Hong Kong Med J 2017 Feb;23(1):6–12 | Epub 9 Dec 2016
DOI: 10.12809/hkmj164875
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Differences in cancer characteristics of Chinese patients with prostate cancer who present with
different symptoms
Samson YS Chan, MB, ChB, MRCS;
CF Ng, MD, FRCSEd (Urol);
Kim WM Lee, BSc, MPH;
CH Yee, MB, BS, FRCSEd (Urol);
Peter KF Chiu, MB, ChB, FRCSEd (Urol);
Jeremy YC Teoh, MB, BS, FRCSEd (Urol);
Simon SM Hou, MB, BS, FHKAM (Surgery)
SH Ho Urology Centre, Division of Urology, Department of Surgery, The
Chinese University of Hong Kong, Prince of Wales Hospital, Shatin, Hong Kong
Corresponding author: Prof CF Ng (ngcf@surgery.cuhk.edu.hk)
Abstract
Introduction: Currently there is no structured
prostate cancer screening programme in Asia.
Early diagnosis of prostate cancer in Asia is by
an opportunistic case-finding approach, that is,
offering prostate-specific antigen testing to an
individual without obvious symptoms of prostate
cancer. In this study, we investigated the
relationship between the mode of presentation and
the characteristics of prostate cancers diagnosed in
our hospital.
Methods: We recruited 120 consecutive Chinese
patients with prostate cancer newly diagnosed from
September 2011 to February 2013 in a regional hospital
in Hong Kong. Patient demographics, symptoms,
presentation, staging, and risk profiles were collected
and analysed.
Results: The number of subjects diagnosed during
a health check (group 1), investigated for symptoms
with no/low suspicion of prostate cancer (group 2),
investigated for symptoms where prostate cancer
was suspected (group 3), or who had undergone
transurethral prostatectomy (group 4) were 12
(10.0%), 53 (44.2%), 46 (38.3%), and nine (7.5%),
respectively. Overall mean age was 71.0 (range, 54-90)
years, and patients in group 3 were significantly
older than those in groups 1 and 2 (P<0.001).
Patients in group 3 had a significantly higher level
of serum prostate-specific antigen, higher incidence
of abnormal digital rectal examination, and more
metastatic disease at presentation than the other
groups. Nonetheless, more than 50% of the prostate
cancers in groups 1 and 2 were of intermediate risk
or higher staging at presentation. After a median
follow-up of 32 months, cancer-specific survival
was 100% for each of groups 1, 2, and 4 but was only
76.8% for group 3 (P=0.006).
Conclusions: Patients with prostate cancer who
presented with prostate cancer–related symptoms
had more metastatic disease and poorer survival
than patients diagnosed by a case-finding approach.
Moreover, more than half of those patients
diagnosed by case finding belonged to intermediate-
or higher-risk groups for which active treatment was
recommended.
New knowledge added by this study
- In the local Chinese population, patients with prostate cancer who presented with prostate cancer–related symptoms had more metastatic disease and poorer survival than asymptomatic patients.
- More than half of those patients with prostate cancer diagnosed by prostate-specific antigen (PSA) testing (case-finding approach) had intermediate- or higher-risk disease warranting treatment.
- Health care professionals could offer PSA testing to appropriate male patients when they are seen for non-prostate-cancer–related symptoms after appropriate counselling. This may help to improve outcome and survival of prostate cancer patients.
Introduction
Prostate cancer is the second most frequently
diagnosed cancer of men worldwide, with the
highest incidence and prevalence rates occurring in
more developed societies.1 The incidence of prostate
cancer is also increasing in Asian countries.2 Many
reasons have contributed to this recent rise in
incidence in Asia, including the increase in the
ageing population, the westernised diet, and also
the increased use of serum prostate-specific antigen
(PSA) for cancer detection.3 4 Although current
evidence supports the use of PSA testing to decrease
the incidence of metastatic disease and prostate
cancer–specific mortality,5 the use of serum PSA
for the early detection of prostate cancer is still
controversial.6 7 One of the concerns is the risk of
overdiagnosis and overtreatment of low-risk cancer
that may result in more potential harm than benefit
to patients.8 9 10 There are many types of prostate cancer screening approaches. Currently, there is no
structured prostate cancer screening programme in
Asia. Therefore, early diagnosis of prostate cancer in
Asia is by an opportunistic case-finding approach,
that is, offering PSA testing to an individual without
obvious signs and symptoms of prostate cancer.
