Hong Kong Med J 2016 Oct;22(5):496–505
DOI: 10.12809/hkmj164920
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
Opioid therapy for chronic non-cancer pain: guidelines for Hong Kong
CW Cheung, MD, FHKAM (Anaesthesiology)1;
Timmy CW Chan, FFPM ANZCA, FHKAM (Anaesthesiology)2;
PP Chen, FFPM ANZCA, FHKAM (Anaesthesiology)3;
MC Chu, FFPM ANZCA, FHKAM (Anaesthesiology)4;
William CM Chui, MSc, BPharm (Hon)5;
PT Ho, FRCPsych, FHKAM (Psychiatry)6;
Flori Lam, BHSc(RN)7;
SW Law, FRCSEd(Orth), FHKCOS8;
Josephine LY Lee, MSc9;
Steven HS Wong, MB, BS, FHKAM (Anaesthesiology)7;
Vincent KC Wong, BCPS, MPharm5
1 Laboratory and Clinical Research Institute for Pain, Department of Anaesthesiology, The University of Hong Kong, Pokfulam, Hong Kong
2 Department of Anaesthesiology, Queen Mary Hospital, Pokfulam, Hong Kong
3 Department of Anaesthesiology and Operating Services, Alice Ho Miu Ling Nethersole Hospital, Tai Po, Hong Kong
4 Department of Anaesthesia, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
5 Department of Pharmacy, Queen Mary Hospital, Pokfulam, Hong Kong
6 Consultation and Liaison Psychiatry Team, Kwai Chung Hospital, Kwai Chung, Hong Kong
7 Department of Anaesthesiology and Operating Theatre Services, Queen Elizabeth Hospital, Jordan, Hong Kong
8 Department of Orthopaedics and Traumatology, Prince of Wales Hospital, Shatin, Hong Kong
9 Occupational Therapy Department, Prince of Wales Hospital, Shatin, Hong Kong
Corresponding author: Dr CW Cheung (cheucw@hku.hk)
Abstract
Opioids are increasingly used to control chronic
non-cancer pain globally. International opioid
guidelines have been issued in many different
countries but a similar document is not generally
available in Hong Kong. Chronic opioid therapy has
a role in multidisciplinary management of chronic
non-cancer pain despite insufficient evidence for
its effectiveness and safety for long-term use. This
document reviews the current literature to inform
Hong Kong practitioners about the rational use of
chronic opioid therapy in chronic non-cancer pain. It
also aims to provide useful recommendations for the
appropriate, effective, and safe use of such therapy
in the management of chronic non-cancer pain in
adults. Physicians should conduct a comprehensive
biopsychosocial evaluation of patients prior to the
commencement of opioid therapy. When opioid use
is deemed appropriate, the patient should provide
informed consent within an agreement that specifies
treatment goals and expectations. A trial of opioid
can be commenced and, provided there is progress
towards treatment goals, then chronic therapy
can be considered at a dose that minimises harm.
Monitoring of effectiveness, safety, and drug misuse
should be continued. Treatment should be stopped
when opioids become ineffective, intolerable,
or misused. The driving principles for opioid
prescription in chronic pain management should
be: start with a low dose, titrate slowly, and maintain
within the shortest possible time.
Introduction
Chronic pain is pain that persists beyond the usual
time of healing, usually marked as 6 months or even
3 months by the International Association for the
Study of Pain (IASP).1 Chronic pain arises from
complex changes to central or peripheral nervous
system signalling, or both. The perception of pain
is modulated by an individual cognitive factors and
the environment,2 and can significantly compromise
daily function, resulting in an important health issue.
Hong Kong survey data estimated the prevalence of
chronic pain to affect 10.8% of the population in 2000
and 35% in 2007.3 4 Survey participants with chronic pain from an earlier study reported a significant
impact on their daily lives.3 Moreover, chronic pain
placed a substantial load on productivity, with an
estimated loss of approximately 0.2 working days per
person in the working population per year, and on
health care resources, with almost three quarters of
respondents consulting a health care practitioner.
The latter survey found that reports of chronic pain
were strongly associated with co-morbid mental
health problems and anxiety.4
Opioid therapy is accepted for acute pain
and cancer pain,5 6 but its effectiveness and safety for chronic non-cancer pain (CNCP) remains
contentious. By definition, CNCP refers to non-malignant
pain that lasts beyond the time of tissue
healing, or longer than 3 months.1 Authors cite weak
evidence for opioid use for CNCP due to the lack of
randomised controlled trials with long follow-up.7 8 Based on systematic reviews, opioids for CNCP—including neuropathic pain, nociceptive pain, and arthritic pain—confer some benefit by reducing
pain intensity and improving functional outcome
compared with placebo and other non-steroidal
anti-inflammatory drugs,9 10 11 12 13 but high-quality studies
are rare, and treatment duration is short, ranging
from 2 weeks to 6 months. A proportion of patients in
the studies reviewed did not progress to long-term
therapy due to adverse effects.12 Discontinuation
rates from adverse effects were almost 30%, with the
most frequently reported events being constipation,
nausea, dizziness, drowsiness, and headache.12
The potential to develop opioid abuse or
addiction with long-term therapy is also a concern.
