to kindergartens.
(2) As the key government agency for infection control and public health practice, CHP should work with the Hospital Authority and other health care providers to obtain consent from parents to gain timely access to the relevant medical records and laboratory results. This would accelerate confirmation of the source and subsequent cases in an outbreak.
(3) Epidemiological investigation is important for risk assessment during a HFMD outbreak to inform subsequent preventive measures. A guideline should be developed on how and when it should be carried out. As with the China CDC, the CHP should initiate an investigation within 24 hours at an affected facility upon the report of a clustered outbreak.
(4) “Enhance health surveillance” is mentioned in the guideline for children without symptoms at the facilities.14 A more active surveillance plan that will enable identification of symptomatic and asymptomatic cases during an HFMD outbreak should be developed.
(5) The government should provide incentives to encourage childcare facilities to train extra staff as ‘infection control specialists’. These specialists will act as the first contact, knowledge, and communication points to exchange information and support stakeholders during a communicable disease epidemic. They should be invited to attend CHP’s regular seminar and training workshops about current communicable diseases and provide a forum to exchange ideas and discuss prevention methods.
(6) Although a response team is available for different communicable diseases, information is not immediately made public. To improve communication and transparency, an online interactive forum should be developed where parents, childcare facilities, and a CHP specialist team can exchange news of communicable diseases and the appropriate measures taken. The 24-hour hotline and notification forms are not interactive nor an effective means of communication across stakeholders.
(1) A clear definition of an outbreak should be developed for individual and clustered outbreaks. Currently, there is only a section for the definition of an outbreak of communicable diseases, and a brief definition provided in a letter to kindergartens.
(2) As the key government agency for infection control and public health practice, CHP should work with the Hospital Authority and other health care providers to obtain consent from parents to gain timely access to the relevant medical records and laboratory results. This would accelerate confirmation of the source and subsequent cases in an outbreak.
(3) Epidemiological investigation is important for risk assessment during a HFMD outbreak to inform subsequent preventive measures. A guideline should be developed on how and when it should be carried out. As with the China CDC, the CHP should initiate an investigation within 24 hours at an affected facility upon the report of a clustered outbreak.
(4) “Enhance health surveillance” is mentioned in the guideline for children without symptoms at the facilities.14 A more active surveillance plan that will enable identification of symptomatic and asymptomatic cases during an HFMD outbreak should be developed.
(5) The government should provide incentives to encourage childcare facilities to train extra staff as ‘infection control specialists’. These specialists will act as the first contact, knowledge, and communication points to exchange information and support stakeholders during a communicable disease epidemic. They should be invited to attend CHP’s regular seminar and training workshops about current communicable diseases and provide a forum to exchange ideas and discuss prevention methods.
(6) Although a response team is available for different communicable diseases, information is not immediately made public. To improve communication and transparency, an online interactive forum should be developed where parents, childcare facilities, and a CHP specialist team can exchange news of communicable diseases and the appropriate measures taken. The 24-hour hotline and notification forms are not interactive nor an effective means of communication across stakeholders.
Conclusions
Timely notification of a clustered outbreak within 24
hours and implementation of isolation measures
according to the CDC guidelines are crucial to
minimise attack rate of HFMD within childcare
facilities. To achieve this, communication between
stakeholders (childcare facilities, CHP, parents, and
health care providers) about outbreak confirmation,
risk assessment, and sentinel surveillance in the
form of regular body checks should be enhanced by
the provision of clear guidelines and an interactive
platform. Findings from this review informed
us of more comprehensive HFMD policy and
practices to apply in Hong Kong that, similar to
the China CDC framework, will improve disease
communication. The government should provide
incentives for childcare facilities to train public
health specialists to coordinate infection control
measures from notification to implementation of
the intervention and enforce the guideline within
the facility. In China, the health specialist facilitates
communication between the CDC and kindergarten,
enables accelerated outbreak notification, and
effectively manages control efforts.25 26 32 In light of
all the missing information in the included studies,
standardised reporting and outbreak investigation
guidelines could be established. It should be
provided in both a paper and electronic format and
shared across all childcare facilities in Hong Kong
monitored by the CHP response team to generate
more timely, comprehensive, and comparable
evidence during outbreaks. Future research could
generate more robust evidence for the effectiveness
of different control practices using a spatial
deterministic epidemic model. Applied across all
kindergartens located in each of the 18 districts in
Hong Kong, it could assess the effectiveness of basic
(eg personal health measures, health education) and
contingency public health control measures.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Western Pacific Regional Office of World Health
Organization. Hand, foot, and mouth disease situation
update. Available from: http://www.wpro.who.int/emerging_diseases/hfmd_biweekly_29dec2014.pdf.
