Hong Kong Med J 2016 Dec;22(6):589–99 | Epub 24 Oct 2016
DOI: 10.12809/hkmj164869
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE CME
Associations between diabetic retinopathy and systemic risk factors
Noel Wat, MB, ChB, BSc1;
Raymond LM Wong, MB, BS, MRCSEd(Ophth)1,2;
Ian YH Wong, MB, BS, FRCOphth3
1 Department of Ophthalmology and Visual Sciences, The Chinese
University of Hong Kong, Shatin, Hong Kong
2 Hong Kong Eye Hospital, Argyle Street, Hong Kong
3 Department of Ophthalmology, The University of Hong Kong, Pokfulam, Hong Kong
2 Hong Kong Eye Hospital, Argyle Street, Hong Kong
3 Department of Ophthalmology, The University of Hong Kong, Pokfulam, Hong Kong
Corresponding author: Dr Raymond LM Wong (raymondwlm@hotmail.com)
Abstract
Introduction: Diabetes mellitus is a systemic disease
with complications that include sight-threatening
diabetic retinopathy. It is essential to understand the
risk factors of diabetic retinopathy before effective
prevention can be implemented. The aim of this
review was to examine the association between
diabetic retinopathy and systemic risk factors.
Methods: A PubMed literature search was
performed up to May 2016 to identify articles
reporting associations between diabetic retinopathy
and systemic risk factors; only publications written
in English were included. Relevant articles were
selected and analysed.
Results: Patients with diabetic retinopathy were more
likely to have poor glycaemic control as reflected by
a higher glycated haemoglobin, longer duration of
diabetes, and use of insulin therapy for treatment.
For other systemic risk factors, hypertension was
positively associated with prevalence and progression
of diabetic retinopathy. No clear association between
obesity, hyperlipidaemia, gender, or smoking
with diabetic retinopathy has been established as
studies reported inconsistent findings. Myopia was
a protective factor for the development of diabetic
retinopathy. Several genetic polymorphisms were
also found to be associated with an increased risk of
development of diabetic retinopathy.
Conclusions: Good glycaemic and blood pressure
control remain the most important modifiable risk
factors to reduce the risk of progression of diabetic
retinopathy and vision loss.
Introduction
Diabetic retinopathy is the leading cause of blindness
in adults living in developed countries.1 Almost all
patients with type 1 diabetes mellitus (DM) and more
than 60% of patients with type 2 DM will develop
some degree of retinopathy after a 20-year history
of diabetes.2 It has also been well established that
DM increases the risk of cardiovascular disease.3
Cheng et al4 found that the prevalence of diabetic
retinopathy associated with one, two, three, or four
cardiometabolic risk factors was 16.0%, 17.6%, 21.3%,
or 25.1%, respectively (P=0.001). This implies a
relationship between systemic health conditions and
diabetic retinopathy. In order to help identify and to
prevent progression of this ophthalmic complication
of diabetes, a better understanding of its association
with systemic risk factors is necessary.
Methods
A PubMed literature search was conducted up
to May 2016 using the following key words:
“diabetic retinopathy”, “prevalence”, “epidemiology”,
“systemic associations”, “risk factors”, “diabetic
control”, “HbA1c”, “blood glucose”, “hypertension”,
“hyperlipidaemia”, “cholesterol”, “obesity”, “smoking”,
“myopia”, and “genetics”. Articles reporting systemic
associations of diabetic retinopathy were selected
and analysed. A search through the references of
the retrieved articles was also performed. Only
articles published in English were reviewed. During
selection of the articles, prospective studies had a
higher ranking than retrospective ones.
Results
Prevalence of diabetic retinopathy in diabetic patients
Approximately a quarter to one third of adults
living with diabetes are reported to have diabetic
retinopathy. In a recent systematic literature
review, Yau et al5 estimated that the overall global
prevalence of diabetic retinopathy was 34.6%. The
Multi-Ethnic Study of Atherosclerosis (MESA)
reported a prevalence of 33.2% in adults within
the US population.6 A more recent study of the US
population reports a lower prevalence of diabetic
retinopathy of 28.5%.7
Within the Caucasian population in the US,
the prevalence of diabetic retinopathy ranges from
24.8% to 26.4%.6 7 This is comparable with studies in
western Europe with largely Caucasian populations,
such as in the Gutenberg Health Study in Germany
with a prevalence of 21.7%8 and Tromsø Eye Study in
Norway with a prevalence of 26.8%.9
Multiple studies have reported a higher
prevalence of diabetic retinopathy in black and
Hispanic populations. Studies performed in the
US reported a prevalence of 36.7%6 and 38.8%7 in
African American populations. Additionally, the
Los Angeles Latino Eye Study (LALES) reported
that 46% of Hispanic Americans with type 2 DM
had diabetic retinopathy.10 The MESA study6 and a
more recent study by Zhang et al7 reported a lower
prevalence of 37.4% and 34.0%, respectively in the
Hispanic American population.
There is considerable variation in prevalence
rates of diabetic retinopathy within the Chinese
population. The prevalence in Hong Kong has been
reported between 28.4% and 39.2% in different
studies.11 12 13 A more recent study by Lian et al14 on a
local screening programme for diabetic retinopathy
reported a prevalence rate of 39.0% (95% confidence
interval [CI], 38.8%-39.2%) in Hong Kong. Chen et al15 reported a prevalence of 35.0% in Taiwanese diabetic patients. Similarly, the comprehensive
Beijing Eye Study covering both urban and rural
Chinese populations reported a prevalence of 37%,16
in comparison with the Handan Eye Study focusing
on rural China that reported a higher prevalence of
43.1%.17 The MESA study included data on Chinese
Americans and reported a prevalence rate of 25.7%,
comparable with their Caucasian counterparts.6
Differences in prevalence rates of diabetic
retinopathy between populations may be due to both
genetic factors and access to health care. Moreover,
ethnicity is complex and multifactorial; alone it may
not fully explain the differences in prevalence rates
of diabetic retinopathy reported. Urbanisation and
socio-economic status may also play a role in the
prevalence of diabetic retinopathy.18 Additionally,
discrepancies between studies of diabetic retinopathy
prevalence may depend on several factors including
study methodology. Some studies may have used
different cut-off values for glycated haemoglobin
(HbA1c), oral glucose tolerance test, and spot glucose
to define a diagnosis of DM, thereby increasing
the heterogeneity in the overall study population.
The method of diabetic retinopathy screening and
reporting logistics also varied among studies. For
studies using retinal photographs as screening tools,
the differences in the number of fundus photographs
taken and whether pupil dilatation was performed
before retinal assessment may have influenced the
number of cases of diabetic retinopathy identified.