In studies reviewed, addiction or abuse rates were
reported to range from 0.27% to 0.43%.12 14 15 Deaths
related to opioid analgesic overdose have been
increasing, and is being linked to an increase in opioid
prescriptions for pain.16 17 Indeed, chronic opioid exposure from prescription appears to be a strong
risk factor for an opioid misuse event in patients just
diagnosed with CNCP.18 Other potential harm from
chronic opioid use includes increased fracture risk,19
androgen deficiency,20 respiratory depression,21 cognitive impairment,22 impaired immunity,23 and opioid-induced hyperalgesia.24 25
Global consumption of opioids for moderate-to-severe pain increased approximately 15-fold from 1980 to 2012.26 Generally, opioid consumption
of countries in Asia, including Hong Kong, is low
relative to the global picture (Fig27), but showing an increasing trend.27 28 Physicians in Hong Kong may be reluctant to prescribe opioids for long-term
therapy due to fear of patient addiction. There
may also be a cultural prejudice against opioid use
stemming from a history of China’s involvement in the
global opium trade in the early part of the century.29
Other possible barriers to prescription and patient
care may include inadequate physician education
about pain management, and lack of physician-patient
communication about the seriousness of
the pain problem. The absence of a central registry
for opioid prescriptions, and the limited number
of addiction specialists are potential local logistical
barriers. With the introduction of opioid choices
in Hong Kong, however, the prescription of strong
opioids for long-term treatment may become more
common. Examples of strong opioids for CNCP
used in Hong Kong are listed in Box 1.30
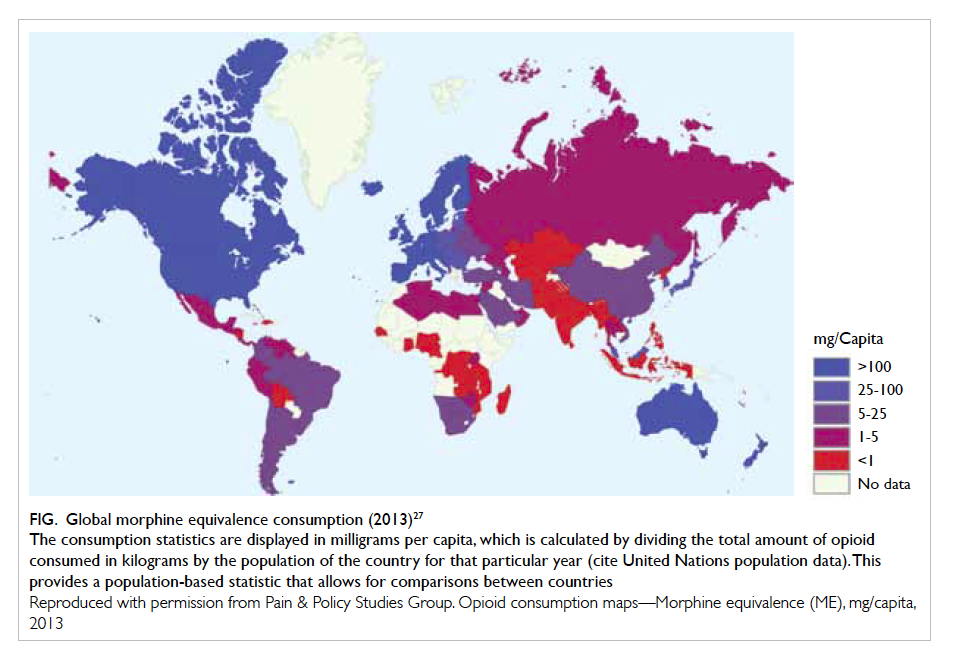
Figure. Global morphine equivalence consumption (2013)27
The consumption statistics are displayed in milligrams per capita, which is calculated by dividing the total amount of opioid consumed in kilograms by the population of the country for that particular year (cite United Nations population data). This provides a population-based statistic that allows for comparisons between countries
Reproduced with permission from Pain & Policy Studies Group. Opioid consumption maps—Morphine equivalence (ME), mg/capita, 2013
Guidelines governing the use of opioids for
patients with CNCP have been issued in different
countries according to their needs.31 32 33 34 35 36 37 38 The Hospital
Authority has recently introduced an opioid
guideline for CNCP for public hospital use. A similar
guide is not readily available for physicians who are
involved in CNCP management but practise outside
Hospital Authority in Hong Kong. This document
aims to serve as a resource and uniform guide for
the appropriate, effective, and safe use of chronic
opioid therapy (COT) in the management of CNCP
in adults based on local considerations; COT is
regarded as the use of strong opioids for more than 3
months.39 This guideline provides recommendations
about patient selection, risk screening, initiation
of COT, and monitoring during COT, based on a
review of the current literature. The target audience
is all physicians, especially non-pain specialists,
and other health care professionals involved in the
multidisciplinary management of the patient with
CNCP, who are considering prescribing COT.