Accessed 29 Mar 2015.
2. Hand, foot and mouth disease (HFMD). Centre for Health
Protection, Department of Health of the Hong Kong SAR
Government. Communicable Diseases Watch 2015;12(4):17.
3. Ruan F, Yang T, Ma H, et al. Risk factors for hand, foot, and
mouth disease and herpangina and the preventive effect of
hand-washing. Pediatrics 2011;127:e898-904. Crossref
4. Hand, foot and mouth disease (HFMD). Centre for Health
Protection, Department of Health of the Hong Kong SAR
Government. Communicable Diseases Watch 2016;13(4):17.
5. Lee MS, Lin TY, Chiang PS, et al. An investigation of
epidemic enterovirus 71 infection in Taiwan, 2008: clinical,
virologic, and serologic features. Pediatr Infect Dis J
2010;29:1030-4. Crossref
6. Western Pacific Regional Office of World Health
Organization. A guide to clinical management and
public health response for hand, foot and mouth disease.
Available from: http://www.wpro.who.int/publications/docs/GuidancefortheclinicalmanagementofHFMD.pdf.
Accessed 16 Mar 2013.
7. Ma E, Chan KC, Cheng P, Wong C, Chuang SK.
The enterovirus 71 epidemic in 2008—public health
implications for Hong Kong. Int J Infect Dis 2010;14:e775-80. Crossref
8. Tsao KC, Chan EC, Chang LY, et al. Responses of IgM for
enterovirus 71 infection. J Med Virol 2002;68:574-80. Crossref
9. Cardosa MJ, Perera D, Brown BA, et al. Molecular
epidemiology of human enterovirus 71 strains and recent
outbreaks in the Asia-Pacific region: comparative analysis
of the VP1 and VP4 genes. Emerg Infect Dis 2003;9:461-8. Crossref
10. McMinn P, Lindsay K, Perera D, Chan HM, Chan KP,
Cardosa MJ. Phylogenetic analysis of enterovirus 71 strains
isolated during linked epidemics in Malaysia, Singapore,
and Western Australia. J Virol 2001;75:7732-8. Crossref
11. Bible JM, Pantelidis P, Chan PK, Tong CY. Genetic evolution
of enterovirus 71: epidemiological and pathological
implications. Rev Med Virol 2007;17:371-9. Crossref
12. Shimizu H, Utama A, Onnimala N, et al. Molecular
epidemiology of enterovirus 71 infection in the Western
Pacific Region. Pediatr Int 2004;46:231-5. Crossref
13. Zhang Y, Tan X, Cui A, et al. Complete genome analysis of
the C4 subgenotype strains of enterovirus 71: predominant
recombination C4 viruses persistently circulating in China
for 14 years. PLoS One 2013;8:e56341. Crossref
14. Centre for Health Protection, Department of Health of the
Hong Kong SAR Government. Guidelines on Prevention
of Communicable Diseases in Schools/ Kindergartens/Kindergartens-cum-Child Care Centers/Child Care
Centres 2011. Available from: http://www.chp.gov.hk/files/pdf/guidelines_on_prevention_of_communicable_
diseases_in_schools_kindergartens_kindergartens_cum_child_care-centres_child_are_centres.pdf. Accessed 20 Sep
2012.