Information on the characteristics of prostate cancer
diagnosed by various approaches in Asia is lacking,
however. We postulated that patients diagnosed by
a case-finding approach, such as during a routine
health check or a consultation for symptoms with
a low suspicion of prostate cancer origin, would
have a better prognosis than those who presented
with symptoms related to prostate cancer, with or
without metastatic disease. We investigated the
relationship between the mode of presentation and
the characteristics of prostate cancers diagnosed in
our hospital.
Methods
This was a prospective cohort study to assess
consecutive adult male patients diagnosed with
prostate cancer at Prince of Wales Hospital, a
regional hospital in Hong Kong, between September
2011 and February 2013. Institutional ethics approval
was obtained for the study. Informed consent was
obtained from all study subjects prior to enrolment
in the study.
All patients aged 18 years or above at our
hospital with histological confirmation of prostate
cancer were identified and approached for inclusion
in this study. After informed consent was obtained,
information on the initial presentation of the
patient’s condition, prostate cancer characteristics
at diagnosis, and the initial treatment plan were
collected. Patients were then followed up for a
minimum of 2 years, and the clinical outcome was
assessed. All cancer was graded using the Gleason
grading system that is based on the histological
pattern of the cancer tissue. The tissue was
graded from 1 (well-differentiated) to 5 (poorly
differentiated). Each biopsy was given two scores,
the first indicated the most common pattern and the
second, the highest grading.11 Our scoring system
for prostate cancer consists of staging according to
TNM staging 201012 and risk stratification according
to the National Comprehensive Cancer Network
(NCCN) guideline.13
Subjects were divided into four groups
according to the initial clinical presentation of
their prostate cancer by two investigators who were
blinded to the clinical outcome during the assessment
and then confirmed by a senior investigator. Any
discrepancy was discussed and a final allocation
made. The health check group (group 1) included
patients in whom a raised PSA was detected during
a routine health check. Group 2 comprised patients
diagnosed with prostate cancer by the case-finding
approach after they presented with symptoms with
no/low clinical suspicion of prostate cancer (eg
renal cysts, non-specific abdominal pain). Group 3
comprised patients with a high clinical suspicion of
prostate cancer or malignant disease, for example,
lower urinary tract symptoms with abnormal digital
rectal examination (DRE), bone pain, or weight loss.
Finally, those patients with a histological diagnosis
of prostate cancer made following transurethral
resection of the prostate (TURP) but with no
preoperative suspicion of prostate cancer were
assigned to the TURP group (group 4).
Since prostate cancer arises mostly from the
peripheral zone (in contrast to benign prostate
hyperplasia [BPH] that commonly arises from the
transition zone), patients with early-stage prostate
cancer are usually asymptomatic.14 Not until the
tumour becomes locally advanced (with clinical
signs of abnormal DRE) do patients have voiding
symptoms attributed to prostate cancer. Therefore,
in a patient who presents with lower urinary tract
symptoms (LUTS) and normal DRE, the symptoms
are more likely related to BPH, not secondary to
prostate cancer. Testing of PSA is not routine for male
patients with LUTS.15 16 According to the Guidelines
from the European Association of Urology, PSA
measurement should only be performed to assess
the risk of progression of LUTS or if a diagnosis of
prostate cancer would change disease management.16
For patients who present with LUTS but with a low
clinical suspicion of prostate cancer (ie normal DRE),
PSA testing is considered case-finding for prostate
cancer. In this study, such patients were assigned
to group 2. This also applied to other presenting
symptoms with no or low suspicion of being related
to prostate cancer. Nonetheless, in subjects with
LUTS and clinical symptoms or signs suspected to be
secondary to prostate cancer, such as abnormal DRE
findings, PSA testing would be part of the diagnostic
process for prostate cancer, not case-finding. As a
result, these patients would be assigned to group 3.
After all data were collected, descriptive
statistics were applied. A Chi squared test or Fisher’s
exact test was used to determine any relationship
between the categorical outcome measures. Analysis
of variance or Kruskal-Wallis test was used for
normal or skewed data, and then followed by post-hoc
comparisons with Bonferroni adjustment.