General considerations for using opioid in patients with chronic non-cancer pain
The biopsychosocial model describes pain as bodily
disruption shaped by an individual’s subjective
perception. Biological processes, emotions, and
social factors all influence the pain experience.2
Consequently, chronic pain is a complex condition
that requires a multidisciplinary approach to
both evaluation and management, preferably in a
coordinated treatment programme.40 41 The IASP recognises the effectiveness of multidisciplinary pain
programmes for chronic pain, and recommended the
establishment of multidisciplinary pain clinics or pain
centres; the latter of which should be associated with
a research or academic programme.40 Staff at both
pain centres and clinics should include practitioners
from various disciplines deemed expert in pain
management. Physicians, nurses, mental health
professionals, and physical therapists are among
those who comprise a team that coordinates diagnosis
and management, with constant communication,
preferably in one setting.40 41 Essential elements of multidisciplinary pain programmes address pain
management, psychosocial recovery, and physical
rehabilitation, and include medication, physical
therapy, and cognitive and behavioural strategies.42
Such programmes have been proven clinically effective and
cost-effective.43 44 45
In fact, COT is just part of the multimodal strategy to manage CNCP. Experts do not
recommend opioids for first-line treatment of
CNCP.34 36 38 Non-opioid treatment options, both
non-pharmacological and pharmacological (eg typical analgesics), should be tried first. Despite
limited evidence to support the safe and effective
use of opioids in CNCP, they may be considered
for selected patients who have moderate-to-severe
pain and who have not responded adequately
to non-opioid therapy.38 In accordance with
the biopsychosocial model, treatment of CNCP
should address the physical, psychological, and
social aspects of the pain problem.41 Treatment
of CNCP should thus aim to reduce pain and
support patients’ physical, psychological, social,
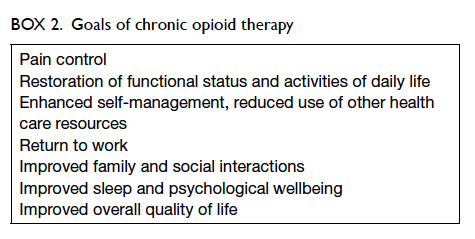
and work functioning.40 The goals of COT include
pain reduction, reduction of pain-associated
symptoms such as anxiety and sleep problems,
and improvement of daily function.46 47 These goals should be individualised and utilise achievable
milestones without excessive use of opioids.46 Indeed,
daily function outcomes were deemed important by
survey participants with chronic pain,48 and should
be targets for improvement along with pain control
(Box 2).
Recommendations
- Chronic opioid therapy should be considered only as part of a multidisciplinary pain management strategy.
- Opioids should be prescribed only after exhausting other pharmacological and non-pharmacological treatment options.
- Treatment goals acceptable to both patients and their physicians should be set when COT is considered.
- When prescribed, COT should aim to reasonably reduce pain and its associated symptoms, as well as improve functional outcomes.
Patient evaluation and selection
Prior to starting COT, a comprehensive patient
evaluation should be carried out to form a diagnosis,
establish the cause of pain, describe pain intensity,
and determine a patient’s risk profile with the
use of opioids.38 46 The evaluation informs on the suitability of a patient for COT, and, if the decision
to pursue COT is made, provides the foundation
for individualising a treatment plan. A thorough
history and physical examination with appropriate
investigations are essential. The pain complaint
should be thoroughly investigated with regard to
characteristics, underlying factors, and effects on the
patient’s functional status.38 46 Assessment should include a medical and psychosocial history, including
previous medication, general health, family support,
and work status. For example, increased use of
opioids for pain control prior to spine surgery was
found to be associated with worse postoperative
outcomes, such as increased demand for opioids
after surgery, and decreased incidence of opioid
independence 1 year after surgery.49 50 This highlights the need for a complete evaluation and screening of
each patient prior to opioid use. Evaluation findings
may impact subsequent patient counselling and
treatment planning.
The rate of problematic opioid use or aberrant
drug–related behaviour in patients with CNCP on
COT is 11.5%.51 Prevalence of substance use disorder
among patients with CNCP, with or without opioid
use, ranges from 3% to 48%.52 Because problematic
opioid use complicates pain treatment and may
cause significant harm, a patient’s risk for opioid
abuse, misuse, or addiction must be assessed prior
to COT.38 46 53 54 Screening helps the physician to
anticipate the patient’s risk of developing aberrant
behaviour while on COT.53 54 Family and personal history of substance abuse, a history of psychiatric
or mood disorders, and younger age have not been
validated as predictive of opioid misuse, but have
been shown to potentially increase risk.52 55 56
The physician can utilise structured clinical
interviews and self-reports to elicit these factors.52 56
Questionnaires such as the Opioid Risk Tool, which
assigns weights to predictive factors of opioid
misuse, may be useful.57 The presence of apparent
predictors of misuse such as a personal or family
history of substance abuse or addiction does not
necessarily preclude the use of opioids, but may
affect structure of therapy (eg closer monitoring,
stricter prescription practice) and require
additional consultation from specialists, including
psychiatrists.54 Urine drug screening (UDS) may be
a useful aid in detection of latent drug abuse. Every
assessment must be thoroughly documented.
According to addiction specialists from the
Hong Kong College of Psychiatrists, screening by
psychiatrists for suitability of high-risk patients
for COT may not be useful, as evidence to support
reduced substance abuse risk through conjoint
selection by pain specialists and psychiatrists is
lacking. There is also inadequate evidence that the
future risk of opioid abuse is reduced by treating
substance abuse prior to COT; thus, the decision to
make this treatment a prerequisite for COT rests on
the pain management team (written communication,
Clinical Division of Substance Abuse & Addiction
Psychiatry, Hong Kong College of Psychiatrists,
2016). Patients with active substance abuse (opioid,
non-opioid, or both) and psychological disturbances
may be referred to substance abuse clinics (SACs)
in Hong Kong for pre-COT psychiatric treatment
(Appendix 1).
