Hong Kong Med J 2017 Feb;23(1):41–7 | Epub 30 Dec 2016
DOI: 10.12809/hkmj154811
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Screening for retinopathy of prematurity and treatment outcome in a tertiary hospital in
Hong Kong
Lawrence PL Iu, FRCSEd (Ophth), FHKAM (Ophthalmology);
Connie HY Lai, FHKAM (Ophthalmology);
Michelle CY Fan, FRCSEd (Ophth), FHKAM (Ophthalmology);
Ian YH Wong, FRCOphth, FHKAM (Ophthalmology);
Jimmy SM Lai, FRCOphth, FHKAM (Ophthalmology)
Department of Ophthalmology, Queen Mary Hospital, The University of Hong Kong, Pokfulam, Hong Kong
Corresponding author: Dr Lawrence PL Iu (lawipl@hku.hk)
Abstract
Introduction: Studies on the prevalence and
severity of retinopathy of prematurity in the local
population are scarce. This study aimed to evaluate
the prevalence, screening, and treatment outcome of
retinopathy of prematurity in a tertiary hospital in
Hong Kong.
Methods: This cross-sectional study with internal
comparison was conducted at Queen Mary Hospital,
Hong Kong. The study evaluated 89 premature infants
who were born at the hospital and were screened
for retinopathy of prematurity, in accordance with
the 2008 British Guidelines, between January 2013
and December 2013. The prevalences of retinopathy
of prematurity and severe retinopathy requiring
treatment were studied.
Results: The mean (± standard deviation) gestational
age at birth was 30+2 weeks ± 16.5 days (range, 24+1
to 35+5 weeks). The mean birth weight was 1285 g ±
328 g (range, 580 g to 2030 g). A total of 15 (16.9%)
infants developed retinopathy of prematurity and
three (3.4%) required treatment. In a subgroup
analysis of extremely-low-birth-weight infants of
<1000 g, 70.6% developed retinopathy of prematurity
and 17.6% required treatment. Multivariate logistic
regression analysis suggested low birth weight and
patent ductus arteriosus were significantly associated
with development of retinopathy of prematurity
(P<0.001 and P=0.035, respectively). Among the
three infants who received treatment for severe
retinopathy of prematurity, all regressed successfully
after one laser treatment.
Conclusions: Retinopathy of prematurity is a
significant problem among premature infants in
Hong Kong, especially those with extremely low
birth weight. Our screening service for retinopathy
of prematurity was satisfactory and treatment
results were good. Strict adherence to international
screening guidelines and vigilance in infants at risk
are key to successful management of retinopathy of
prematurity.
New knowledge added by this study
- Low birth weight and patent ductus arteriosus were significantly associated with the development of retinopathy of prematurity (ROP).
- ROP is a significant problem among premature infants in Hong Kong, especially those with extremely low birth weight.
- Strict adherence to international screening guidelines and vigilance in high-risk infants are key to successful ROP management.
