DOI: 10.12809/hkmj154670
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Retrievable endoscopic stenting for tuberculous
oesophagopleural fistula with empyema
Brian YO Chan, MBChB; Canon KO Chan, FRCS, FHKAM (Surgery)
Department of Surgery, Queen Elizabeth Hospital, Jordan, Hong Kong
Corresponding author: Dr Canon KO Chan (cko296@ha.org.hk)
Case report
A 23-year-old female was admitted for fever, cough,
and right pleuritic pain in January 2015. Chest
X-ray revealed right pleural effusion. Subsequent
computed tomography of the thorax demonstrated
necrotising pneumonitis in the right lower lobe,
with large right empyema and multiple left lung
centrilobular nodules suspicious of tuberculosis
(Fig 1a). On admission, haemoglobin level was only 84 g/L. Oesophagogastroduodenoscopy (OGD)
found a linear deep oesophageal ulceration with
suspected fistula opening (Fig 1b), biopsy of which
yielded Mycobacterium tuberculosis. Gastrografin
swallow confirmed contrast leakage at the lower
oesophagus with fistulation to the right hemithorax
(Fig 1c), suggesting the occurrence of tuberculous oesophagopleural fistula (OPF) and resulting empyema.
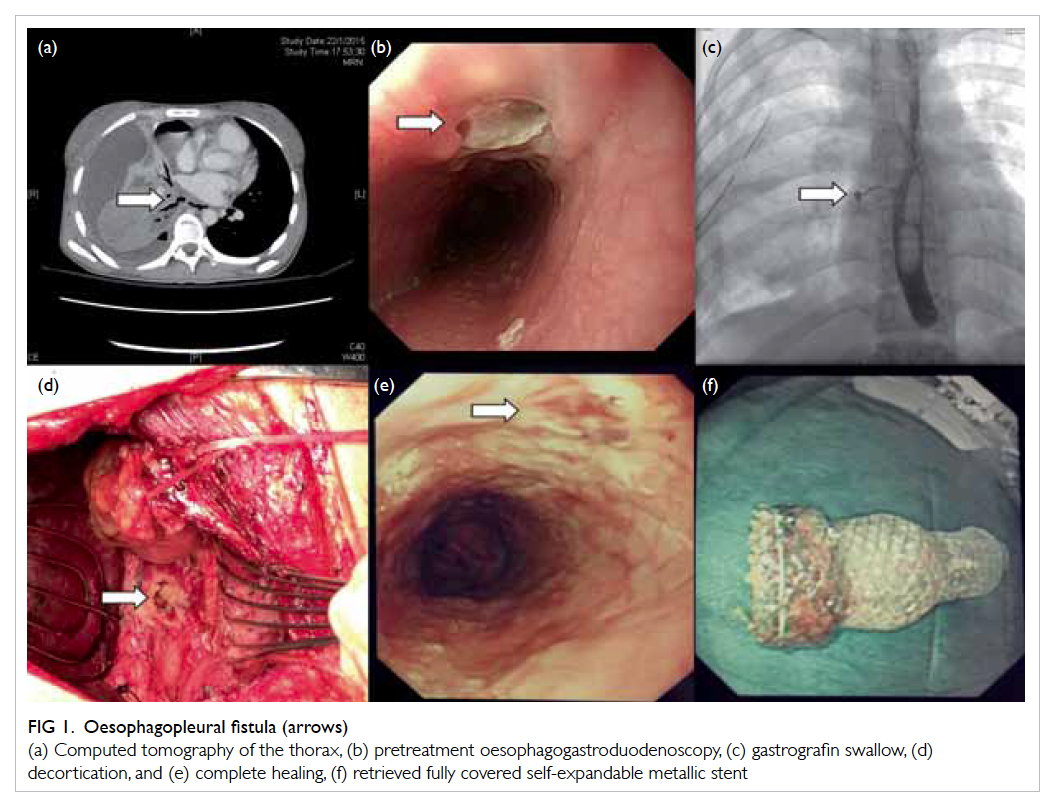
Figure 1. Oesophagopleural fistula (arrows)
(a) Computed tomography of the thorax, (b) pretreatment oesophagogastroduodenoscopy, (c) gastrografin swallow, (d) decortication, and (e) complete healing, (f) retrieved fully covered self-expandable metallic stent
Prompt chest drainage and systemic antibiotics
were initiated, targeting the polymicrobial and smear
positive tuberculous tissue samples. Subsequent
therapeutic OGD with endoscopic stenting was
performed. The patient was placed in the left lateral
decubitus position and sedated. The linear ulcer at
24 to 32 cm from incisor, with 5-mm fistula opening
at 24 cm was visualised. Submucosal lipiodol
injection under fluoroscopy marked the margins of
the fistulated ulcer, and the most distal end of the
linear ulcer (Fig 2a).