Classification of diabetic retinopathy
The Early Treatment Diabetic Retinopathy Study
(ETDRS) Diabetic Retinopathy Grading Scale
(Modified Airlie House) and International Clinical
Diabetic Retinopathy (ICDR) Severity Scale are the
two major and most commonly used classification
systems for diabetic retinopathy. The ETDRS Grading
Scale is more commonly used in research contexts
whereas the ICDR Severity Scale is more commonly
used in the clinical context. Additionally, the United
Kingdom National Screening Committee (UK NSC)
diabetic retinopathy grading system is notable for
its widespread use in digital fundus photo screening
programmes worldwide.
In general, diabetic retinopathy can be
classified as non-proliferative or proliferative.
Non-proliferative can then be further classified by
severity ranging from mild to moderate and severe.
Non-proliferative diabetic retinopathy (NPDR) is
characterised by the presence of microaneurysms,
hard exudates, cotton-wool spots, and/or
retinal haemorrhages. Pre-proliferative diabetic
retinopathy changes include vasculopathies such
as intraretinal microvascular abnormality (IRMA)
whereas proliferative diabetic retinopathy is defined
by the presence of neovascularisation or vitreous
haemorrhage or preretinal haemorrhage.
The ETDRS Diabetic Retinopathy Grading
Scale assigns a retinopathy severity score from
level 10 to 85. If no abnormality is found, ETDRS
level 10 is assigned. If only microaneurysms are
present, ETDRS level 20 (very mild NPDR) is
assigned. The ETDRS level 35 is equivalent to
mild NPDR, and is characterised by the presence
of hard exudates, cotton-wool spots, and/or mild
retinal haemorrhages. Standard retinal photographs
of retinal haemorrhages and IRMA are used to
define moderate and severe NPDR in the ETDRS
grading. The number of quadrants in which those
signs are present is also taken into account. In
contrast, a simple 4-2-1 rule is used in the ICDR
Severity Scale: mild NPDR is defined as the
presence of microaneurysms only. Severe NPDR is
diagnosed when there are ≥20 diffuse intraretinal
haemorrhages and/or microaneurysms in all four
quadrants, venous beading in at least two quadrants,
or intraretinal microvascular abnormalities in at
least one quadrant. Moderate NPDR on the other
hand has severity falling below the 4-2-1 rule but
more than merely microaneurysms. In both grading
scales, proliferative diabetic retinopathy is defined
as the presence of neovascularisation. High-risk
and advanced proliferative diabetic retinopathy
(ETDRS level, 71-85) refers to the presence of
neovascularisation complications including preretinal
haemorrhage, vitreous haemorrhage, or
retinal detachment.19
Under the UK NSC diabetic retinopathy
screening system, fundus photos are given a
letter and numerical grading: R0 is assigned if no
abnormality is found; R1 is given in early diabetic
retinopathy with evidence of microaneurysms,
retinal haemorrhage, or other features of diabetic
retinopathy; R2 and R3 ratings are assigned in pre-proliferative
and proliferative diabetic retinopathy,
respectively. M1 is designated in cases with
maculopathy, the presence of which is used to predict
the presence of clinically significant macular oedema
that requires treatment. P1 is assigned if the fundus
photograph shows evidence of previous panretinal
laser photocoagulation. Accordingly M0 and P0 are
given respectively if the above characteristics are
absent. Finally, a grading of U is given if an image is
regarded as ungradable,20 common reasons include
dense cataract and corneal scars.
Diabetes and diabetic retinopathy
Glycaemic control
Glycated haemoglobin is a commonly used marker
for monitoring glycaemic control. Multiple studies
have consistently shown HbA1c to be an independent
risk factor for diabetic retinopathy.6 7 10 21 22 A higher
HbA1c is associated with both increased incidence
as well as progression of diabetic retinopathy.21 The
LALES study found a 22% increase in prevalence
of diabetic retinopathy with 1% increase in HbA1c.
Data suggested a plateau of the curve at HbA1c of
≥11%, however.10 Elevated HbA1c reflects poorly
controlled diabetes, which is one of the major
causes of complications in DM including diabetic
retinopathy. Nonetheless, the United Kingdom
Prospective Diabetes Study (UKPDS) demonstrated
that even a good HbA1c level of 7.0% had an
absolute risk of 7.9 per 1000 patient-years for retinal
laser photocoagulation.10 23 This emphasises the
importance of optimal glycaemic control in diabetic
patients in order to prevent diabetic retinopathy. In
contrast, this association was not seen in young-onset
type 1 DM,24 a difference that may be attributed
to the role of hyperglycaemic memory.25
The ACCORD study26 compared the effects of
intensive glycaemic treatment with target HbA1c
of <6.0% compared with standard treatment with
target HbA1c of 7.0% to 7.9%. The authors found a
decreased rate of progression of diabetic retinopathy
in the intensive treatment group, 7.3%, versus 10.4%
in the standard therapy group (adjusted odds ratio
[OR]=0.67; 95% CI, 0.51-0.87; P=0.003).26 Despite the
perceived benefit of reduced progression of diabetic
retinopathy, intensive glycaemic control is not
without risk. The ACCORD study found an increased
risk of hypoglycaemia requiring medical assistance
(10.5% vs 3.5%; P=0.001), weight gain of >10 kg (27.8%
vs 14.1%; P=0.001), and all-cause mortality (hazard
ratio=1.22; 95% CI, 1.01-1.46; P=0.04).27 Similar findings
have also been demonstrated in the Diabetes Control
and Complications Trial (DCCT).28 The authors
reported that intensive diabetes control therapy
reduced the adjusted mean risk for development of
retinopathy by 76%, but at a cost of a 33% increased
risk of weight gain and a 3-times higher risk of
severe hypoglycaemia.28 Thus, one must balance the
benefit of reducing the risk of diabetic retinopathy
and the risk of tight glycaemic control when treating
DM. As demonstrated in this review, however,
multiple factors play a role in the development
and progression of diabetic retinopathy, hence
tight blood glucose control alone may not entirely
prevent the development of diabetic retinopathy.
Furthermore, Mohamed et al29 have recommended
that in patients with diabetic retinopathy, a HbA1c
level of 7% is ideal in reducing progression of and new
development of diabetic retinopathy.
Studies have also found a significantly higher
fasting plasma glucose in subjects with diabetic
retinopathy.6 16 Xie et al16 found that patients with
diabetic retinopathy had a mean (± standard
deviation) fasting plasma glucose level of 8.88 ± 4.56
mmol/L compared with 7.70 ± 2.80 mmol/L in those
without diabetic retinopathy. This association may
also be due to the effects of hyperglycaemia causing
retinopathy.
Duration of diabetes
An unmodifiable risk factor, prolonged duration
of diabetes, has been consistently demonstrated to
be a risk factor for diabetic retinopathy.6 8 16 21 24 30 In fact, one study reported that patients with diabetic
retinopathy had longer duration of diabetes, double than those without retinopathy (25 ± 10 vs 12 ±
8 years; P<0.0001).30 This was corroborated by Zhang et al7 in a large-scale study which found that patients with diabetic retinopathy had a
longer duration of diabetes (15.0 ± 1.6 years vs 7.3 ± 0.8 years; P<0.001). Furthermore, Wong et al31 reported the OR of diabetic retinopathy increased by
1.07 ± 0.2 per year of duration of disease. The LALES
study found that each year of increased history of
diabetes was associated with an 8% increased risk
of having diabetic retinopathy.10 This association
can be explained by a prolonged exposure to the
hyperglycaemic state that may increase the risk of
vascular injury, leading to diabetic retinopathy and
other complications.