15. Hand, foot and mouth disease (HFMD). Centre for Health Protection, Department of Health of the Hong Kong SAR Government. Communicable Diseases Watch 2017;14(2):6-7.
16. Reed Z, Cardosa MJ. Status of research and development
of vaccines for enterovirus 71. Vaccine 2016;34:2967-70. Crossref
17. Lu S. EV71 vaccines: a milestone in the history of global
vaccine development. Emerg Microbes Infect 2014;3:e27. Crossref
18. Kim KH. Enterovirus 71 infection: an experience in Korea,
2009. Korean J Pediatr 2010;53:616-22. Crossref
19. Chang LY, King CC, Hsu KH, et al. Risk factors of
enterovirus 71 infection and associated hand, foot, and
mouth disease/herpangina in children during an epidemic
in Taiwan. Pediatrics 2002;109:e88. Crossref
20. Critical Appraisal Skills Programme: Making sense of
evidence. Available from: http://www.casp-uk.net/.
Accessed 12 Feb 2013.
21. Wong WC, Cheung CS, Hart GJ. Development of a quality
assessment tool for systematic reviews of observational
studies (QATSO) of HIV prevalence in men having sex
with men and associated risk behaviours. Emerg Themes
Epidemiol 2008;5:23. CrossRef
22. Duan SB, Ren LQ, Shi ZM. Analysis of clustered
outbreaks of hand foot and mouth disease in Jining City in 2009 [in Chinese]. South China J Prev Med
2010;36(2):37-9,41.
23. Qu H. Analysis of emergency of hand foot and mouth
disease in Zhongshan District in Dalian City in 2009 [in
Chinese]. Prev Med Trib 2010;16(3):277-8.
24. Jiang QS. Outbreak investigation of an outbreak of hand
foot and mouth disease in a kindergarten in Zhengzhou
City [in Chinese]. China Mod Doct 2011;49(2):85,120.
25. Li MZ, Chen YZ, Li P. Epidemiological investigation of an
outbreak of hand foot and mouth disease in a kindergarten
in Zhengzhou City [in Chinese]. Henan J Prev Med
2010;21(6):461-2,473.
26. Li XY, Si XH, Li LF. Investigation of an outbreak of hand,
foot and mouth disease in a kindergarten in Futian District
of Shenzhen [in Chinese]. Pract Prev Med 2008;15(6):1787-8.
27. Lu GY, Liu GP, Yue JL. Report on an outbreak of the hand-foot-and-mouth disease epidemic situation [in Chinese]. J
Public Health Prev Med 2008;19(5):55-6.
28. Tao T, Liu L, Song SP, Zheng JF, Hao YJ. Investigation of an
outbreak of hand, foot and mouth disease in a kindergarten
of army unit [in Chinese]. Occup Heal 2009;25(19):2087.
29. Wang G, Liu Y, Luo S, Wang T, Zhan J. Epidemiological
investigation of an outbreak of hand foot and mouth
disease in a kindergarten in Shenzhen, Guangdong [in
Chinese]. China Heal Mon 2010;29:82-3.
30. Wu C. Epidemiological survey of an outbreak of HFMD
in a kindergarten of Baoshan District [in Chinese]. Occup
Heal 2011;27(20):2334-5.
31. Yu HZ, Xiang N, Cui LM, et al. An outbreak of hand-foot-mouth
disease caused by EV71 virus in a kindergarten of
Fangshan District in Beijing [in Chinese]. China J Infect
Control 2009;8(2):113-4.
32. Zhang HJ, Qin ZK. Epidemiological investigation of an
outbreak of hand foot and mouth disease in a kindergarten
[in Chinese]. Jiangsu J Prev Med 2007;18(2):15.