Kaplan-Meier survival analysis was applied to analyse
survival among the four groups. Data management
and analysis were performed using the Statistical
Package for the Social Sciences (Windows version
22.0; SPSS Inc, Chicago [IL], US). A two-tailed test
was used with significance set at P<0.05.
Results
From September 2011 to February 2013, 126
consecutive patients with newly diagnosed,
histologically confirmed prostate cancer were
managed in our centre. One patient refused to
participate in this study, and five patients were not
capable of providing informed consent. Therefore 120
patients were enrolled in this study: group 1 (n=12),
group 2 (n=53), group 3 (n=46), and group 4 (n=9)
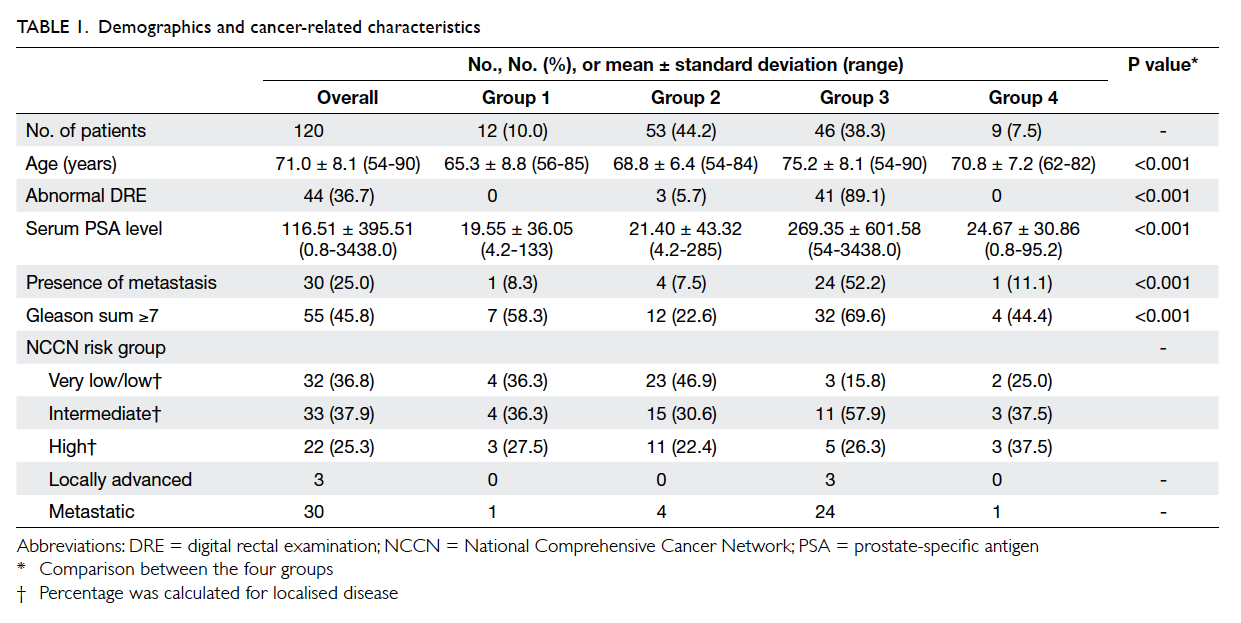
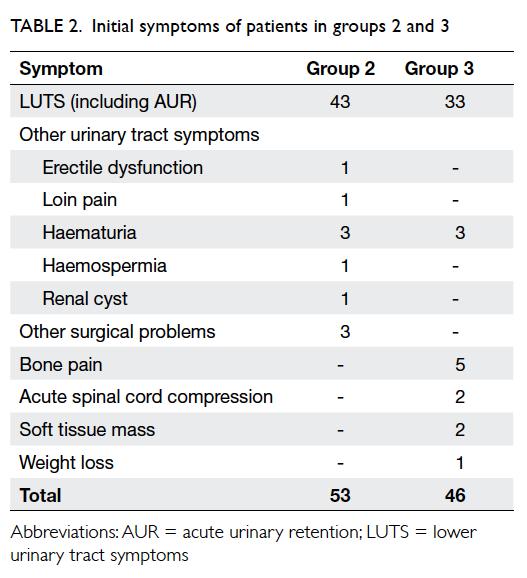
[Table 1]. The initial presenting symptoms of patients in groups 2 and 3 are listed in Table 2. In group 2, 43 patients presented with LUTS (including three with
acute urinary retention) with low clinical suspicion
of prostate cancer. Ten patients presented with other
symptoms—seven with other urological symptoms
and three with other general surgical problems.