Diabetic drug use
Patients with diabetic retinopathy are also more likely
to require medication such as oral hypoglycaemic
agents or insulin to control their diabetes. In other
words, known and treated diabetes is a predictor of
diabetic retinopathy compared with unknown and
untreated diabetes.32 A large-scale study performed
in the Chinese population found that 90% of diabetic
patients without retinopathy were either not on
treatment, diet control, or oral hypoglycaemic
agents.16 In contrast, almost 80% of patients with
diabetic retinopathy required oral hypoglycaemic
agents or insulin injections for diabetic control.16
Diabetic patients with diabetic retinopathy were
statistically significantly more likely to use insulin
(47.4 ± 8.3% vs 26.7 ± 4.8%; OR=3.23).7 One study
found the prevalence of diabetic retinopathy to
be 70% in patients with type 2 DM using insulin,
compared with only 39% in those not receiving
insulin treatment.33 These findings are consistent
with several other studies.10 34 This relationship
between insulin use and diabetic retinopathy may be
explained by the severity and level of blood glucose
control in patients. In other words, patients who
did not require medication are likely those with
borderline diabetes or relatively well controlled
blood glucose profile, and thus have less risk of
developing diabetic retinopathy.
The associations between diabetic risk factors
and diabetic retinopathy are summarised in Table 1.6 7 8 16 21 24 29 30
Systemic risk factors and diabetic retinopathy
Hypertension
Hypertension has been consistently demonstrated to
have a positive association with the development of
diabetic retinopathy.4 6 7 8 30 The LALES study found an
OR of 1.26 (P=0.002) for every 20 mm Hg increase
in blood pressure.10 The Hoorn study estimated that
patients with hypertension had more than double
the risk of developing retinopathy after 10 years
when compared with diabetic patients with normal
blood pressure.22 Stratton et al35 found that the
incidence of developing new retinopathy increased
from 17% to 32% when comparing the lowest
tertile with the top third mean blood pressure in
patients with diabetic retinopathy (P<0.0001). This
gradient was less pronounced with 26% and 36% of
progression in retinopathy when the bottom third
was compared with the top tertile of blood pressure,
respectively (P=0.005).35 This marked association
between hypertension and diabetic retinopathy may
be explained by the clinical finding that hypertension
and diabetes frequently co-exist. Hypertension may
cause morphological changes in the retinal vessels
that are similar to those seen in mild-to-moderate
NPDR such as hard exudates, cotton-wool spots, and
retinal haemorrhages.34
The landmark UKPDS 69 study has outlined
the importance of blood pressure control in
patients with diabetic retinopathy.21 The authors
demonstrated that tight control of blood pressure
with a target level of 150/85 mm Hg, rather
than loose control of less than 180/105 mm Hg,
statistically significantly decreased the development
of microaneurysms (relative risk [RR]=0.66;
P<0.001), hard exudates (RR=0.53; P<0.001), and
cotton-wool spots (RR=0.53; P<0.001). These
effects were evident by 4.5 years of follow-up and
persisted for up to 7.5 years. Furthermore, there
were no detectable differences between blood
pressure control by atenolol or captopril therapy,
nor in primary or secondary prevention groups.
Additionally, the loose blood pressure
control group had an absolute risk of 4.1 per 1000
patient-years for blindness in one eye due to all
causes (P=0.046; RR=0.76; 99% CI, 0.29-1.99), in
comparison with an absolute risk of 3.1 per 1000
patient-years risk of blindness in the tight blood
pressure control group.21
The importance of tight blood pressure
control could not be clearly demonstrated in
both the Appropriate Blood Pressure Control in
Diabetes (ABCD) Trial36 or the ACCORD study.26
In the ABCD Trial, subjects were randomised to
intensive control group (diastolic blood pressure
of 75 mm Hg) or moderate control group (diastolic
blood pressure of 80-89 mm Hg).36 The authors
found no statistically significant difference in the
progression of diabetic retinopathy over a 5-year
follow-up period. Discrepancies in findings between
the studies may be partially due to the difference in
blood pressure targets. The authors of the ABCD
Trial found that both groups had poor glycaemic
control despite optimal blood pressure control
and this may have accounted for the progression
of diabetic retinopathy.36 This may further suggest
the importance of glycaemic control in diabetic
retinopathy. Likewise, the ACCORD study found
no statistically significant relationship between
intensive blood pressure therapy (median systolic
blood pressure, 117 mm Hg) and standard therapy
(median systolic blood pressure, 133 mm Hg)
[10.4% vs 8.8%; adjusted OR=1.23; 95% CI, 0.84-1.79; P=0.29].26 The authors hypothesised that these findings may be attributable to the small difference
between blood pressure in the two groups.
Obesity
Obesity is another risk factor commonly associated
with cardiovascular disease. It can be defined by
waist-hip ratio, waist circumference, and body mass
index (BMI). Both greater waist-hip ratio and waist
circumference are positively associated with diabetic
retinopathy.6 22 24 The OR of diabetic retinopathy is 1.28 per 5-cm increase in waist circumference
(OR=1.28; 95% CI, 1.05-1.56; P=0.014).24
The association between BMI and diabetic
retinopathy has been variable among studies. A
study looking specifically at patients with type 1 DM
found that obesity with a BMI of >30 kg/m2 was the
predominant risk factor for diabetic retinopathy,
even when controlling for other risk factors such as
HbA1c and the use of cardioprotective drugs.37 In
another study, obesity was associated with increased
prevalence of retinopathy and patients with
retinopathy were more likely to be obese, although
this association was not sustained after adjusting for
confounding factors such as blood pressure.30 Yet
among patients with retinopathy, higher BMI was
noted to be positively associated with more severe
retinopathy and vision-threatening retinopathy.8
Some studies have reported no increased risk
between elevated BMI and retinopathy.38 39 On the
contrary, some studies have even reported an inverse
relationship between BMI and diabetic retinopathy.
The Wisconsin Epidemiologic Study of Diabetic
Retinopathy found that underweight patients (BMI
<20 kg/m2) with diabetes had a higher incidence of
retinopathy compared with obese patients (RR=1.99;
95% CI, 1.21-3.26 vs RR=1.27; 95% CI, 1.00-1.61).40 In fact,
100% of the underweight patients in this study
developed retinopathy by 10 years. This observation
held true for the progression of retinopathy as
well. The authors observed that the underweight
patients had a longer duration of diabetes and
were also more likely to be taking insulin than the
obese subjects. Thus, they hypothesised that the
underweight patients might have had poorer overall
glycaemic control and hence were in a more ‘severe’
phase of diabetes when compared with their obese
counterparts.40 This hypothesis also corroborates
the findings of the DCCT study that tight glycaemic
control increases the risk of being overweight.28
Although not statistically significant, Wong et al31
reported that a lower BMI was associated with
diabetic retinopathy.