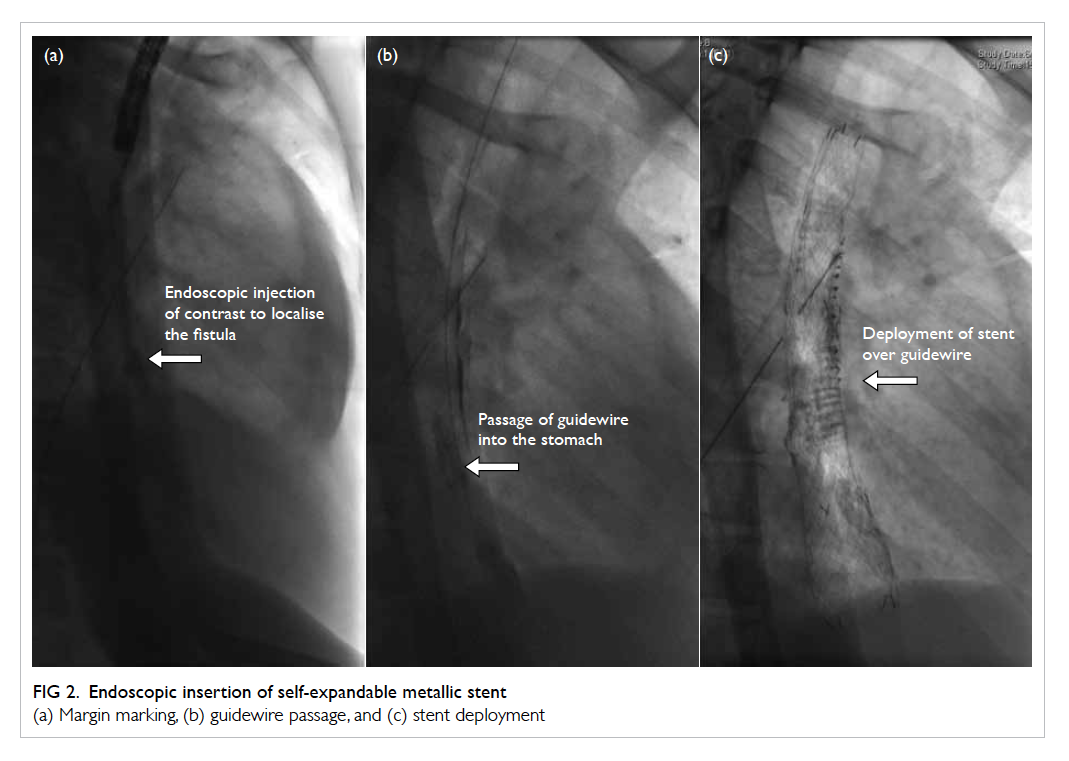
Figure 2. Endoscopic insertion of self-expandable metallic stent
(a) Margin marking, (b) guidewire passage, and (c) stent deployment
A 12-cm 22-Fr retrievable, fully covered, self-expandable
metallic stent (SEMS) with an over-the-wire distal delivery stent deployment system
(Taewoong Medical, South Korea) was selected. The
shoulders of the stent were placed across the fistula
opening, being deployed over the guidewire (Fig 2b and 2c). All positions were confirmed by fluoroscopy
and the proximal stent secured with two endoclips at
12 and 6 o’clock position.