Introduction
Retinopathy of prematurity (ROP) is a retinal vascular
disease that affects premature infants in whom the
retinal vasculature is not fully developed at the time
of birth. The hyperoxic environment after birth—inclusive of room air—compared with the relative
hypoxic intra-uterine environment suppresses the
growth of retinal vessels (phase 1). Subsequently,
growth of the retina increases metabolic demand
and, in the background of incomplete retinal
vascularisation, results in retinal hypoxia and ROP
development (phase 2).1 2 3 Retinopathy of prematurity
is characterised by the presence of abnormal retinal
fibrovascular proliferation. Severe disease will
progress to total retinal detachment and blindness if
there is no intervention during the critical treatment
period. Such retinopathy is one of the leading causes
of visual impairment in children.4 5 6
The risk factors for ROP include low gestational
age (GA), low birth weight (BW), presence of co-morbidities
(eg patent ductus arteriosus [PDA],
necrotising enterocolitis (NEC), intraventricular
haemorrhage [IVH]), and a high level of supplemental
oxygen.7 8 9 Those born at extreme prematurity and
with extremely low birth weight (ELBW) are at
high risk of severe ROP development. The British
Guidelines published by the Royal College of
Paediatrics and Child Health in 2008 recommended
screening for ROP in premature infants with GA
of <32 weeks or BW of <1501 g.10 The American
Guidelines published by the American Academy of
Pediatrics in 2013 recommended screening for ROP
if GA was ≤30 weeks or BW ≤1500 g. Selected infants
with BW of 1500 to 2000 g or GA of >30 weeks with
an unstable clinical course should also be screened if
they were assessed by the attending neonatologist to
be at high risk of ROP.11 Since neonatal intensive care
units and standards of health care have improved
significantly in the past decade, more extreme
preterm infants are surviving and a higher risk of
ROP development is to be expected.12 13
The revised International Classification
of Retinopathy of Prematurity was published in
2005.14 In this revised version, ROP was classified
into five stages depending on the severity of retinal
fibrovascular proliferation and into three zones
depending on the location of vascularisation.14 Plus
disease is characterised by the presence of severe
retinal venous dilatation and arteriolar tortuosity, iris
vascular engorgement, poor pupillary dilatation, and
vitreous haze.14 Presence of plus disease indicates
high disease activity.14 Aggressive posterior ROP is
an uncommon, severe form of ROP characterised by
posterior vascularisation, prominent plus disease,
and rapid progression.14 Timely treatment is required
for severe ROP to prevent retinal detachment and
vision loss. Current guidelines suggest treatment if
the ROP is type 1 pre-threshold defined by the Early
Treatment for Retinopathy of Prematurity (ETROP)
study,15 that is: (i) zone I, any stage of ROP, with plus
disease; (ii) zone I, stage 3 ROP, with or without plus
disease; or (iii) zone II, stage 2 or 3 ROP, with plus
disease.10 11
The traditional mainstay of treatment is laser
photocoagulation to ablate all avascular areas and
reduce the ischaemic stress to allow the retinal
fibrovascular proliferation to regress.10 11 Intravitreal injection of anti–vascular endothelial growth factor
(anti-VEGF) agent has recently been advocated
if ROP is in zone I, stage 3 with plus disease
or aggressive posterior ROP.16 Operation with
vitrectomy or scleral buckle surgery is required if
retinal detachment has occurred but the results are
often unsatisfactory.17 18 Proper screening and timely treatment are thus important measures to prevent
retinal detachment and vision loss.
The prevalence of ROP and severe ROP that
requires treatment vary among different countries.
The reported prevalence of ROP ranged from 12.6%
to 44.5%13 19 20 21 22 23 and that of severe ROP requiring
treatment ranged from 1.5% to 11.7% in other
countries.13 19 20 21 22 23 In Hong Kong, studies that report
the prevalence and severity of ROP are scarce.24 25 The aim of this study was to evaluate the ROP
prevalence, screening, and treatment outcome in a
tertiary hospital in Hong Kong.
Methods
This was a retrospective cross-sectional study with
internal comparison in which the medical records of
eligible subjects were reviewed. All premature infants
who were born at Queen Mary Hospital and had ROP
screening performed between 1 January 2013 and 31
December 2013 were included. In this hospital, all
infants are screened if the British screening criteria
are met—GA of <32 weeks or BW of <1501 g. Those
who died before ROP screening could be performed
were excluded from this study. This study was done
in accordance with the principles outlined in the
Declaration of Helsinki.
All ROP screening was performed by two
ophthalmologists who had experience in screening
and treating ROP. All examinations were performed
with binocular indirect ophthalmoscopy following
pupil dilatation by topical mydriatic medication.
The severity of ROP was graded according to the
revised International Classification of Retinopathy
of Prematurity.14 The screening protocol followed
the British Guidelines published in 200810:
- First ROP screening was performed at 30 to 31 weeks postmenstrual age (PMA) for infants born before 27 weeks GA, and at 4 to 5 weeks postnatal age for infants born at or after 27 weeks GA.