Sex
Male sex is an independent risk factor for diabetic
retinopathy. A large-scale study performed in the
United States revealed that in diabetic patients
over the age of 40 years, 38% ± 5.5% of men
compared with 27.1% ± 4.7% of women had diabetic
retinopathy (OR=2.07; 95% CI, 1.39-3.10).7 While
the LALES study showed no statistically significant
difference in the incidence of diabetic retinopathy
between different sexes, their stepwise multivariate
model demonstrated that men had a 50% higher risk
of having any diabetic retinopathy when compared
with females (OR=1.50; P=0.006).10 This finding
was echoed by the UKPDS 50 study that also found
no difference in incidence between the two sexes
(P=0.67), but a multivariate model showed that
women had a lower RR of progression of diabetic
retinopathy (RR=0.54; CI, 0.37-0.80; P=0.0016).35
Despite the above evidence, several other studies
have found no statistically significant associations
between diabetic retinopathy and sex.22 30
Hyperlipidaemia
Studies have found varying relationships between
elevated cholesterol and diabetic retinopathy.
While Yau et al5 reported that elevated total serum
cholesterol was associated with a higher prevalence
of diabetic macular oedema and vision-threatening
diabetic retinopathy, other studies have been
unable to reproduce similar results. Tomić et al34
demonstrated no statistically significant difference
in cholesterol level between patients with different
severity of diabetic retinopathy. The Hoorn study
also found no relationship between total cholesterol
level and incidence of diabetic retinopathy,22 but
demonstrated that elevated serum lipid level is
associated with increased prevalence of hard
exudates characterising NPDR.41 This association
of elevated serum lipid levels and hard exudates
was observed in other studies as well.42 43 44 Patients
with type 1 DM with diabetic retinopathy have been
shown to have statistically significantly higher total
cholesterol level than those without diabetic retinopathy (199 ± 35 mg/dL vs 188 ± 36 mg/dL;
P=0.001), although after logistic regression analysis
this was not shown to be an independent risk factor
for diabetic retinopathy.30 On the contrary, Wong et al31 reported higher total cholesterol level to be
protective of diabetic retinopathy (OR=0.73, per 1
mmol/L increase).
Studies showed inconsistent results in the
relationship between serum triglycerides and diabetic
retinopathy. The Hoorn study,22 De Block et al,30
and Tomić et al34 have all demonstrated that serum
triglyceride levels are not an independent risk factor
for diabetic retinopathy. Likewise, in a large-scale
study of 2535 patients with type 2 DM, retinopathy
was not significantly associated with triglyceride
or high-density lipoprotein (HDL) cholesterol
levels after adjustment of confounding factors.45 In
contrast, Cheng at al4 found that in overweight type
2 DM patients, elevated triacylglycerol levels were
significantly associated with diabetic retinopathy
(OR=1.29; 95% CI, 1.05-1.58; P<0.05).
Despite the conflicting data, there appears to
be merit in treating hyperlipidaemia in patients with
diabetic retinopathy. The ACCORD trial investigated
the effects of intensive treatment of dyslipidaemia on
the progression of diabetic retinopathy.26 Patients in
the intensive treatment group were given 160 mg of
fenofibrate daily plus simvastatin, while patients in
the standard treatment group were given placebo
with simvastatin. The authors found a significant
improvement in HDL cholesterol (P=0.002) and
significant decrease in triglyceride level (P<0.001)
in the intensive treatment group. As expected,
fall in low-density lipoprotein cholesterol level
was comparable between the two groups (P=0.68)
as both received simvastatin. With the
significant improvement in lipid profile, the authors
found that intensive therapy decreased the risk of
progression of diabetic retinopathy compared with simvastatin alone, 6.5% vs 10.2%
(adjusted OR=0.60; 95% CI, 0.42-0.87; P=0.006).26 A
more recent meta-analysis by Das et al46 found no
statistically significant improvement in severity of
diabetic macular oedema or decrease in progression
of hard exudates in patients receiving lipid-lowering
drugs compared with placebo. The authors, however,
remarked that studies included in the meta-analysis
were of questionable quality, calling to the necessity
for further high-quality research. Studies included
in the meta-analysis by Das et al46 had small sample
sizes, underpowered studies, and poor subject
selection criteria since patients included had early-stage
diabetic retinopathy and hence were at low risk
of progression.
Chronic kidney disease
Both retinopathy and nephropathy are microvascular
complications of diabetic retinopathy. Multiple
studies have demonstrated the association between
diabetic retinopathy and chronic kidney disease.
In a study by Park et al47 in a Korean population,
the authors defined chronic kidney disease as
estimated glomerular filtration rate (eGFR) of
<60 mL/min/1.73 m3. They reported that even
after controlling for confounders, both chronic
kidney disease (OR=2.34; 95% CI, 1.04-5.28) and
proteinuria (OR=4.56; 95% CI, 1.51-13.77) were
significantly associated with diabetic retinopathy.47
Additionally, Zhang et al48 found that in the Chinese
population, lower eGFR was significantly associated
with increasing severity of diabetic retinopathy in
patients with diabetic retinopathy (mean eGFR, 93
mL/min/1.73 m3) compared with patients without (mean eGFR, 116 mL/min/1.73
m3; P<0.0001), independent of hypertension and
diabetes duration. Diabetic retinopathy was also
associated with microalbuminuria (P<0.0001) and
higher albumin-to-creatinine ratio (6.4 vs 0.63 in
patients with and without diabetic retinopathy,
respectively). The authors suggested that in
diabetic patients, chronic hyperglycaemia causes
microvascular changes in both the glomerulus of
the kidney and retina of the eye. Over time, these
microvascular changes lead to narrowing and
occlusion of the vascular lumina, and eventually
cause inadequate perfusion of affected tissues
leading to retinopathy and nephropathy.48 These
findings were corroborated by Penno et al49 who
reported that a high urine albumin-to-creatinine
ratio of ≥300 mg/g was associated with diabetic
retinopathy (OR=2.9; 95% CI, 2.1-4.0). Likewise,
Rodríguez-Poncelas et al50 found that increasing
urine albumin-to-creatinine ratio was significantly
correlated with rising diabetic retinopathy
prevalence, and this association was significant even
at urine albumin-to-creatinine levels of ≥10 mg/g
(OR=1.2; 95% CI, 1.1-1.4).