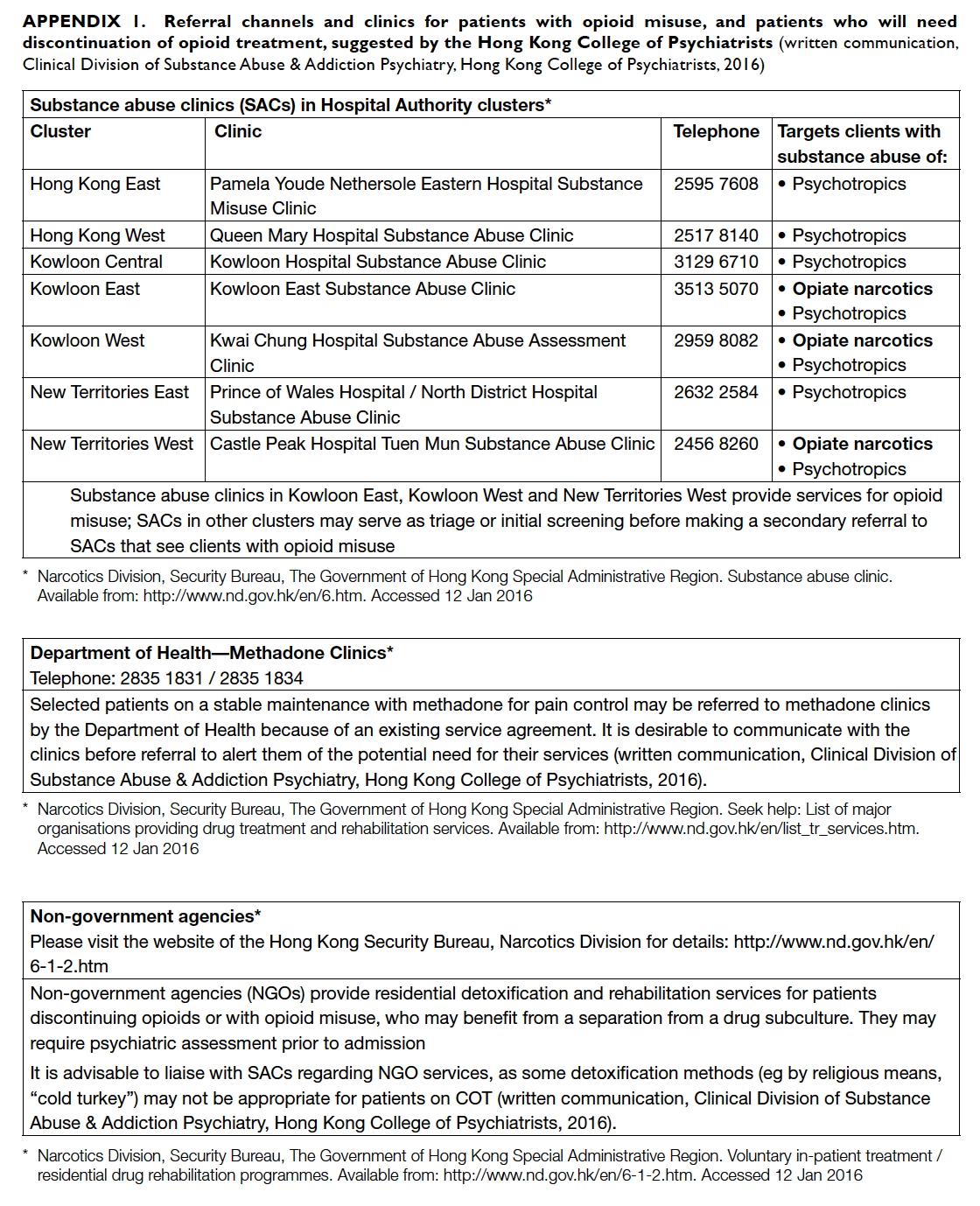
Appendix 1. Referral channels and clinics for patients with opioid misuse, and patients who will need discontinuation of opioid treatment, suggested by the Hong Kong College of Psychiatrists
(written communication, Clinical Division of Substance Abuse & Addiction Psychiatry, Hong Kong College of Psychiatrists, 2016)
Recommendations
- Patients considered for COT should have a thorough physical, psychological, and social assessment.
- Risk of substance misuse or addiction should also be assessed for appropriate treatment planning.