The patient tolerated the procedure well. She
was put on total parenteral nutrition for 2 weeks,
then resumed enteral feeding via a nasoduodenal
tube inserted during a separate OGD session.
Decortication was later performed to clear the
empyema cavity (Fig 1d). Follow-up OGD at 8 weeks demonstrated no stent migration and stent
was removed uneventfully by pulling on the purse-string suture
with endoscopic forceps. Check endoscopy
confirmed complete healing of the ulcer by scarring
(Fig 1e). The retrieved fully covered SEMS was intact and without tissue ingrowth (Fig 1f).
Recovery of the patient was satisfactory.
She achieved complete resolution of sepsis and
tolerated an oral diet after stent removal. Follow-up
computed tomography of the thorax in May 2015
showed minimal thoracic collection and no lung
consolidation.
Discussion
Oesophageal stenting has significantly matured since
its inception. In unresectable malignant obstruction,
SEMS has become the standard of care, with high
technical success (83%-100%) in positioning and
effective dysphagia palliation (80%-95%).1 Emerging
roles are also seen in benign conditions like
anastomotic leaks or perforations, with promising
81.4% and 86% success in defect closure, respectively.2
Success is nonetheless variable in treating benign
strictures and refractory oesophageal variceal
bleeding.1 In malignant oesophagorespiratory
fistulae (ORF), 80% of cases achieve complete healing
with SEMS.3 Little is known about the efficacy in
benign ORF, OPF in particular, due to the clinical
rarity and inherent heterogeneity.
Oesophagorespiratory fistulae is an uncommon
entity comprising all pathological connections
between the oesophagus and airway. Malignant
oesophageal, lung, or mediastinal tumours are
the most common causes. Fistulation occurs
secondary to tumour invasion or inflammation
post-radiotherapy, laser, chemotherapy or stenting.
Benign ORF is rare, with major causes being
trauma and infection.4 Regarding fistula subtypes,
only 3% to 11% represent oesophagopulmonary
fistula or OPF that communicate peripherally. The
rest are either oesophagotracheal (52%-57%) or
oesophagobronchial (37%-40%) in location.5 It is
easy to understand why few reports exist of OPF and
its treatment.
In general, OPF has a similar aetiology to
ORF. Malignancies aside, it is a well-recognised
complication in 0.2% to 1% of pneumonectomies.
Other traumatic causes include endoscopic
sclerotherapy, gunshot wound, and foreign
body ingestion.5 Infective causes range from
tuberculosis to mycotic disease, also candidiasis in
immunocompromised individuals. Other reported
cases include Crohn’s disease, perforated Barrett’s
ulcer, and ruptured diverticulum.6 7 8 Like postsurgical
leaks and perforations, OPF is a sinister condition
associated with high morbidity and mortality.2 3
Significant mediastinal and pleural contamination,
polymicrobial sepsis and abscesses often ensue,
warranting multiple drainage procedures and
surgery, as well as intensive life support.
Oesophagopleural fistula rarely heals
spontaneously. Principles of management include
drainage of collection, exclusion of fistulation,
aggressive antimicrobials, and lastly, intensive
nutritional and organ support. Established abscesses
persist despite spillage control by stenting, hence
necessitating drainage procedures.9 Pleural drainage
is possible via tube thoracostomy or image-guided
pigtail catheter insertion. Yet if multiloculated
collections exist, open or thoracoscopic drainage is
preferred since it permits septa breakdown under
direct vision, thorough irrigation, and placement of
a drain in an ideal position.10 Early use of broad-spectrum
intravenous antibiotics with aerobic
and anaerobic coverage is equally important.