- Regular ROP screening was performed every 1 to 2 weeks, and more frequent examinations at 1 week or less if the following features were present: (i) vascularisation ending in zone I or posterior zone II; (ii) presence of plus or pre-plus disease; or (iii) presence of stage 3 ROP.
- Treatment was initiated within 48 to 72 hours if the ROP was type 1 pre-threshold defined by the ETROP study15 with the following features: (i) zone I, any stage of ROP, with plus disease; (ii) zone I, stage 3 ROP, with or without plus disease; or (iii) zone II, stage 2 or 3 ROP, with plus disease.
- ROP screening was terminated in infants who did not develop ROP and in whom vascularisation had extended into zone III after 36 weeks PMA, or in those who developed ROP that did not meet treatment criteria and had subsequently regressed.
Data recorded included GA, BW, presence
of co-morbidities, most severe ROP stage, any
treatment given, and the treatment outcome. If the
ROP stage was asymmetrical between the two eyes
in an individual infant, the more severe ROP stage
was measured.
Primary outcome measures included the
prevalence of ROP of any stage and severe ROP that
required treatment. Secondary outcome measures
included association between risk factors of interest
and risk of ROP development, and treatment
outcome. The risk factors of interest studied included
low GA, low BW, presence of respiratory distress
syndrome (RDS), PDA, sepsis, NEC, IVH and the
need for blood transfusion.
Statistical analysis
The Statistical Package for the Social Sciences
(Windows version 23.0; SPSS Inc, Chicago [IL],
US) was used to perform the statistical analysis.
All continuous demographic data are expressed as
mean ± standard deviation and categorical data are
expressed as number (%). Further, GA and PMA are
represented as number of weeks and the remaining
days not completing a week written in superscript,
eg 32 weeks and 5 days represented by 32+5 weeks.
Chi squared test was used to evaluate the difference
among subgroups for ROP development. Fisher’s
exact test was used when the expected frequency of a
cell in a table was <5. Risk factors that might predict
ROP development were evaluated in univariate
logistic regression analyses to calculate the odds
ratio (OR) and 95% confidence interval. If there
was more than one factor associated with a P value
of <0.05 in univariate level, the risk factors would
be entered into a multivariate logistic regression
analysis with backward stepwise method. P<0.05
was considered to be statistically significant. All tests
were two-sided.
Results
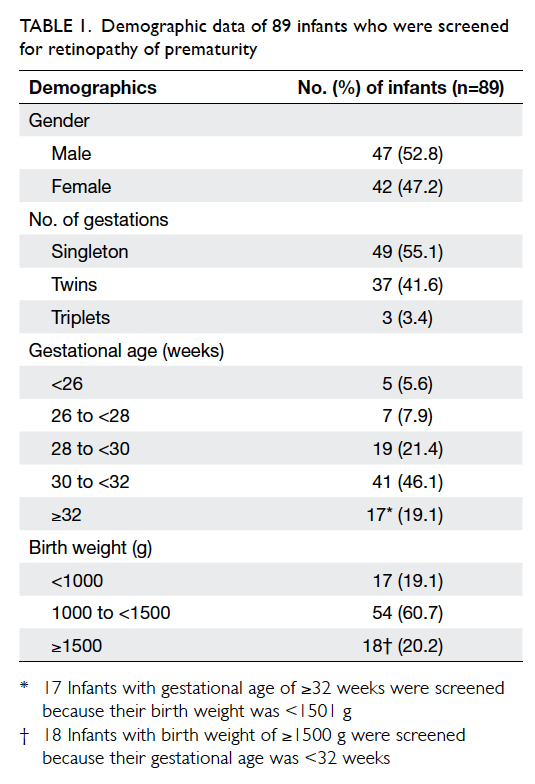
Demographic data
A total of 92 infants met the British screening
criteria during the study period of whom three died
before ROP screening could be performed and were
excluded from this study. Among the 89 infants
screened, 52.8% were male. There were 49 (55.1%)
singletons, 37 (41.6%) twins, and three (3.4%) triplets.