Among them, three patients (one with loin pain, one
with erectile dysfunction, and one with hernia) were
found to have abnormal DRE during consultation.
In group 3, 33 patients presented with LUTS (nine
patients with acute urinary retention) and abnormal
DRE. Three patients presented with haematuria
and DRE during initial workup was abnormal and a
subsequent diagnosis was made of prostate cancer.
Nine patients presented with metastatic symptoms,
eg bone pain, acute spinal cord compression, and
abnormal soft tissue mass. One patient presented
with weight loss and was subsequently diagnosed to
have non-metastatic prostate cancer (Table 2).
The overall mean age was 71.0 (range, 54-90)
years (Table 1). Age and serum PSA level were statistically significantly different across groups. Multiple
comparisons with Bonferroni correction revealed
that patients in group 3 were significantly older
than those in groups 1 and 2 (P<0.001). Patients in
group 3 also had a significantly higher serum PSA
level compared with those in group 1 (P=0.044) and
group 2 (P=0.045) by Kruskal-Wallis test. In group
3, 41 (89.1%) patients had an abnormal DRE
(P<0.001).
With regard to disease status, the numbers
of patients with a Gleason sum of ≥7 were seven
(58.3%) in group 1, 12 (22.6%) in group 2, 32
(69.6%) in group 3, and four (44.4%) in group 4. In
accordance with the NCCN guideline, the number
of patients with disease more severe than very low
or low risk were eight (66.7%) in group 1, 30 (56.6%)
in group 2, 43 (93.5%) in group 3, and seven (77.8%)
in group 4 (Table 1). In group 3, 24 (52.2%) patients had metastatic disease at initial presentation, a
much higher rate than in the other groups (P<0.001,
Fisher’s exact test).
Since both groups 1 and 2 had no prostate
cancer–related symptoms, we tried to combine the
two groups to assess the characteristics of prostate
cancer diagnosed by a case-finding approach. Group
3 patients had significantly older age, higher serum