Fistula exclusion may be operative, fluoroscopic,
or endoscopic. Traditional surgical approaches
involve either primary suturing or segmental
oesophagectomy, but are complex and associated
with high morbidity (30%-50%) and mortality
(10%).11 Bypass and muscle flap buttressing are
newer operations possible for the debilitated.12 13 Embolisation with vascular plug and coils under
fluoroscopy has been reported in one article.
Endoscopic fibrin glue application represents a
viable option in small-calibre fistulae. Limited
reports also exist for other endoscopic measures
like argon plasma or electrocoagulation, suturing, or
clipping devices.11
Our patient represented a promising candidate
for oesophageal stenting. Initially performed with
rigid plastic tubes, development has evolved to
SEMS with easier insertion and less migration. Most
commonly made of nitinol, SEMS exhibits great
flexibility and radial force to maintain patency and
position. They come uncovered, partially, or fully
covered, with a plastic or silicon membrane. Fully
covered SEMS is the stent-of-choice in managing
fistulae, since the covering is vital for leakage
prevention and easy retrievability.1 Most published
studies suggest stent placement immediately after
leakage confirmation to minimise mediastinal
contamination.3 Insertion is via upper endoscopy
under sedation, with the patient in a left lateral or
prone position to minimise aspiration. Stent size
is chosen according to oesophageal diameter and
defect size. The proximal and distal ends of the
lesion are identified endoscopically, marked by
radiopaque dye, then a guidewire advanced over the
defect, stent positioned across and deployed over-the-wire, all under endoscopic and fluoroscopic
guidance. Potential stent shrinkage or migration is
counteracted by allowing 4-6 cm proximal and distal
coverage.1 10 A feeding tube is routinely inserted for prompt enteral nutrition. No data are available for
optimal timing of when to resume an enteral diet,
but in oesophageal leaks, Rajan et al10 demonstrated
fitness on day 2 following stent placement. Oral
intake is not encouraged though due to potential
peristalsis-inducing stent migration and hence
leakage. No conclusive timing for stent removal
exists, with studies in general reporting intervals
of 4 to 6 weeks or 6 to 8 weeks.10 In our case, stent
removal at 8 weeks was without difficulty, with no
tissue ingrowth or stent disintegration.
Only one review of SEMS placement in
oesophageal fistulae exists. It is reported that 64.7%
clinical success was achieved in defect closure,
which is significantly lower than the over 80% rates
in malignant ORF, perforations, and post-surgical
leaks.2 3 Detailed scrutiny of the seven studies
pooled, however, revealed that aortoesophageal and
oesophagoatrial fistulae were also included.13 These
conditions present with life-threatening torrential
haemorrhage, and are undoubtedly different in terms
of pathogenesis and survival to the majority of ORF
where sepsis is the main issue. Moreover, fistulae
subtype or location was mostly not reported, and
if present, mostly oesophagotracheal. Significant
heterogeneity exists. Only one of 24 fistulae was
identified as OPF. It is questionable how applicable
the data are.
Specific literature is lacking. Our case report
aside, Kang et al4 represent the only article reporting
endoscopic SEMS for OPF as far as we are aware.
Successful technical and clinical outcomes were
achieved in both studies. To take this further,
considering the similarities in terms of pleural or
mediastinal sepsis and treatment goals in minimising
further contamination, we believe it is reasonable to
extrapolate the success in managing perforations
and postsurgical leaks to ORF.
Potential SEMS-related complications
include retrosternal pain, oesophageal perforation,
bronchoesophageal fistulae, stent migration,
obstruction, or fracture. Migration of the stent
contributes to clinical failure, and appears to be a
particularly troubling issue for fully covered SEMS,
with 10% to 25% occurrence in covered stents and
2% to 5% in uncovered.1 It is also more frequent
in placements for fistulae (33.3%) compared with
benign strictures (15.8%).14 Appropriate stent size
and length reduce the risk of migration, balancing
the larger radial force to oppose migration against the
risk of pressure necrosis. To counteract migration,
some stents come with flared proximal ends.3
Endoscopic clip application of the proximal stent end
to the oesophageal wall has also been reported to be
an effective strategy.15 Such technological advances
may further improve outcomes.