Prescription of chronic opioids
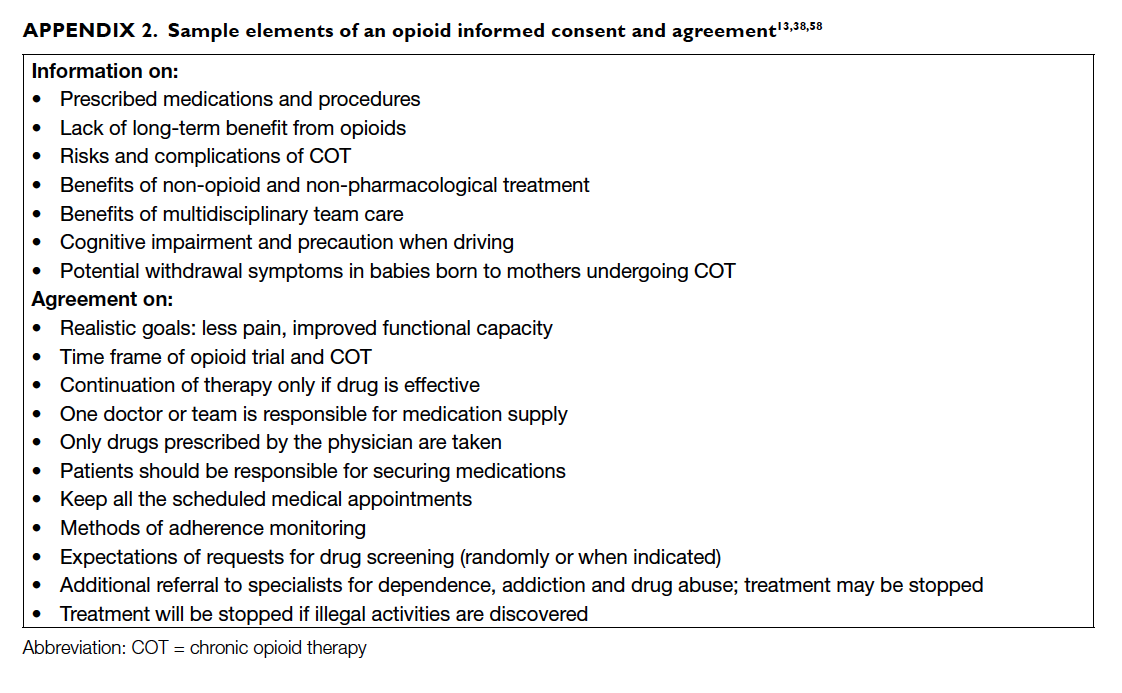
Informed consent and documentation
Guides on COT for CNCP recognise the need for
informed consent to explain the benefits, risks,
and complications of COT before a trial of opioid
therapy, and when the decision to use COT is
reached.38 46 Consent in the form of a written plan or treatment agreement, or opioid contract, can
set expectations between the physician and patient
regarding their actions and treatment targets.13 38 46
The usefulness of a contract in promoting adherence
to a COT regimen lacks evidence, however. Also,
use of a contract has underlying ethical issues
relating to patient autonomy and the assumption of
potential opioid abuse.13 58 Nevertheless, if utilised, an agreement should outline information on COT
and alternative treatment options, benefits, risks
(which include adverse effects, behavioural risks,
and medical complications), steps to reach treatment
goals, means of monitoring improvements, and
conditions for continuing or discontinuing therapy
(Appendix 2).13 38 58 Specifically, it may include from whom and where the patients should obtain
their prescription, the number and time of office
follow-ups, and expectations on use of UDS.46 58 The agreement should be reviewed continually during
treatment.46
Trial of opioid therapy
An opioid trial assesses patients’ responsiveness
to opioids. Dose titration during an opioid trial
determines the lowest effective dose with the least
or minimal side-effects for an individually tailored
programme.59 The choice of opioid should be based
on factors related to the individual patient, such as
previous response to opioids, health status, treatment
goals, predicted risks; on medication-related factors,
such as availability and pharmacological and adverse
effect profiles; and on physician experience and
expertise.33 36 59 Parenteral forms of opioids are not
recommended for an opioid trial due to risk of
abuse.33 36
Opioids should be titrated slowly, for example,
at increments of 10 to 50 mg equivalent per day
of a long-acting opioid, until the optimal dose
that provides benefit with the least side-effects is
attained.33 47 60 The optimal dose is suggested to lead
to a 30% pain reduction (based on an 11-point scale)
that does not cause significant adverse effects.33 61 A trial of therapy may last weeks or months. Duration
can be set from 4 to 6 weeks, or up to 90 days in some
pain centres.46 47 60 The target results and duration of
the trial period should be understood and agreed by
the patient.46 60
Prescription for special concerns
Driving and work safety are potential concerns when
COT is used. Studies have not shown impairment
of driving-related activities with opioids,13 62 63 but
patients should be informed of factors related to
opioid use that may cause impairment, including
initiating opioids or changing opioid dose, poor
sleep, severe pain, and concomitant intake of
alcohol or sedating medications.13 33 38 Patients
should be advised to avoid driving or participating
in potentially dangerous activities if they show signs
of impaired cognition or psychomotor ability, such
as somnolence, poor coordination, or decreased
concentration.33 38
Pregnant patients or patients planning to get
pregnant should be counselled on the risks of COT
during pregnancy. Such therapy during pregnancy
has been found to be associated with neonatal
withdrawal syndrome, poor birth outcome, and
certain birth defects.64 65 66 Because of this, COT during
pregnancy is not encouraged unless the potential
benefits outweigh the risks.38 47
Recommendations
- Informed consent should be obtained before an opioid trial as well as before the start of COT.
- Prior to COT, an opioid trial should be conducted to assess patient response and to determine an optimal opioid dose.
- The choice of drugs and initial dosing should be individualised according to the drug profile, patient characteristics and goals, and physician experience.
- Patients on opioids who show signs of impaired cognition or psychomotor activity should be cautioned against driving and other potentially dangerous activities.
- Use of COT during pregnancy is discouraged because opioids may lead to poor birth outcomes.