PSA level, more aggressive disease (Gleason sum
≥7), and more metastatic disease at presentation
than the combined groups 1 and 2 patients (P<0.001
for all parameters; Table 3).
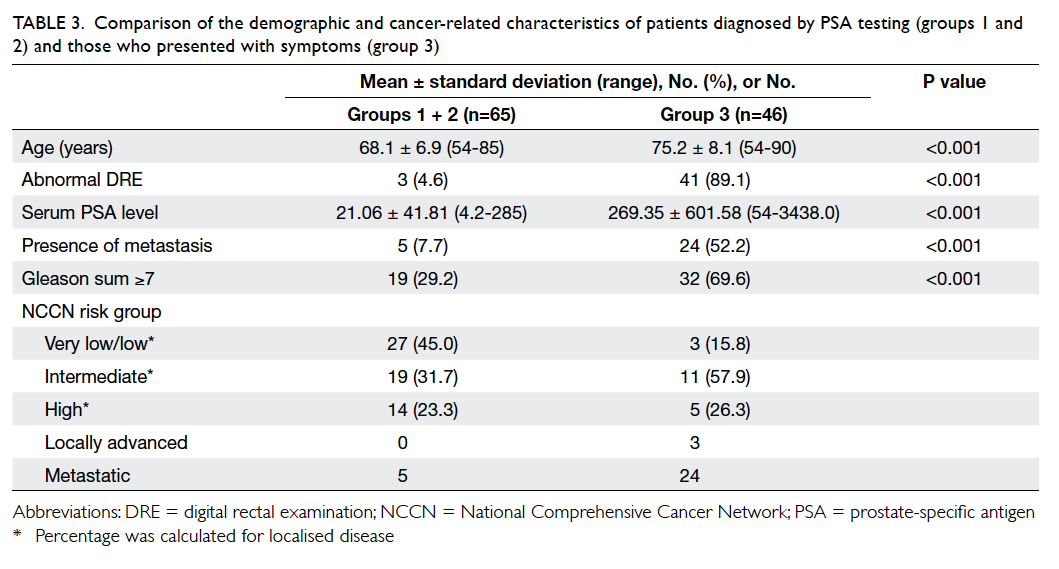
Table 3. Comparison of the demographic and cancer-related characteristics of patients diagnosed by PSA testing (groups 1 and 2) and those who presented with symptoms (group 3)
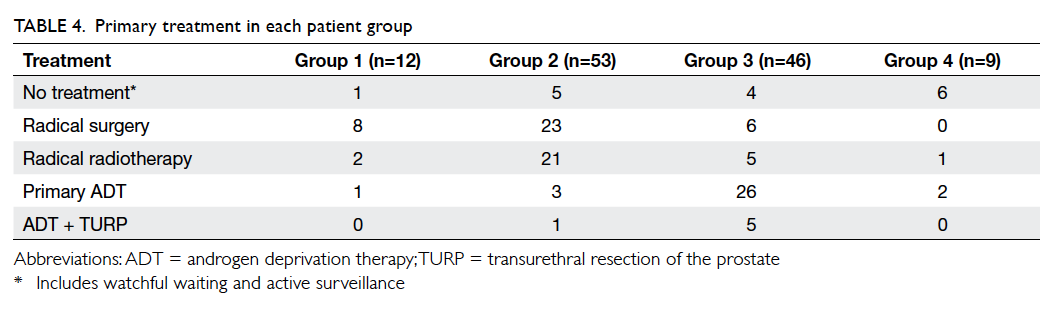
The types of primary treatment administered
are listed in Table 4. The number of patients receiving radical local therapy (either surgery or
radiotherapy) was 10 (83.3%) in group 1, 44 (83.0%)
in group 2, 11 (23.9%) in group 3, and one (11.1%) in
group 4. Systemic androgen deprivation therapy was
prescribed to one (8.3%) patient in group 1, three
(5.7%) in group 2, 26 (56.5%) in group 3, and two
(22.2%) in group 4 (P<0.0005). Because group 3 had
significantly more patients with locally advanced
and metastatic disease, significantly fewer could be
managed with curative-intent local therapy. Among
those patients with very low– or low-risk disease in
groups 1 and 2, one (25%) and five (21.7%) respectively
elected to have conservative management, either
watchful waiting or active surveillance.
The median follow-up period was 32 months
(interquartile range, 28-35 months). No patients were
lost to follow-up. Eleven (9.2%) patients died—10
(21.7%) in group 3 and one (11.1%) in group 4. No
patient in groups 1 or 2 died during the follow-up
period. The causes of death in group 3 patients were
directly related to prostate cancer in seven patients,
metastatic bladder cancer in one patient, and acute
myocardial infarction in two patients. The cause of
death of the patient in group 4 was secondary to
advanced rectosigmoid carcinoma. Therefore, the
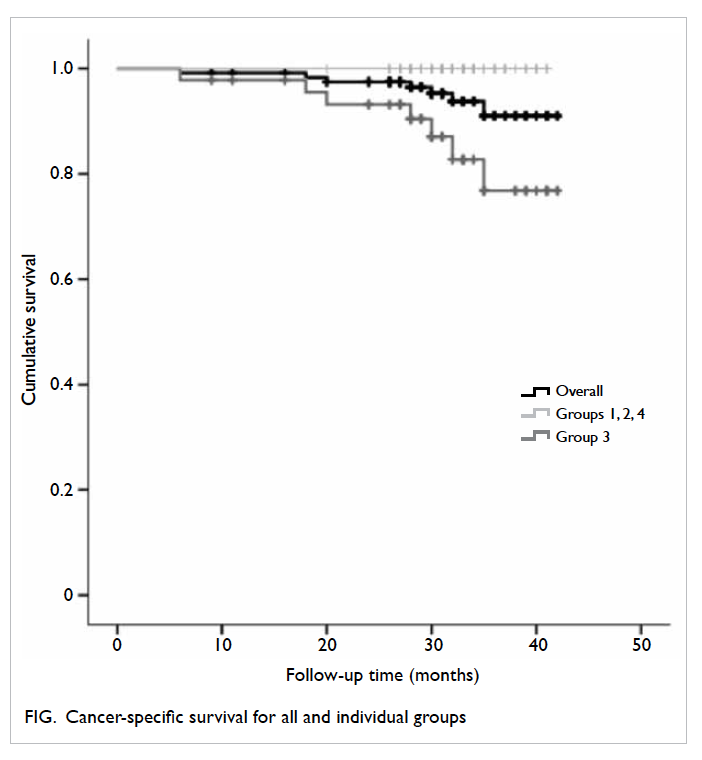
overall rate of cancer-specific survival for the total
cohort was 91.0%, but 100% for each of groups
1, 2, and 4 compared with only 76.8% for group 3
(P=0.006, log-rank test; Fig).