The mean GA was 30+2 weeks ± 16.5 days (range,
24+1 weeks to 35+5 weeks; median, 30+4 weeks). The
mean BW was 1285 g ± 328 g (range, 580 g to 2030 g;
median, 1340 g). The distribution of infants in
relation to GA and BW is shown in Table 1.
Prevalence of retinopathy of prematurity
Of the 89 infants screened, 15 (16.9%) developed
ROP at a mean time of 34+1 weeks ± 13.0 days (range,
31+5 weeks to 38+4 weeks; median, 33+4 weeks), and
three (3.4%) required treatment at a mean time
of 40+2 weeks ± 9.6 days (range, 39+2 weeks to 41+6
weeks; median, 39+5 weeks). Nine (10.1%) infants
developed stage 1 ROP, three (3.4%) developed stage
2 ROP, three (3.4%) developed stage 3 ROP, and none
developed stage 4 or 5 ROP (Table 2).
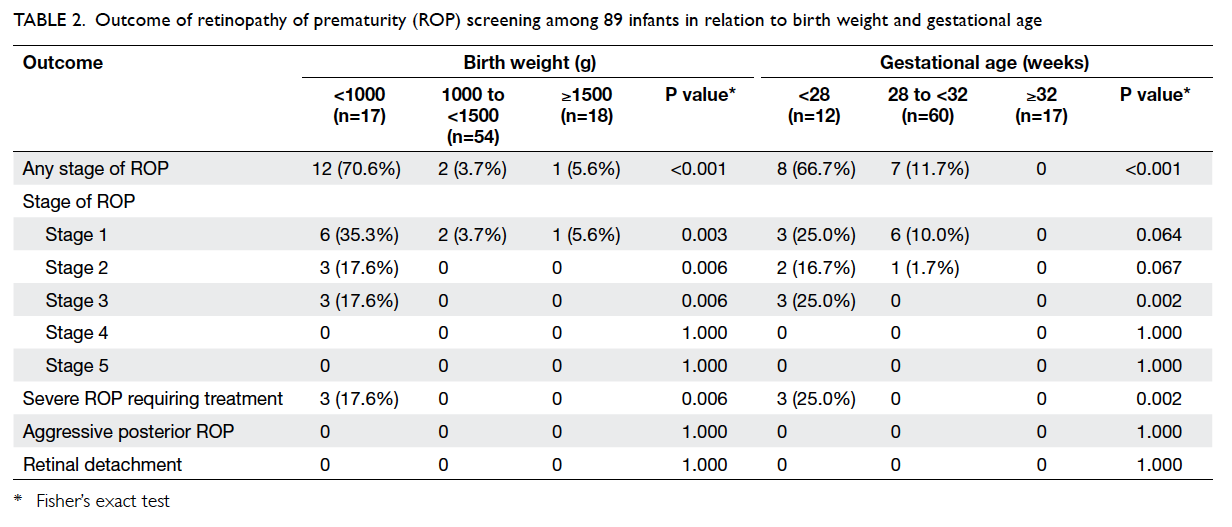
Table 2. Outcome of retinopathy of prematurity (ROP) screening among 89 infants in relation to birth weight and gestational age
Among the 15 infants who developed ROP,
their mean GA was 27+1 weeks ± 14.4 days (range,
24+1 weeks to 30+2 weeks; median, 27+5 weeks) and
mean BW was 846 g ± 276 g (range, 580 g to 1530 g;
median, 790 g).
In subgroup analysis, among the 17 ELBW
infants of <1000 g, 12 (70.6%) developed ROP and
three (17.6%) required treatment. Among the 12
extremely preterm infants with GA of <28 weeks,
eight (66.7%) developed ROP and three (25.0%)
required treatment (Table 2).
When the 2013 American screening criteria
were applied retrospectively, 78 (87.6%) infants met
the criteria. In the 11 (12.4%) infants whose GA and
BW exceeded the 2013 American screening criteria,
none of them developed any ROP.