33. Zhang J, Ren HY. Epidemiological investigation of an
outbreak of hand foot and mouth disease in a kindergarten
[in Chinese]. Occup Heal 2010;26(3):328-9.
34. Zhang A, Li Y, Zhang J, et al. Investigation of an outbreak of
EV71 hand foot and mouth disease [in Chinese]. Shanghai
J Prev Med 2001;13(12):587-8.
35. Ge GX, Lu L. Survey on outbreak of hand-foot-mouth
disease at a kindergarten in Gangcheng District, Laiwu City,
2008 [in Chinese]. Lit Inf Prev Med 2010;16(9):764,847.
36. Chen H, Situ CM, Tian HW. Investigation of an outbreak
of hand foot and mouth disease in children [in Chinese]. J
Prev Med Inf 2007;23:364-5.
37. Li J, Jia L, Liu JR, et al. Epidemiological and etiological
characteristics of hand foot and mouth disease cluster in a
kindergarten in Beijing, 2010. Dis Surveill 2011;26:790-3.
38. Merlin T, Weston A, Tooher R. Extending an evidence
hierarchy to include topics other than treatment: revising
the Australian ‘levels of evidence’. BMC Med Res Methodol
2009;9:34. Crossref
39. China Internet Information Center. Administrative
division system 2016. Available from: http://www.china.org.cn/english/feature/38436.htm. Accessed 21 Apr 2016.
40. Chinese Center for Disease Control and Prevention.
Management guide for clustered and epidemic hand, foot
and mouth disease outbreak (2012 version) [in Chinese].
Available from: http://www.chinacdc.cn/jkzt/crb/szkb/jszl_2275/201206/t20120629_63852.htm. Accessed 15 Jul
2012.
41. Chinese Center for Disease Control and Prevention. EV71
infection diagnostic and treatment guide (2008 version)
[in Chinese]. Available from: http://www.chinacdc.cn/jkzt/crb/szkb/jszl_2275/200805/t20080506_24703.htm.
Accessed 5 Jul 2012.
42. Wong SS, Yip CC, Lau SK, Yuen KY. Human enterovirus
71 and hand, foot and mouth disease. Epidemiol Infect
2010;138:1071-89. Crossref
43. Chan YF, Abu Bakar S. Virucidal activity of Virkon S on
human enterovirus. Med J Malaysia 2005;60:246-8.
44. Kadurugamuwa JL, Shaheen E. Inactivation of human
enterovirus 71 and coxsackie virus A16 and hand, foot, and
mouth disease. Am J Infect Control 2011;39:788-9. Crossref
45. Tan EL, Yong LL, Quak SH, Yeo WC, Chow VT, Poh CL.
Rapid detection of enterovirus 71 by real-time TaqMan
RT-PCR. J Clin Virol 2008;42:203-6. Crossref
46. Fujimoto T, Yoshida S, Munemura T, et al. Detection and
quantification of enterovirus 71 genome from cerebrospinal
fluid of an encephalitis patient by PCR applications. Jpn J
Infect Dis 2008;61:497-9.
47. Xiao XL, He YQ, Yu YG, et al. Simultaneous detection of
human enterovirus 71 and coxsackievirus A16 in clinical
specimens by multiplex real-time PCR with an internal
amplification control. Arch Virol 2009;154:121-5. Crossref
48. Kennedy KJ. Immigration and Hong Kong: “New
immigrants” and ethnic minorities. Hong Kong report
prepared for the UNESCO-KEDI Regional Policy Seminar
2012—Education policy: Making in the age of migration in
Asia and the Pacific; 2012 Jul 10-12: 8.
49. The Basic Law of the Hong Kong SAR Government.
Chapter III: Fundamental rights and duties of the
residents. Available from: http://www.basiclaw.gov.hk/en/basiclawtext/chapter_3.html. Accessed 10 Jan 2017.
50. One-way Permit Scheme [press release]. The Hong Kong
SAR Government; 2014 Jan 22.