Discussion
Since the introduction of PSA testing, there has
been a worldwide change in the presentation of
prostate cancer. More and more prostate cancers
are diagnosed at a lower risk level and earlier stage
for which curative treatment can be offered.17 18 As a
result, the use of PSA testing for early detection of
prostate cancer is believed to be one of the factors
that has led to the decrease in overall prostate
cancer mortality in many developed areas.2 From
our cohort, we also observed that patients with
prostate cancer diagnosed by case-finding approach
using PSA testing (groups 1 and 2) had significantly
more clinically localised disease and hence a higher
chance of receiving curative-intent treatment than
those patients who presented with prostate cancer–related symptoms (group 3).
We also observed that the short-term cancer-specific
mortality rate of patients who presented
with prostate cancer–related symptoms (group
3) was significantly higher than that in the other
groups. In our cohort, more than half of the patients
in group 3 already had metastasis at diagnosis.
Because patients presenting with metastasis have a
much poorer outcome than other patients,19 20 it was
not surprising that the mortality rate for patients
who presented with symptoms was also higher. This
indirectly supports the case-finding approach by
PSA testing in patients with symptoms but no/low
clinical suspicion of prostate cancer, as it might help
to decrease the incidence of metastatic disease and
hence the mortality related to prostate cancer.21
Although PSA testing is widely performed in
western countries to detect early prostate cancer, its
use in Asian countries is still not a common practice.
From a population-based telephone survey involving
1002 Chinese men aged ≥50 years in Hong Kong,
only 9.5% had ever had a PSA test, and only 3.7% of
the total sample had PSA test done during a routine
health check.22 Even in more developed Asian
countries such as Japan and South Korea, only 15% to
20% of the population had had a PSA test.23 Only 10%
of the prostate cancers in our cohort were diagnosed
during a self-initiated health check with PSA testing.
Therefore, offering a PSA test as case-finding for
prostate cancer during a patient’s consultation
for non-prostate-cancer–related symptoms is an
alternative approach for early detection of prostate
cancer. We believe that this case-finding approach is
feasible for the detection of early prostate cancer in
our region, where public awareness and use of PSA
testing is still low. Certainly, patients need to be well
informed about the nature and implications of PSA
testing before the test is performed.24 25
The main concerns surrounding the use of
PSA testing for the detection of early prostate
cancer are overdiagnosis and overtreatment.8 9 10 Only
approximately 36% of patients in the study cohort
had very low– or low-risk disease that might not
require aggressive intervention.13 26 Even in those
patients with prostate cancer diagnosed by PSA
testing (ie groups 1 and 2), more than 50% were in
the intermediate- or higher-risk groups. Testing of PSA
level did help to detect patients with significantly
high-risk prostate cancer that warranted further
treatment. To minimise the risk of overtreatment,
the Melbourne Consensus Statement advises
uncoupling of the prostate cancer diagnosis from
the intervention.5 Offering active surveillance to
patients with low-risk disease will help to minimise
the potential harm of overtreatment. In our cohort,
for patients in groups 1 and 2 with very low– or low-risk
disease, six (22.2%) opted for observation with
no active treatment. We believe this concept should
be promoted to both clinicians and patients, rather
than limiting the use of PSA testing for the case
finding of prostate cancer.
Currently, some newly proposed strategies,
such as determination of the baseline PSA level
earlier in life27 28 and the use of newer diagnostic
tools,29 30 might help to reduce unnecessary prostatic
biopsies and overdiagnosis of low-risk prostate
cancer. Nonetheless, since most of these studies were
conducted in Caucasian-based populations, further
studies in Asian populations are necessary to verify
their suitability in our region.
Although our data show that the short-term
outcome of patients who present with prostate
cancer–related symptoms seems to be worse than
those diagnosed by PSA testing, this might be due
to potential lead-time bias, that is, the increase in
survival is actually due to the length of time between
the detection of a disease by PSA testing and its usual
clinical presentation and diagnosis. This will result
in an increase in survival time for patients diagnosed
by PSA testing. Other potential bias is length-time
bias which suggests that annual PSA may only detect
slow-growing tumours, that screening for prostate
cancer does not detect the very tumour for which it
is intended.