Risk factors for development of retinopathy of prematurity
In univariate logistic regression analysis, factors
associated with risk of ROP development included
low GA (OR=1.129 for each day decrease; P<0.001),
low BW (OR=1.007 for each g decrease; P<0.001),
RDS (OR=4.952; P=0.044), PDA (OR=12.904;
P<0.001), sepsis (OR=4.787; P=0.013), and need for
blood transfusion (OR=11.786; P<0.001) [Table 3].
In multivariate logistic regression analysis,
factors associated with risk of ROP development
included low BW (OR=1.006 for each g decrease;
P<0.001) and PDA (OR=5.749; P=0.035) [Table 3].
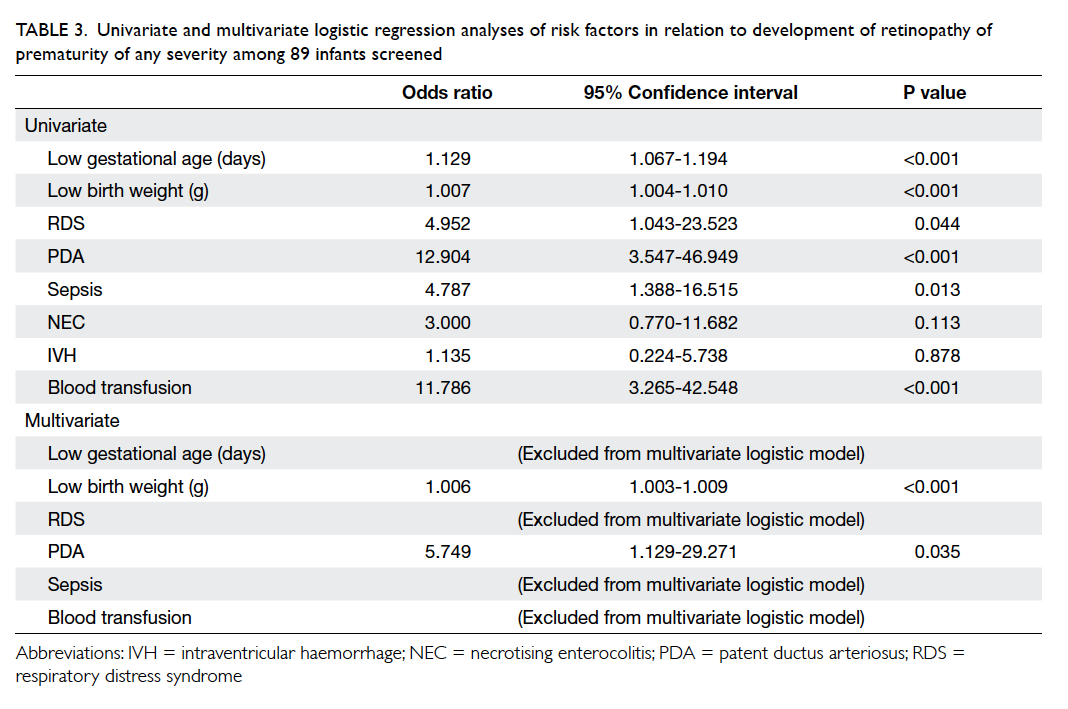
Table 3. Univariate and multivariate logistic regression analyses of risk factors in relation to development of retinopathy of prematurity of any severity among 89 infants screened
Treatment of retinopathy of prematurity
Three infants developed severe ROP that required
treatment (Table 4). Their mean GA was 25+1 weeks
± 7.5 days (range, 24+1 weeks to 26+2 weeks; median,
25+1 weeks) and mean BW was 708 g ± 79 g (range,
660 g to 800 g; median, 665 g). All received
indirect diode laser photocoagulation treatment
and all regressed after one laser treatment. No
supplementary laser, intravitreal injection of anti-VEGF agent, or surgery was necessary.