Monitoring during chronic opioid therapy
Ongoing assessment and regular monitoring during
COT allows physicians to characterise patients who
continue to benefit from opioids or who may need
changes in their treatment programme. During
an opioid trial, the patient is regularly monitored,
usually at weekly intervals, to assess the four A’s:
analgesia, activity, adverse effects, and aberrant
behaviour.33 60 67 The dose is adjusted based on
changes in pain intensity, improvements in daily
function, and development of side-effects and of
aberrant behaviour.33 35 46 Validated pain assessment
tools such as the Brief Pain Inventory or the Pain,
Enjoyment, and General Activity scale may be used
at baseline and at regular intervals thereafter to
describe changes in pain relief and function.33 46 47
Drug-related behaviour can be monitored through
patient interviews, observation, and UDS.
Evidence is lacking on the reliability of UDS in
predicting aberrant behaviour, and UDS still needs
to be evaluated if it improves clinical outcomes.37
Nonetheless, UDS provides important information
on adherence to the treatment plan and existence
of possible drug misuse when appropriately used
with other monitoring tools. Although other
biological specimens may be tested, obtaining
urine is considered practical and convenient, and
results can be obtained within a few days to allow
modification of patient care.68 Physicians should
be aware of limitations in interpreting results
and should maintain communication with testing
laboratories to resolve any doubts.68 In addition,
differential diagnoses for each result should be
considered, for example, absence of prescribed drug
in the urine could indicate non-access to required
prescription, or diversion of the prescription.37 69 These considerations should facilitate a discussion
with the patient in order to improve care. Prior to
the onset of opioid therapy, it is important to educate
the patient and specify the objectives of UDS in the
treatment agreement to avoid confrontation and
uphold a strong physician-patient relationship.69
There is no agreement on the frequency of UDS, but it should be performed as frequently
as necessary according to the patient’s risk for
misuse, occurrence of aberrant behaviour, and on
availability of the test.37 47 If aberrant drug behaviour is noticed, the patient may need more intensive
monitoring and referral to a pain or substance abuse
specialist.47 60 Continuation of opioid therapy may be agreed upon by physician and patient if there is note
of progress towards the patient’s goals as determined
from regular monitoring.60 If the patient is deemed
suitable to continue opioids in the long term after an
opioid trial has established a stable dose, monitoring
of the four A’s is undertaken at regular intervals.60
Intervals can be as far apart as 6 months for patients
with no risk issues, or as frequent as monthly for
those at risk of opioid misuse, or those taking doses
near the threshold level.47
Recommendations
- Monitoring during COT should include documentation of pain intensity, level of functioning, presence of adverse events, and adherence to prescribed therapies.
- Validated pain assessment tools may be used at baseline and regularly thereafter to describe changes in pain and function.
Opioid rotation
Patients who initially responded but have become
tolerant despite escalating doses, and those in whom
side-effects limit dose increases, may consider
an opioid switch or rotation.70 Despite the lack of
evidence from controlled studies for the clinical
effectiveness of opioid rotation,71 72 the strategy may be useful based on observed differences in individual
responses to various opioids.73 This occurrence
may be explained by genetic variations in receptor
subtypes that modulate drug effects, and that also
potentially promote incomplete cross-tolerance
among opioids.74
Opioid rotation involves selection of a new
opioid, determination of its appropriate initial dose,
and subsequent titration for a satisfactory balance of
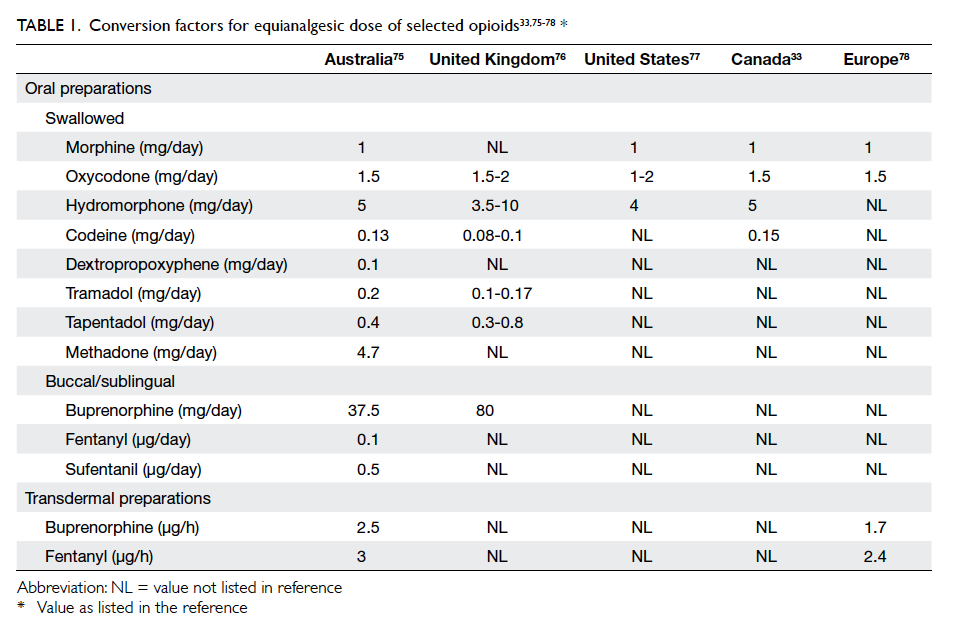
efficacy and side-effects.70 Use of an equianalgesic
table can facilitate estimation of the new drug’s
initial dose.33 75 76 77 78 Its dose should approximate the dose of the previous drug (Table 133 75 76 77 78). Drug
potencies reflected by the reference table may be
underestimated74; therefore, experts support an initial
automatic reduction in the estimated equianalgesic
dose of the new drug by 25% to 50% for safety.70 If the
previous dose was high, a reduction by at least 50% is
recommended by some authors.33 Exceptions to this
include a switch to methadone, which should use a
reduction of 75% to 90%, and switch to transdermal
fentanyl, which does not require a reduction.70 This
initial computation should be adjusted or retained
based on the patient’s clinical situation. Subsequent
drug titration should be based on this initial dose.70
Recommendations
- When switching to a new opioid, calculate the equianalgesic dose using a reference equianalgesic table.