The aim of our study was not to assess the role of
PSA testing in the detection of early prostate cancer
or its effect on long-term outcome and survival of
patients. Rather, we aimed only to compare cancer
characteristics and short-term outcome among
patients with different presentations. We also did
not analyse the potential harm of PSA testing,
prostatic biopsy, or morbidities related to treatment.
The positive rate for prostatic biopsy depends on
the level of serum PSA and DRE finding. From
local experience, for patients with a normal DRE,
the positive rate of prostatic biopsy for serum PSA
level of 4-10 ng/mL and 10-20 ng/mL was only 6.7%-13.4% and 10.3%-21.8%, respectively.31 32 33 Therefore,
information on this would be helpful during patient
counselling for prostatic biopsy. Another limitation
of our study was the relatively small sample size from
a single centre in Hong Kong, therefore our results
might not represent the general situation in Hong
Kong. Further studies, especially with multicentre
collaboration, may help to confirm the applicability
of our results in the local population.
Conclusions
Patients with prostate cancer presenting with related
symptoms had more metastatic disease and poorer
survival than those diagnosed by a case-finding
approach using PSA testing during a health check
or management of symptoms with a low suspicion
of prostate cancer. More than half of the patients
diagnosed by this case-finding approach belonged to
intermediate- or higher-risk groups for which active
treatment was recommended. Apart from a self-initiated
health check with PSA testing, offering PSA
testing to appropriate patients who present with
symptoms with no/low clinical suspicion of prostate
cancer is an alternative approach to early diagnosis
of prostate cancer. Pre-test counselling, including
the discussion of potential bias (such as lead time
or length-time bias), is essential. This may hopefully
help to improve the short-term outcome for these
patients.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J,
Jemal A. Global cancer statistics, 2012. CA Cancer J Clin
2015;65:87-108. Crossref
2. Center MM, Jemal A, Lortet-Tieulent J, et al. International
variation in prostate cancer incidence and mortality rates.
Eur Urol 2012;61:1079-92. Crossref
3. Sim HG, Cheng CW. Changing demography of prostate
cancer in Asia. Eur J Cancer 2005;41:834-45. Crossref
4. Pu YS, Chiang HS, Lin CC, Huang CY, Huang KH, Chen
J. Changing trends of prostate cancer in Asia. Aging Male
2004;7:120-32. Crossref
5. Murphy DG, Ahlering T, Catalona WJ, et al. The Melbourne
Consensus Statement on the early detection of prostate
cancer. BJU Int 2014;113:186-8. Crossref
6. Schröder FH. Landmarks in prostate cancer screening.
BJU Int 2012;110 Suppl 1:3-7. Crossref
7. Alberts AR, Schoots IG, Roobol MJ. Prostate-specific
antigen-based prostate cancer screening: past and future.
Int J Urol 2015;22:524-32. Crossref
8. Welch HG, Black WC. Overdiagnosis in cancer. J Natl
Cancer Inst 2010;102:605-13. Crossref
9. Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and
overtreatment of prostate cancer. Eur Urol 2014;65:1046-55. Crossref
10. Stone NN, Crawford ED. To screen or not to screen: the
prostate cancer dilemma. Asian J Androl 2015;17:44-5. Crossref
11. Epstein JI. An update of the Gleason grading system. J Urol
2010;183:433-40. Crossref
12. Edge S, Byrd DR, Compton CC, Fitz AG, Greene FL, Trotti
A. AJCC cancer staging manual. 7th ed. New York, NY:
Springer; 2010.
13. National Comprehensive Cancer Network. NCCN Clinical
Practice Guidelines in Oncology—Prostate cancer, version
1.2015. Available from: http://www.nccn.org. Accessed 1
Jul 2015.
14. Loeb S, Carter HB. Early detection, diagnosis, and staging
of prostate cancer. In: Wein AJ, Kavoussi LR, Novick AC, et
al, editors. Campbell-Walsh Urology. 10th ed. Philadelphia,
PA: Elsevier Saunders; 2012: 2763-70. Crossref
15. European Association of Urology. Guidelines on the
management of non-neurogenic male lower urinary tract
symptoms (LUTS), incl. benign prostatic obstruction
(BPO). Available from: http://uroweb.org/wp-content/uploads/EAU-Guidelines-Non-Neurogenic-Male-LUTS_LR.pdf. Accessed 1 Jul 2015.