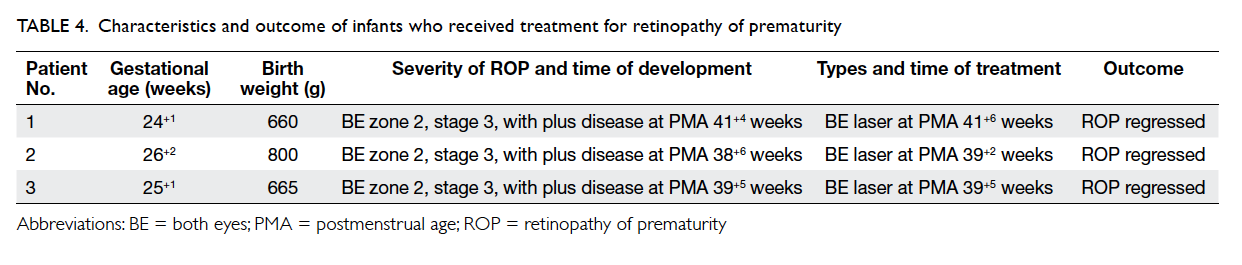
Table 4. Characteristics and outcome of infants who received treatment for retinopathy of prematurity
Discussion
This retrospective study identified the prevalence
of ROP and severe ROP requiring treatment among
premature infants in a tertiary hospital in Hong
Kong. We observed a prevalence of ROP of 16.9%
and that of severe ROP requiring treatment was
3.4%. Our prevalence was comparable to or less than
that reported in most other countries. The reported
prevalences of ROP were 29.2% in Singapore,19 37.8%
in Southern Taiwan,20 21.6% in Southern India,21 12.6% in England,13 21.9% in Netherlands,22 and 44.5% in Brazil.23 The reported prevalences of severe ROP
requiring treatment were 4.8% to 5.0% in Singapore,19
11.7% in Southern Taiwan,20 6.7% in Southern
India,21 1.5% in England,13 and 1.8% in Brazil.23 In mainland China, the ROP screening included bigger
infants with BW of up to 2000 g and GA of up to
34 weeks, and the reported prevalences of ROP and
severe ROP requiring treatment were 17.8% and 6.8%,
respectively.26 Since the risk of ROP among large
infants is known to be small, the prevalence of ROP
in mainland China could not be directly compared
with our study.
Severe ROP is more prevalent in ELBW infants.
Our study showed the prevalence of ROP was
70.6% and that of severe ROP requiring treatment
was 17.6% in ELBW infants of <1000 g. This was
comparable to another local study in Hong Kong in
which 53.4% of ELBW infants developed ROP and
14.5% developed severe ROP requiring treatment.25
Our results are also comparable to those of other
countries, where the prevalences of ROP and severe
ROP requiring treatment in ELBW infants were 55.4%
and 13.7% respectively in Singapore,19 70.7% and
29.3% respectively in Southern Taiwan,20 61.3% and
28.4% respectively in Northern Taiwan,27 and 55.9%
and 19.4% respectively in Turkey.28 In this study,
multivariate logistic regression analysis showed the
risk of ROP development was significantly associated
with low BW and presence of PDA. The association
between PDA and risk of ROP development has been
shown in previous studies.8 9 It has been postulated that persistent left-to-right shunt results in low
systemic blood flow and retinal ischaemia, and thus
is associated with higher risk of ROP development.8 29 In addition, use of indomethacin to close PDA might
reduce retinal blood flow and contribute to ROP
development.8 30
Dilated fundal examination in ROP screening
is a stressful event for premature infants. It is
important to screen only those who are at risk to
avoid unnecessary examination and stress. In this
study, 11 infants would not have been screened if
the 2013 American screening criteria were used
and none of them developed any ROP. This may
suggest that the 2013 American Guidelines are more
appropriate than the 2008 British Guidelines in
reducing unnecessary examination.
In our study, all severe ROP (100%) regressed
after one laser treatment without the need for repeat
treatment. No infants developed stage 4 or above
ROP. This reflects a good standard of neonatal care in
Hong Kong. In Southern Taiwan, 16.9% progressed
to stage 4 or 5 ROP requiring further intervention
after initial treatment.20 In mainland China, 4.2%
of stage 3 ROP and 28.6% of aggressive posterior
ROP progressed to retinal detachment after initial
treatment.26
Our study highlights the importance
of proper screening and timely treatment to prevent
retinal detachment and severe vision loss due to ROP.