- Reduction of the calculated dose is recommended for safety.
- Prior to a new opioid trial, adjust the dose further after reassessment of the patient’s clinical situation.
Management of side-effects and problematic opioid use
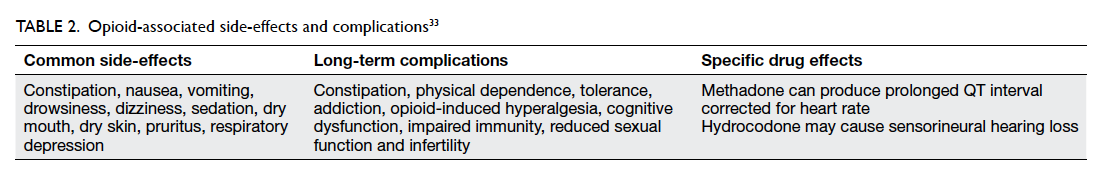
Constipation, nausea, headache, and sedation are
the most frequently reported opioid side-effects
in clinical trials.12 Many side-effects reportedly
diminish over time,12 but some side-effects such as
constipation and vomiting may be severe enough to
prompt discontinuation from trials. Constipation, in
particular, may not diminish and cause significant
severe discomfort.79 Opioid dose adjustment, opioid
rotation, and proactive therapy (eg stool softeners
for constipation) are strategies that can minimise
severity of adverse effects.21 79 Common opioid-associated side-effects should be anticipated and
managed appropriately when identified to maintain
compliance (Table 2).33
Problematic drug use or aberrant drug
behaviour may arise from opioid use. As described
previously, patients on COT who are at high
risk of opioid misuse or addiction may need
additional consultation with addiction experts,
and a restructuring of their programme to include
frequent, close monitoring.33 46 47 54 In addition
to patient interviews, proper drug use may be
monitored through methods such as regular visits,
pill counts, and UDS.80 Physicians should attempt
to identify the cause of behaviour that suggests
the possibility of opioid misuse. It is important to
be aware of pseudo-addiction, which apparently
exhibits the same compulsive behaviours for opioids
as in addiction, but is due to inadequate pain relief
from undermedication.81
When drug misuse is identified, the patient
should undergo a complete re-evaluation for
treatment modification.33 Repeated, serious
aberrant behaviour requires discontinuation of
COT and referral to substance abuse specialists
for detoxification.33 47 Referral channels and treatment through SACs for patients with significant
psychological disturbance or addiction features (eg
aberrant drug behaviour, other substance abuse such
as benzodiazepines) are available in Hong Kong
(Appendix 1).
Recommendations
- Anticipate common side-effects and manage appropriately.
- If problematic drug use is recognised, re-evaluate the patient and modify treatment.
- Discontinue COT if aberrant behaviour is present. Referral to substance abuse specialists is warranted.
Upper dose limit and exit strategy
The goals of COT should be revisited at regular
intervals to see if patients can meet their targets
within a defined dose range, determined from the
initial daily dose titrated to the lowest effective dose.
It is recommended that the upper dose titration
limit should not exceed 120 mg of oral morphine or
its equivalent, or 200 mg in some centres.33 38 47 60 If
the daily dose exceeds this limit, a reassessment of
the pain condition, potential for misuse, and need
for more frequent monitoring is warranted.33 38 Indications for discontinuation include no change or
improvement in therapeutic goals despite escalating
doses, intolerance to side-effects, and persistence of
aberrant behaviour. If the decision to discontinue
opioids is reached, the opioid should be tapered to
avoid withdrawal problems.33 46 47 60
The tapering plan is variable, but generally, a
reduction of 10% per week from the original dose
is well tolerated.33 47 60 A faster or slower rate may
be suitable depending on the patient’s situation.
Physicians should monitor patients for changes
in pain and for the appearance of side-effects,
withdrawal symptoms, and behavioural issues. These
concerns should be properly managed. In some
cases, a referral to the appropriate specialists may
be warranted. Likewise, patients who fail to benefit
from COT and who need discontinuation may be
referred to SACs in Hong Kong for detoxification
and rehabilitation services (written communication,
Clinical Division of Substance Abuse & Addiction
Psychiatry, Hong Kong College of Psychiatrists,
2016).
Recommendations
- Chronic opioid therapy should be stopped if patients experience no progress towards treatment goals, experience intolerable side-effects, or are engaged in repeated aberrant drug-related behaviours.
- Opioids should be tapered to avoid withdrawal.