Smoking
Smoking was shown to have neither a statistically
significantly positive nor negative association with
diabetic retinopathy. The Hoorn study demonstrated
a non-significant trend for increased OR of diabetic
retinopathy incidence in cigarette smokers and ex-smokers.22 A 25-year follow-up study showed a non-significant trend that current smokers were more
likely to develop proliferative diabetic retinopathy
than never smokers, yet was unable to establish a
statistically significant association between smoking
status or pack-years of smoking and proliferative
diabetic retinopathy. The authors found that mild
NPDR was more common among current smokers
than former smokers (28.4% vs 13.0%; P=0.038) and
may suggest that smoking is indeed related to early
forms of diabetic retinopathy.51 On the contrary,
the UKPDS 50 study demonstrated a protective
effect of smoking—current smokers had a reduced
incidence of retinopathy with a RR of 0.63 (95% CI,
0.4-0.82; P=0.0043) as well as reduced progression
of retinopathy with a RR of 0.50 (95% CI, 0.36-0.71,
P=0.0045) when compared with never smokers.35
Myopia
Long-sightedness is prevalent among Asians and
it has become a significant problem in this locality
in terms of resources for glasses prescriptions,
refractive surgeries, and the management of visual-threatening
complications such as myopic macular
degeneration, myopic tractional maculopathy, and
choroidal neovascularisation. Although myopia
in most cases is harmful to ocular health, it has
long been observed that the prevalence of diabetic
retinopathy is low in myopic patients. This protective
effect of myopia against diabetic retinopathy has
recently been proven by various meta-analysis,52 53
revealing the OR of diabetic retinopathy in myopic
diabetic patients versus non-myopic diabetic
patients to be 0.70 (95% CI, 0.58-0.85; P<0.001). On
the contrary, axial length, the major cause of myopia,
was found to be associated with diabetic retinopathy
as well, in which each millimetre increase in axial
length is associated with decreased risk of diabetic
retinopathy (OR=0.75; 95% CI, 0.65-0.86; P<0.001).
The relationships between systemic risk factors
and diabetic retinopathy are summarised in Table 2.4 5 6 7 8 10 19 21 22 24 28 30 34 38 39 40 42 43 44 47 48 49 50 51 52 53
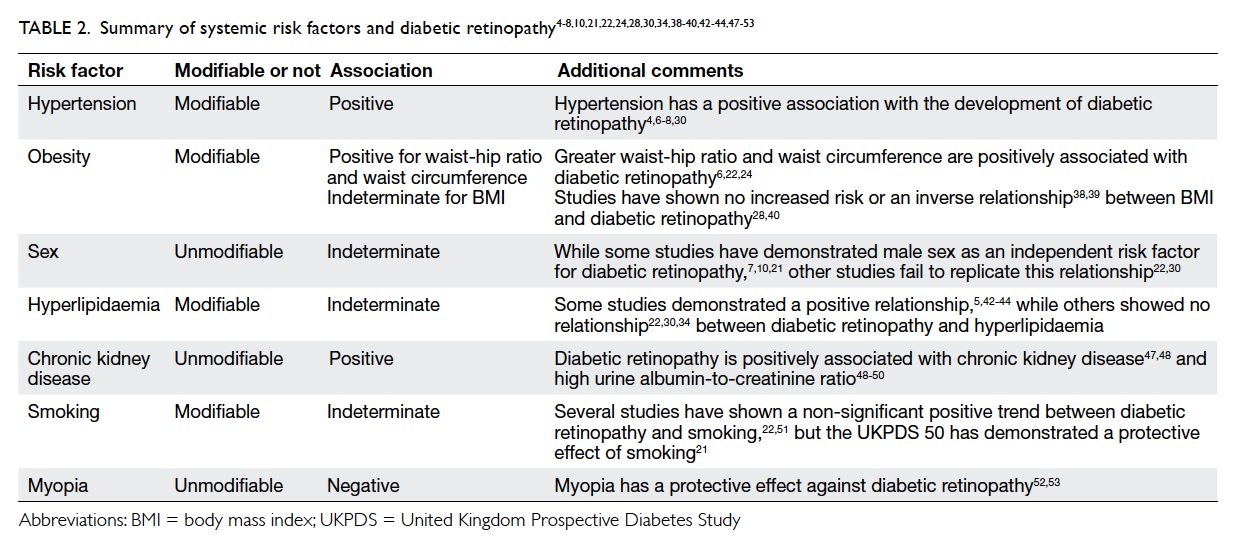
Table 2. Summary of systemic risk factors and diabetic retinopathy4 5 6 7 8 10 21 22 24 28 30 34 38 39 40 42 43 44 47 48 49 50 51 52 53
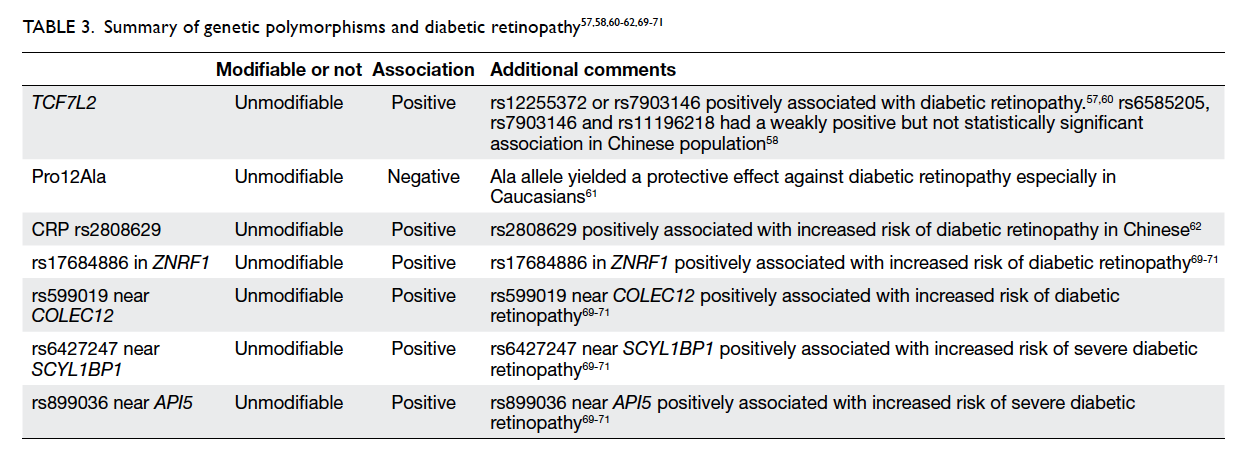
Genetic polymorphisms and diabetic retinopathy
While genetic polymorphism is not a modifiable risk
factor, various genetic polymorphisms may cause a
predisposition to the development and progression
of diabetic retinopathy. Multiple studies have
demonstrated the relationship between TCF7L2 and
the development of type 2 diabetes54 55 56 as increased
expression of this gene is associated with poor serum
glucose control. Ciccacci et al57 found that patients
with rs12255372 or rs7903146 variants of TCF7L2
were at higher risk of developing diabetic retinopathy.