We recommend strict adherence to international
screening guidelines, and all ROP screening should
be performed by ophthalmologists with dilated
fundal examination.10 Since ELBW infants have
a high risk of ROP and need for treatment, early
parent education and good communication with
anticipation for treatment will be helpful. For
premature infants transferred from other hospitals
for non-ophthalmological conditions, attention
should be paid to the ROP screening record and
examinations should be performed if the screening
criteria are met. Good communication between
hospital units is crucial to ensure continuous care
and that these infants do not miss ROP screening
and thus the window of opportunity for treatment.
There were several limitations in this study.
First, the sample size was small. Second, due to
the retrospective design, this study was not able
to evaluate other potential risk factors that might
increase the risk of ROP development. The level of
oxygen therapy was not evaluated because it could
not be assessed accurately in view of frequent
changing of arterial oxygen saturation level and
percentage of oxygen administered. Last, this
study reviewed only those who had received ROP
screening or treatment, therefore we were not able
to evaluate those who died before being screened.
Conclusions
Retinopathy of prematurity is an important health
problem among premature infants in Hong Kong,
especially those with ELBW. The results of our
study suggest that the current screening service
and treatment outcome are satisfactory. Strict
adherence to international screening guidelines and
vigilance in infants at risk are key to successful ROP
management.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Hartnett ME, Penn JS. Mechanisms and management of
retinopathy of prematurity. N Engl J Med 2012;367:2515-26. Crossref
2. Hartnett ME. Pathophysiology and mechanisms of severe
retinopathy of prematurity. Ophthalmology 2015;122:200-10. Crossref
3. Hellström A, Smith LE, Dammann O. Retinopathy of
prematurity. Lancet 2013;382:1445-57. Crossref
4. Kong L, Fry M, Al-Samarraie M, Gilbert C, Steinkuller PG.
An update on progress and the changing epidemiology
of causes of childhood blindness worldwide. J AAPOS
2012;16:501-7. Crossref
5. Furtado JM, Lansingh VC, Carter MJ, et al. Causes of
blindness and visual impairment in Latin America. Surv
Ophthalmol 2012;57:149-77. Crossref
6. Haddad MA, Sei M, Sampaio MW, Kara-José N. Causes
of visual impairment in children: a study of 3,210 cases. J
Pediatr Ophthalmol Strabismus 2007;44:232-40.
7. Sylvester CL. Retinopathy of prematurity. Semin
Ophthalmol 2008;23:318-23. Crossref
8. Thomas K, Shah PS, Canning R, Harrison A, Lee SK, Dow
KE. Retinopathy of prematurity: Risk factors and variability
in Canadian neonatal intensive care units. J Neonatal
Perinatal Med 2015;8:207-14. Crossref
9. Hadi AM, Hamdy IS. Correlation between risk factors
during the neonatal period and appearance of retinopathy
of prematurity in preterm infants in neonatal intensive care
units in Alexandria, Egypt. Clin Ophthalmol 2013;7:831-7. Crossref
10. Wilkinson AR, Haines L, Head K, Fielder AR. UK
retinopathy of prematurity guideline. Early Hum Dev
2008;84:71-4. Crossref
11. Fierson WM; American Academy of Pediatrics Section on
Ophthalmology; American Academy of Ophthalmology;
American Association for Pediatric Ophthalmology
and Strabismus; American Association of Certified
Orthoptists. Screening examination of premature infants
for retinopathy of prematurity. Pediatrics 2013;131:189-95. Crossref
12. Chamney S, McGrory L, McCall E, et al. Treatment of
retinopathy of prematurity in Northern Ireland, 2000-2011: a population-based study. J AAPOS 2015;19:223-7. Crossref
13. Painter SL, Wilkinson AR, Desai P, Goldacre MJ, Patel CK.
Incidence and treatment of retinopathy of prematurity
in England between 1990 and 2011: database study. Br J
Ophthalmol 2015;99:807-11. Crossref
14. International Committee for the Classification of
Retinopathy of Prematurity. The International Classification
of Retinopathy of Prematurity revisited. Arch Ophthalmol
2005;123:991-9. Crossref
15. Good WV; Early Treatment for Retinopathy of Prematurity
Cooperative Group. Final results of the Early Treatment
for Retinopathy of Prematurity (ETROP) randomized trial.