16. Arianayagam M, Arianayagam R, Rashid P. Lower urinary
tract symptoms—current management in older men. Aust
Fam Physician 2011;40:758-67.
17. Cooperberg MR, Lubeck DP, Mehta SS, Carroll PR,
CaPSURE. Time trends in clinical risk stratification for
prostate cancer: implications for outcomes (Data from
CaPSURE). J Urol 2003;170(6 Pt 2):S21-7. Crossref
18. Crawford ED. Epidemiology of prostate cancer. Urology
2003;62(6 Suppl 1):3-12. Crossref
19. Chowdhury S, Robinson D, Cahill D, Rodriguez-Vida
A, Holmberg L, Møller H. Causes of death in men with
prostate cancer: an analysis of 50,000 men from the Thames
Cancer Registry. BJU Int 2013;112:182-9. Crossref
20. Patrikidou A, Loriot Y, Eymard JC, et al. Who dies
from prostate cancer? Prostate Cancer Prostatic Dis
2014;17:348-52. Crossref
21. Wu JN, Fish KM, Evans CP, Devere White RW, Dall’Era
MA. No improvement noted in overall or cause-specific
survival for men presenting with metastatic prostate
cancer over a 20-year period. Cancer 2014;120:818-23. Crossref
22. So WK, Choi KC, Tang WP, et al. Uptake of prostate cancer
screening and associated factors among Chinese men aged
50 or more: a population-based survey. Cancer Biol Med
2014;11:56-63.
23. Baade PD, Youlden DR, Cramb SM, Dunn J, Gardiner RA.
Epidemiology of prostate cancer in the Asia-Pacific region.
Prostate Int 2013;1:47-58. Crossref
24. Carter HB, Albertsen PC, Barry MJ, et al. Early detection of
prostate cancer: AUA Guideline. J Urol 2013;190:419-26. Crossref
25. Basch E, Oliver TK, Vickers A, et al. Screening for prostate
cancer with prostate-specific antigen testing: American
Society of Clinical Oncology Provisional Clinical Opinion.
J Clin Oncol 2012;30:3020-5. Crossref
26. European Association of Urology. Guidelines on prostate
cancer. Available from: http://uroweb.org/wp-content/uploads/09-Prostate-Cancer_LRV2-2015.pdf. Accessed 1
Jul 2015.
27. Lilja H, Cronin AM, Dahlin A, et al. Prediction of significant
prostate cancer diagnosed 20 to 30 years later with a single
measure of prostate-specific antigen at or before age 50.
Cancer 2011;117:1210-9. Crossref
28. Vickers AJ, Ulmert D, Sjoberg DD, et al. Strategy for
detection of prostate cancer based on relation between
prostate specific antigen at age 40-55 and long term risk of
metastasis: case-control study. BMJ 2013;346:f2023. Crossref
29. Braun K, Sjoberg DD, Vickers AJ, Lilja H, Bjartell AS. A
four-kallikrein panel predicts high-grade cancer on biopsy:
independent validation in a community cohort. Eur Urol
2016;69:505-11. Crossref
30. Stattin P, Vickers AJ, Sjoberg DD, et al. Improving the
specificity of screening for lethal prostate cancer using
prostate-specific antigen and a panel of kallikrein markers:
a nested case-control study. Eur Urol 2015;68:207-13. Crossref
31. Ng CF, Ng MT, Yip SK. Urology update 2—Management of
uro-oncology and associated urological symptoms. Hong
Kong Pract 2009;31:128-32.
32. Ng CF, Chiu PK, Lam NY, Lam HC, Lee KW, Hou SS. The
Prostate Health Index in predicting initial prostate biopsy
outcomes in Asian men with prostate-specific antigen
levels of 4-10 ng/mL. Int Urol Nephrol 2014;46:711-7. Crossref
33. Teoh JY, Yuen SK, Tsu JH, et al. Prostate cancer detection
upon transrectal ultrasound-guided biopsy in relation to
digital rectal examination and prostate-specific antigen
level: what to expect in the Chinese population? Asian J
Androl 2015;17:821-5.