Within the Chinese population, patients with
rs6585205, rs7903146, and rs11196218 had a weakly
positive but not statistically significant association
with development of diabetic retinopathy.58 A
meta-analysis conducted by Ding et al59 found
that the rs7903146 variant (T allele) of TCF7L2
was significantly associated with increased risk of
development of diabetic retinopathy (OR=1.47; 95%
CI, 1.19-1.81; P≤0.001 for TT vs CC when comparing
genotype polymorphism TT, TC, and CC) especially
within the Caucasian population. Due to rarity of the
T allele within East Asian populations (estimated
at 4.47%60 and 6.997%58 in two different studies),
however, this may be not applicable to this locality.
A meta-analysis by Ma et al61 reported a positive
association between Pro12Ala polymorphism of
the peroxisome proliferator–activated receptor γ2
(PPARγ2) gene. The PPARγ2 gene plays a key role
in multiple pathways including glucose metabolism,
angiogenesis, and inflammation. The authors
found that the Ala allele yielded a protective effect
against diabetic retinopathy in patients with type
2 DM (OR=0.81; 95% CI, 0.68-0.98; P=0.03). This
association was stronger in Caucasian subgroups
compared with Asian subgroups, possibly due to
differences in allele frequencies and study design.61
A recent study by Peng et al62 demonstrated
that the C-reactive protein (CRP) variant rs2808629
is statistically significantly associated with increased
risk of diabetic retinopathy (OR=1.296; 95% CI,
1.076-1.561; P=0.006 for G allele) in Chinese patients
with type 2 DM. Even after adjusting for confounding
factors associated with diabetic retinopathy, this
variant of CRP remained an independent genetic
risk factor for development of diabetic retinopathy.62
Patients with rs2808629 have been demonstrated to
have higher levels of serum CRP.63 Yet, there have
been no conclusive findings of the relationship
between high CRP levels and the development of
diabetic retinopathy as studies have demonstrated
contradicting results.64 65 66 67 68 The exact mechanism of
how rs2808629 causes increased risk of diabetic
retinopathy warrants further research.
Another comprehensive study by Peng et al69
sought to establish the relationship between 40 single
nucleotide polymorphisms and diabetic retinopathy
in Chinese patients with type 2 DM. The authors found
that rs17684886 in ZNRF1 (OR=0.812; P=0.0039)
and rs599019 near COLEC12 (OR=0.835; P=0.0116)
were associated with an increased risk of diabetic
retinopathy. rs6427247 near SCYL1BP1 (OR=1.368;
P=0.0333) and rs899036 near API5 (OR=0.340;
P=0.0005) were associated with increased risk of
severe diabetic retinopathy.69 This is consistent with
other genome-wide association studies in Caucasian
and Mexican-American patients.70 71
As the relationship between genetic
polymorphisms and diabetic retinopathy is still a new
and emerging field, some studies have demonstrated
new associations between single nucleotide
polymorphisms, but these studies have not yet been
replicable. Further studies are thus warranted.
Conclusions
The importance of glycaemic control and duration of
diabetes with diabetic retinopathy have been clearly
established. Additionally, the modality of diabetic
treatment may reflect the severity of diabetes and
risk of developing diabetic retinopathy. Patients with
DM should be encouraged to optimise their control
of the disease in order to prevent the development
and progression of diabetic retinopathy. Of the
systemic risk factors studied, multiple studies
clearly establish a positive association between
hypertension and diabetic retinopathy. Studies have
shown varying results for the association of diabetic
retinopathy with obesity, male sex, hyperlipidaemia,
and smoking. Additionally, declining renal function
and microalbuminuria have been demonstrated to
be associated with increased prevalence of diabetic
retinopathy. In contrast, myopia is protective
against development of diabetic retinopathy.
Despite inconsistent findings for the association of
systemic risk factors with diabetic retinopathy, it is
still important for clinicians to encourage patients
to optimise their body weight, lipid profile, and to
abstain from smoking due to their associations
with risk for cardiovascular disease as well as other
complications of diabetes. New research has also
demonstrated an increasing number of genetic
polymorphisms associated with risk of type 2
DM and the development of diabetic retinopathy.
Investigating the polymorphisms associated with
diabetic retinopathy may help us better understand
the developmental pathway of diabetic retinopathy
and lead to new targeted therapy in treating diabetes
and preventing diabetic retinopathy.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy.
Lancet 2010;376:124-36. Crossref
2. Klein R, Klein BE, Moss SE, Cruickshanks KJ. The
Wisconsin Epidemiologic Study of Diabetic Retinopathy:
XVII. The 14-year incidence and progression of diabetic
retinopathy and associated risk factors in type 1 diabetes.
Ophthalmology 1998;105:1801-15. Crossref
3. Kannel WB, McGee DL. Diabetes and cardiovascular risk
factors: the Framingham study. Circulation 1979;59:8-13. Crossref
4. Cheng Y, Zhang H, Chen R, et al. Cardiometabolic
risk profiles associated with chronic complications in
overweight and obese type 2 diabetes patients in South
China. PLoS One 2014;9:e101289. Crossref
5. Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence
and major risk factors of diabetic retinopathy. Diabetes
Care 2012;35:556-64. Crossref
6. Wong TY, Klein R, Islam FM, et al. Diabetic retinopathy in a
multi-ethnic cohort in the United States. Am J Ophthalmol
2006;141:446-55. Crossref
7. Zhang X, Saaddine JB, Chou CF, et al. Prevalence of
diabetic retinopathy in the United States, 2005-2008.
JAMA 2010;304:649-56. Crossref
8. Raum P, Lamparter J, Ponto KA, et al. Prevalence and
cardiovascular associations of diabetic retinopathy and
maculopathy: results from the Gutenberg Health Study.
PLoS One 2015;10:e0127188. Crossref
9. Bertelsen G, Peto T, Lindekleiv H, et al. Sex differences
in risk factors for retinopathy in non-diabetic men
and women: the Tromsø Eye Study. Acta Ophthalmol
2014;92:316-22. Crossref
10. Varma R, Macias GL, Torres M, et al. Biologic risk factors
associated with diabetic retinopathy: the Los Angeles
Latino Eye Study. Ophthalmology 2007;114:1332-40. Crossref
11. Tam TK, Lau CM, Tsang LC, Ng KK, Ho KS, Lai TC.
Epidemiological study of diabetic retinopathy in a primary
care setting in Hong Kong. Hong Kong Med J 2005;11:438-44.