Trans Am Ophthalmol Soc 2004;102:233-50.
16. Mintz-Hittner HA, Kennedy KA, Chuang AZ; BEAT-ROP
Cooperative Group. Efficacy of intravitreal bevacizumab
for stage 3+ retinopathy of prematurity. N Engl J Med
2011;364:603-15. Crossref
17. Asano MK, Papakostas TD, Palma CV, Skondra D. Visual
outcomes of surgery for stage 4 and 5 retinopathy of
prematurity. Int Ophthalmol Clin 2014;54:225-37. Crossref
18. Yu YS, Kim SJ, Kim SY, Choung HK, Park GH, Heo JW.
Lens-sparing vitrectomy for stage 4 and stage 5 retinopathy
of prematurity. Korean J Ophthalmol 2006;20:113-7. Crossref
19. Shah VA, Yeo CL, Ling YL, Ho LY. Incidence, risk factors
of retinopathy of prematurity among very low birth
weight infants in Singapore. Ann Acad Med Singapore
2005;34:169-78.
20. Li ML, Hsu SM, Chang YS, et al. Retinopathy of prematurity
in southern Taiwan: a 10-year tertiary medical center
study. J Formos Med Assoc 2013;112:445-53. Crossref
21. Rao KA, Purkayastha J, Hazarika M, Chaitra R, Adith
KM. Analysis of prenatal and postnatal risk factors of
retinopathy of prematurity in a tertiary care hospital in
South India. Indian J Ophthalmol 2013;61:640-4. Crossref
22. van Sorge AJ, Termote JU, Kerkhoff FT, et al. Nationwide
inventory of risk factors for retinopathy of prematurity in
the Netherlands. J Pediatr 2014;164:494-8.e1. Crossref
23. Gonçalves E, Násser LS, Martelli DR, et al. Incidence and
risk factors for retinopathy of prematurity in a Brazilian
reference service. Sao Paulo Med J 2014;132:85-91. Crossref
24. Yau GS, Lee JW, Tam VT, Liu CC, Wong IY. Risk factors for
retinopathy of prematurity in extremely preterm Chinese
infants. Medicine (Baltimore) 2014;93:e314. Crossref
25. Yau GS, Lee JW, Tam VT, Liu CC, Chu BC, Yuen CY.
Incidence and risk factors for retinopathy of prematurity in
extreme low birth weight Chinese infants. Int Ophthalmol
2015;35:365-73. Crossref
26. Xu Y, Zhou X, Zhang Q, et al. Screening for retinopathy of
prematurity in China: a neonatal units–based prospective
study. Invest Ophthalmol Vis Sci 2013;54:8229-36. Crossref
27. Yang CY, Lien R, Yang PH, et al. Analysis of incidence and
risk factors of retinopathy of prematurity among very-low-birth-weight infants in North Taiwan. Pediatr Neonatol
2011;52:321-6. Crossref
28. Bas AY, Koc E, Dilmen U; ROP Neonatal Study Group.
Incidence and severity of retinopathy of prematurity in
Turkey. Br J Ophthalmol 2015;99:1311-4. Crossref
29. Saldeño YP, Favareto V, Mirpuri J. Prolonged persistent
patent ductus arteriosus: potential perdurable anomalies
in premature infants. J Perinatol 2012;32:953-8. Crossref
30. Jegatheesan P, Ianus V, Buchh B, et al. Increased
indomethacin dosing for persistent patent ductus
arteriosus in preterm infants: a multicenter, randomized,
controlled trial. J Pediatr 2008;153:183-9. Crossref