Our case represents the first in local medical
literature to report SEMS use in benign OPF. With the
global trend towards minimally invasive therapies,
we expect a paradigm shift towards effective novel
techniques such as endoscopic stenting or repair. In
this patient with tuberculous fistulae, we favoured
stenting over any endoscopic suturing or clipping to
avoid the tension exhibited in inflamed tissue.
References
1. Kang HW, Kim SG. Upper gastrointestinal stent insertion
in malignant and benign disorders. Clin Endosc
2015;48:187-93. Crossref
2. van Halsema EE, van Hooft JE. Clinical outcomes of self-expandable
stent placement for benign esophageal diseases:
a pooled analysis of the literature. World J Gastrointest
Endosc 2015;7:135-53. Crossref
3. Mangiavillano B, Pagano N, Arena M, et al. Role of stenting
in gastrointestinal benign and malignant diseases. World J
Gastrointest Endosc 2015;7:460-80.
4. Kang GH, Yoon BY, Kim BH, et al. A case of spontaneous
esophagopleural fistula successfully treated by endoscopic
stent insertion. Clin Endosc 2013;46:91-4. Crossref
5. Shin JH, Kim JH, Song HY. Interventional management of
esophagorespiratory fistula. Korean J Radiol 2010;11:133-40. Crossref
6. Albuquerque A, Ramalho R, Macedo G. Multiple
esophagopleural and esophagobronchial fistulas in a
patient with Crohn’s disease. Endoscopy 2012;44 Suppl
2:E114-5. Crossref
7. Andersson R, Nilsson S. Perforated Barrett’s ulcer with
esophago-pleural fistula. A case report. Acta Chir Scand
1985;151:495-6.
8. Agarwal V, Singh SK, Siddiqi MS, Joshi LM, Tandon S.
Esophagopleural fistula following spontaneous rupture
of traction diverticulum. Asian Cardiovasc Thorac Ann
2003;11:344-5. Crossref
9. Kim KR, Shin JH, Song HY, et al. Palliative treatment of
malignant esophagopulmonary fistulas with covered
expandable metallic stents. AJR Am J Roentgenol
2009;193:W278-82. Crossref
10. Rajan PS, Bansal S, Balaji NS, et al. Role of endoscopic
stents and selective minimal access drainage in oesophageal
leaks: feasibility and outcome. Surg Endosc 2014;28:2368-73. Crossref
11. Koo JH, Park KB, Choo SW, Kim K, Do YS. Embolization
of postsurgical esophagopleural fistula with AMPLATZER
vascular plug, coils, and Histoacryl glue. J Vasc Interv
Radiol 2010;21:1905-10. Crossref
12. Kim JJ, Park JK, Moon SW, Park K. A new surgical
technique for spontaneous esophagopleural fistula after
pneumonectomy: cervical esophagogastrostomy via
presternal and subcutaneous route, using a thoracic
esophageal mucosal stripping. Thorac Cardiovasc Surg
2013;61:496-8. Crossref
13. David EA, Kim MP, Blackmon SH. Esophageal salvage
with removable covered self-expanding metal stents in
the setting of intrathoracic esophageal leakage. Am J Surg
2011;202:796-801. Crossref
14. Buscaglia JM, Ho S, Sethi A, et al. Fully covered self-expandable
metal stents for benign esophageal disease:
a multicenter retrospective case series of 31 patients.
Gastrointest Endosc 2011;74:207-11. Crossref
15. Park SY, Park CH, Cho SB, et al. The usefulness of clip
application in preventing migration of self-expandable
metal stents in patients with malignant gastrointestinal
obstruction [in Korean]. Korean J Gastroenterol 2007;49:4-9.