12. Tam VH, Lam EP, Chu BC, Tse KK, Fung LM. Incidence and
progression of diabetic retinopathy in Hong Kong Chinese
with type 2 diabetes mellitus. J Diabetes Complications
2009;23:185-93. Crossref
13. Wang WQ, Ip TP, Lam KS. Changing prevalence of
retinopathy in newly diagnosed non-insulin dependent
diabetes mellitus patients in Hong Kong. Diabetes Res Clin
Pract 1998;39:185-91. Crossref
14. Lian JX, Gangwani RA, McGhee SM, et al. Systematic
screening for diabetic retinopathy (DR) in Hong Kong:
prevalence of DR and visual impairment among diabetic
population. Br J Ophthalmol 2016;100:151-5. Crossref
15. Chen MS, Kao CS, Chang CJ, et al. Prevalence and
risk factors of diabetic retinopathy among noninsulin-dependent
diabetic subjects. Am J Ophthalmol 1992;114:723-30. Crossref
16. Xie XW, Xu L, Wang YX, Jonas JB. Prevalence and
associated factors of diabetic retinopathy. The Beijing
Eye Study 2006. Graefes Arch Clin Exp Ophthalmol
2008;246:1519-26. Crossref
17. Wang FH, Liang YB, Zhang F, et al. Prevalence of diabetic
retinopathy in rural China: the Handan Eye Study.
Ophthalmology 2009;116:461-7. Crossref
18. Ramachandran A, Snehalatha C, Vijay V, King H. Impact of
poverty on the prevalence of diabetes and its complications
in urban southern India. Diabet Med 2002;19:130-5. Crossref
19. Grading diabetic retinopathy from stereoscopic color
fundus photographs—an extension of the modified Airlie
House classification. ETDRS report number 10. Early
Treatment Diabetic Retinopathy Study Research Group.
Ophthalmology 1991;98(5 Suppl):786S-806S. Crossref
20. Core NDESP team. Diabetic eye screening feature based
grading forms. NHS Diabetic Eye Screening Programme;
2012.
21. Matthews DR, Stratton IM, Aldington SJ, Holman RR,
Kohner EM, UK Prospective Diabetes Study Group. Risks
of progression of retinopathy and vision loss related to
tight blood pressure control in type 2 diabetes mellitus:
UKPDS 69. Arch Ophthalmol 2004;122:1631-40. Crossref
22. van Leiden HA, Dekker JM, Moll AC, et al. Risk factors
for incident retinopathy in a diabetic and nondiabetic
population: the Hoorn study. Arch Ophthalmol
2003;121:245-51. Crossref
23. Intensive blood-glucose control with sulphonylureas or
insulin compared with conventional treatment and risk of
complications in patients with type 2 diabetes (UKPDS 33).
UK Prospective Diabetes Study (UKPDS) Group. Lancet
1998;352:837-53. Crossref
24. Rajalakshmi R, Amutha A, Ranjani H, et al. Prevalence
and risk factors for diabetic retinopathy in Asian Indians
with young onset type 1 and type 2 diabetes. J Diabetes
Complications 2014;28:291-7. Crossref
25. Zhang L, Chen B, Tang L. Metabolic memory: mechanisms
and implications for diabetic retinopathy. Diabetes Res
Clin Pract 2012;96:286-93. Crossref
26. ACCORD Study Group, ACCORD Eye Study Group,
Chew EY, et al. Effects of medical therapies on retinopathy
progression in type 2 diabetes. N Engl J Med 2010;363:233-44. Crossref
27. Action to Control Cardiovascular Risk in Diabetes Study
Group, Gerstein HC, Miller ME, et al. Effects of intensive
glucose lowering in type 2 diabetes. N Engl J Med
2008;358:2545-59. Crossref
28. The effect of intensive treatment of diabetes on the
development and progression of long-term complications
in insulin-dependent diabetes mellitus. The Diabetes
Control and Complications Trial Research Group. N Engl J
Med 1993;329:977-86. Crossref
29. Mohamed Q, Gillies MC, Wong TY. Management
of diabetic retinopathy: a systematic review. JAMA
2007;298:902-16. Crossref
30. De Block CE, De Leeuw IH, Van Gaal LF. Impact of
overweight on chronic microvascular complications in
type 1 diabetic patients. Diabetes Care 2005;28:1649-55. Crossref
31. Wong TY, Cheung N, Tay WT, et al. Prevalence and risk
factors for diabetic retinopathy: the Singapore Malay Eye
Study. Ophthalmology 2008;115:1869-75. Crossref
32. Lim MC, Lee SY, Cheng BC, et al. Diabetic retinopathy in
diabetics referred to a tertiary centre from a nationwide
screening programme. Ann Acad Med Singapore
2008;37:753-9.
33. Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The
Wisconsin epidemiologic study of diabetic retinopathy.
III. Prevalence and risk of diabetic retinopathy when
age at diagnosis is 30 or more years. Arch Ophthalmol
1984;102:527-32. Crossref
34. Tomić M, Ljubić S, Kaštelan S, Gverović Antunica A,
Jazbec A, Poljičanin T. Inflammation, haemostatic
disturbance, and obesity: possible link to pathogenesis of
diabetic retinopathy in type 2 diabetes. Mediators Inflamm
2013;2013:818671. Crossref
35. Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50:
risk factors for incidence and progression of retinopathy in
type II diabetes over 6 years from diagnosis. Diabetologia
2001;44:156-63. Crossref
36. Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect
of blood pressure control on diabetic microvascular
complications in patients with hypertension and type 2
diabetes. Diabetes Care 2000;23 Suppl 2:B54-64.
37. Price SA, Gorelik A, Fourlanos S, Colman PG, Wentworth
JM. Obesity is associated with retinopathy and
macrovascular disease in type 1 diabetes. Obes Res Clin
Pract 2014;8:e178-82. Crossref
38. Nelson RG, Wolfe JA, Horton MB, Pettitt DJ, Bennett
PH, Knowler WC. Proliferative retinopathy in NIDDM.
Incidence and risk factors in Pima Indians. Diabetes
1989;38:435-40. Crossref
39. Lee ET, Lee VS, Lu M, Russell D. Development of
proliferative retinopathy in NIDDM. A follow-up study of
American Indians in Oklahoma. Diabetes 1992;41:359-67. Crossref
40. Klein R, Klein BE, Moss SE. Is obesity related to
microvascular and macrovascular complications in
diabetes? The Wisconsin Epidemiologic Study of Diabetic
Retinopathy. Arch Intern Med 1997;157:650-6. Crossref
41. van Leiden HA, Dekker JM, Moll AC, et al. Blood pressure,
lipids, and obesity are associated with retinopathy: the
Hoorn study. Diabetes Care 2002;25:1320-5. Crossref
42. Klein BE, Moss SE, Klein R, Surawicz TS. The Wisconsin
Epidemiologic Study of Diabetic Retinopathy. XIII.