51. Government reaffirms its strict enforcement of the
“zero quota” policy [press release]. The Hong Kong SAR
Government; 2012 Dec 28.
52. Cross-border students up 118% in five years. Harbour
Times. 2016 Jun 23.
53. Education Bureau of the Hong Kong SAR Government.
Student enrolment statistics, 2015/16 (kindergarten,
primary and secondary levels). Available from: http://www.edb.gov.hk/attachment/en/about-edb/publications-stat/figures/Enrol_2015.pdf. Accessed 10 Jan 2017.
54. Education Bureau of the Hong Kong SAR Government.
Overview of kindergarten education in Hong Kong.
Available from: http://www.edb.gov.hk/en/edu-system/preprimary-kindergarten/overview/index.html. Accessed
26 Aug 2015.
55. Chinese Center for Disease Control and Prevention.
Hand, foot and mouth disease prevention and control
guideline (2009 version) [in Chinese]. Available from:
http://www.chinacdc.cn/jkzt/crb/szkb/jszl_2275/200906/t20090612_24707.htm. Accessed 10 Jul 2012.
56. Chinese Center for Disease Control and Prevention. Hand
foot and mouth disease as notifiable disease notification
by Health Bureau [in Chinese]. Available from: http://www.chinacdc.cn/jkzt/crb/szkb/jszl_2275/200805/t20080506_24699.htm. Accessed 3 Jul 2012.
57. Goh KT, Doraisingham S, Tan JL, Lim GN, Chew SE. An
outbreak of hand, foot, and mouth disease in Singapore.
Bull World Heal Organ 1982;60:965-9.
58. Chen KT, Chang HL, Wang ST, Cheng YT, Yang JY.
Epidemiologic features of hand-foot-mouth disease and
herpangina caused by enterovirus 71 in Taiwan, 1998-2005. Pediatrics 2007;120:e244-52. Crossref
59. Nguyen NT, Pham HV, Hoang CQ, et al. Epidemiological
and clinical characteristics of children who died from hand,
foot and mouth disease in Vietnam, 2011. BMC Infect Dis
2014;14:341. Crossref
60. Edmond M, Wong C, Chuang SK. Evaluation of sentinel
surveillance system for monitoring hand, foot and mouth
disease in Hong Kong. Public Health 2011;125:777-83. Crossref
61. Sarma N, Sarkar A, Mukherjee A, Ghosh A, Dhar S,
Malakar R. Epidemic of hand, foot and mouth disease in
West Bengal, India in August, 2007: a multicentric study.
Indian J Dermatol 2009;54:26-30. Crossref
62. Ooi EE, Phoon MC, Ishak B, Chan SH. Seroepidemiology
of human enterovirus 71, Singapore. Emerg Infect Dis
2002;8:995-7. Crossref
63. Chakraborty R, Iturriza-Gómara M, Musoke R, Palakudy
T, D’Agostino A, Gray J. An epidemic of enterovirus 71
infection among HIV-1-infected orphans in Nairobi. AIDS
2004;18:1968-70. Crossref
64. Shimizu H, Okuyama K, Hirai Y. Epidemic of hand, foot
and mouth disease in Kawasaki City, Japan. Jpn J Infect Dis
2005;58:330-1.
65. Wu PC, Huang LM, Kao CL, Fan TY, Cheng AL, Chang LY.
An outbreak of coxsackievirus A16 infection: comparison
with other enteroviruses in a preschool in Taipei. J
Microbiol Immunol Infect 2010;43:271-7. Crossref
66. Roy N, Halder N. Compartmental modeling of hand, foot
and mouth infectious disease (HFMD). Res J Appl Sci
2010;5:177-82. Crossref
67. Chowell G, Hyman JM. Mathematical and statistical
modeling for emerging and re-emerging infectious diseases.
US: Springer International Publishing Switzerland; 2016. Crossref