Relationship of serum cholesterol to retinopathy and hard
exudate. Ophthalmology 1991;98:1261-5. Crossref
43. Chew EY, Klein ML, Ferris FL 3rd, et al. Association
of elevated serum lipid levels with retinal hard exudate
in diabetic retinopathy. Early Treatment Diabetic
Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol
1996;114:1079-84. Crossref
44. Chaturvedi N, Sjoelie AK, Porta M, et al. Markers of insulin
resistance are strong risk factors for retinopathy incidence
in type 1 diabetes. Diabetes Care 2001;24:284-9. Crossref
45. Sacks FM, Hermans MP, Fioretto P, et al. Association
between plasma triglycerides and high-density lipoprotein
cholesterol and microvascular kidney disease and
retinopathy in type 2 diabetes mellitus: a global case-control
study in 13 countries. Circulation 2014;129:999-1008. Crossref
46. Das R, Kerr R, Chakravarthy U, Hogg RE. Dyslipidemia
and diabetic macular edema: a systematic review and
meta-analysis. Ophthalmology 2015;122:1820-7. Crossref
47. Park YH, Shin JA, Han JH, Park YM, Yim HW. The
association between chronic kidney disease and
diabetic retinopathy: the Korea national health and
nutrition examination survey 2008-2010. PLoS One
2015;10:e0125338. Crossref
48. Zhang H, Wang J, Ying GS, Shen L, Zhang Z. Diabetic
retinopathy and renal function in Chinese type 2 diabetic
patients. Int Urol Nephrol 2014;46:1375-81. Crossref
49. Penno G, Solini A, Zoppini G, et al. Rate and determinants
of association between advanced retinopathy and chronic
kidney disease in patients with type 2 diabetes: the Renal
Insufficiency And Cardiovascular Events (RIACE) Italian
multicenter study. Diabetes Care 2012;35:2317-23. Crossref
50. Rodríguez-Poncelas A, Mundet-Tudurí X, Miravet-Jiménez
S, et al. Chronic kidney disease and diabetic retinopathy in
patients with type 2 diabetes. PLoS One 2016;11:e0149448. Crossref
51. Gaedt Thorlund M, Borg Madsen M, Green A, Sjølie
AK, Grauslund J. Is smoking a risk factor for proliferative
diabetic retinopathy in type 1 diabetes? Ophthalmologica
2013;230:50-4. Crossref
52. Fu Y, Geng D, Liu H, Che H. Myopia and/or longer axial
length are protective against diabetic retinopathy: a meta-analysis.
Acta Ophthalmol 2016;94:346-52. Crossref
53. Wang X, Tang L, Gao L, Yang Y, Cao D, Li Y. Myopia
and diabetic retinopathy: a systematic review and meta-analysis.
Diabetes Res Clin Pract 2016;111:1-9. Crossref
54. Grant RW, Moore AF, Florez JC. Genetic architecture of
type 2 diabetes: recent progress and clinical implications.
Diabetes Care 2009;32:1107-14. Crossref
55. Grant SF, Thorleifsson G, Reynisdottir I, et al. Variant of
transcription factor 7-like 2 (TCF7L2) gene confers risk of
type 2 diabetes. Nat Genet 2006;38:320-3. Crossref
56. Cauchi S, Froguel P. TCF7L2 genetic defect and type 2
diabetes. Curr Diab Rep 2008;8:149-55. Crossref
57. Ciccacci C, Di Fusco D, Cacciotti L, et al. TCF7L2 gene
polymorphisms and type 2 diabetes: association with
diabetic retinopathy and cardiovascular autonomic
neuropathy. Acta Diabetol 2013;50:789-99. Crossref
58. Fu LL, Lin Y, Yang ZL, Yin YB. Association analysis of
genetic polymorphisms of TCF7L2, CDKAL1, SLC30A8,
HHEX genes and microvascular complications of type 2
diabetes mellitus [in Chinese]. Zhonghua Yi Xue Yi Chuan
Xue Za Zhi 2012;29:194-9.
59. Ding Y, Hu Z, Yuan S, Xie P, Liu Q. Association between
transcription factor 7-like 2 rs7903146 polymorphism and
diabetic retinopathy in type 2 diabetes mellitus: A meta-analysis.
Diab Vasc Dis Res 2015;12:436-44. Crossref
60. Choi HJ, Lee DH, Jeon HJ, Kim DS, Lee YH, Oh T.
Transcription factor 7-like 2 (TCF7L2) gene polymorphism
rs7903146 is associated with stroke in type 2 diabetes
patients with long disease duration. Diabetes Res Clin
Pract 2014;103:e3-6. Crossref
61. Ma J, Li Y, Zhou F, Xu X, Guo G, Qu Y. Meta-analysis of
association between the Pro12Ala polymorphism of the
peroxisome proliferator–activated receptor-gamma2 gene
and diabetic retinopathy in Caucasians and Asians. Mol
Vis 2012;18:2352-60.
62. Peng D, Wang J, Zhang R, et al. C-reactive protein genetic
variant is associated with diabetic retinopathy in Chinese
patients with type 2 diabetes. BMC Endocr Disord
2015;15:8. Crossref
63. Benjamin EJ, Dupuis J, Larson MG, et al. Genome-wide
association with select biomarker traits in the Framingham
Heart Study. BMC Med Genet 2007;8 Suppl 1:11S. Crossref
64. Klein BE, Knudtson MD, Tsai MY, Klein R. The relation
of markers of inflammation and endothelial dysfunction
to the prevalence and progression of diabetic retinopathy:
Wisconsin epidemiologic study of diabetic retinopathy.
Arch Ophthalmol 2009;127:1175-82. Crossref
65. Lim LS, Tai ES, Mitchell P, et al. C-reactive protein, body
mass index, and diabetic retinopathy. Invest Ophthalmol
Vis Sci 2010;51:4458-63. Crossref
66. Muni RH, Kohly RP, Lee EQ, Manson JE, Semba RD,
Schaumberg DA. Prospective study of inflammatory
biomarkers and risk of diabetic retinopathy in the diabetes
control and complications trial. JAMA Ophthalmol
2013;131:514-21. Crossref
67. Nguyen TT, Alibrahim E, Islam FM, et al. Inflammatory,
hemostatic, and other novel biomarkers for diabetic
retinopathy: the multi-ethnic study of atherosclerosis.
Diabetes Care 2009;32:1704-9. Crossref
68. van Hecke MV, Dekker JM, Nijpels G, et al. Inflammation
and endothelial dysfunction are associated with
retinopathy: the Hoorn Study. Diabetologia 2005;48:1300-6. Crossref
69. Peng D, Wang J, Zhang R, et al. Common variants in or near
ZNRF1, COLEC12, SCYL1BP1 and API5 are associated
with diabetic retinopathy in Chinese patients with type 2
diabetes. Diabetologia 2015;58:1231-8. Crossref
70. Fu YP, Hallman DM, Gonzalez VH, et al. Identification
of diabetic retinopathy genes through a genome-wide
association study among Mexican-Americans from Starr
County, Texas. J Ophthalmol 2010;2010.pii: 861291.
71. Grassi MA, Tikhomirov A, Ramalingam S, Below JE, Cox
NJ, Nicolae DL. Genome-wide meta-analysis for severe
diabetic retinopathy. Hum Mol Genet 2011;20:2472-81. Crossref