Conclusion
Strong opioids play a role in the multimodal
management of CNCP. They may be appropriate
for selected patients despite insufficient evidence
of effectiveness. Opioid therapy is potentially
associated with common side-effects and significant
harm; thus, careful patient selection by a thorough
patient evaluation prior to treatment, and careful
dose titration and monitoring during initiation and
long-term therapy, are all recommended steps for
rational opioid use in CNCP. Whenever opioid is
prescribed for chronic pain management, it should
be started with a low dose and titrated slowly, as well
as maintained for the shortest possible time.
Acknowledgements
We would like to thank the Clinical Division of
Substance Abuse & Addiction Psychiatry, Hong
Kong College of Psychiatrists for their advice and
opinions.
Declaration
All authors are members of the working group
for Guideline for Chronic Opioid Therapy in
Chronic Noncancer Pain, Hospital Authority
Multidisciplinary Committee on Pain Medicine,
Hospital Authority, Hong Kong. All authors have
disclosed no conflicts of interest.
References
1. Merskey H, Bogduk N, editors. International Association
for the Study of Pain. Task Force on Taxonomy.
Classification of chronic pain: descriptions of chronic pain
syndromes and definitions of pain terms. 2nd ed. Seattle:
IASP Press; 2002.
2. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC.
The biopsychosocial approach to chronic pain: scientific
advances and future directions. Psychol Bull 2007;133:581-624. Crossref
3. Ng KF, Tsui SL, Chan WS. Prevalence of common chronic
pain in Hong Kong adults. Clin J Pain 2002;18:275-81. Crossref
4. Wong WS, Fielding R. Prevalence and characteristics of
chronic pain in the general population of Hong Kong. J
Pain 2011;12:236-45. Crossref
5. Vallejo R, Barkin RL, Wang VC. Pharmacology of opioids
in the treatment of chronic pain syndromes. Pain Physician
2011;14:E343-60.
6. World Health Organization. WHO’s cancer pain ladder
for adults. Available from: http://www.who.int/cancer/palliative/painladder/en/. Accessed 28 Jul 2015.
7. Manchikanti L, Vallejo R, Manchikanti KN, Benyamin
RM, Datta S, Christo PJ. Effectiveness of long-term opioid
therapy for chronic non-cancer pain. Pain Physician
2011;14:E133-56.
8. Chou R, Turner JA, Devine EB, et al. The effectiveness
and risks of long-term opioid therapy for chronic pain:
a systematic review for a National Institutes of Health
Pathways to Prevention Workshop. Ann Intern Med
2015;162:276-86. Crossref
9. Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in
chronic non-cancer pain: systematic review of efficacy and
safety. Pain 2004;112:372-80. Crossref
10. Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E.
Opioids for chronic noncancer pain: a meta-analysis of
effectiveness and side effects. CMAJ 2006;174:1589-94. Crossref
11. Papaleontiou M, Henderson CR Jr, Turner BJ, et al.
Outcomes associated with opioid use in the treatment of
chronic noncancer pain in older adults: a systematic review
and meta-analysis. J Am Geriatr Soc 2010;58:1353-69. Crossref
12. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid
management for chronic noncancer pain. Cochrane Database Syst
Rev 2010;(1):CD006605. Crossref
13. Chan BK, Tam LK, Wat CY, Chung YF, Tsui SL, Cheung
CW. Opioids in chronic non-cancer pain. Expert Opin
Pharmacother 2011;12:705-20. Crossref
14. Moore RA, McQuay HJ. Prevalence of opioid adverse
events in chronic non-malignant pain: systematic review
of randomised trials of oral opioids. Arthritis Res Ther
2005;7:R1046-51. Crossref
15. Noble M, Tregear SJ, Treadwell JR, Schoelles K. Long-term
opioid therapy for chronic noncancer pain: a systematic
review and meta-analysis of efficacy and safety. J Pain
Symptom Manage 2008;35:214-28. Crossref
16. Dhalla IA, Mamdani MM, Sivilotti ML, Kopp A, Qureshi
O, Juurlink DN. Prescribing of opioid analgesics and related
mortality before and after the introduction of long-acting
oxycodone. CMAJ 2009;181:891-6. Crossref
17. Okie S. A flood of opioids, a rising tide of deaths. N Engl J
Med 2010;363:1981-5. Crossref
18. Edlund MJ, Martin BC, Russo JE, DeVries A, Braden JB,
Sullivan MD. The role of opioid prescription in incident
opioid abuse and dependence among individuals with
chronic noncancer pain: the role of opioid prescription.
Clin J Pain 2014;30:557-64.
19. Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk
associated with the use of morphine and opiates. J Intern
Med 2006;260:76-87. Crossref
20. Smith HS, Elliott JA. Opioid-induced androgen deficiency
(OPIAD). Pain Physician 2012;15(3 Suppl):ES145-56.
21. Inturrisi CE. Clinical pharmacology of opioids for pain.
Clin J Pain 2002;18(4 Suppl):3S-13S. Crossref
22. Ersek M, Cherrier MM, Overman SS, Irving GA. The
cognitive effects of opioids. Pain Manag Nurs 2004;5:75-93. Crossref
23. Roy S, Loh HH. Effects of opioids on the immune system.
Neurochem Res 1996;21:1375-86. Crossref
24. Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L.
A comprehensive review of opioid-induced hyperalgesia.
Pain Physician 2011;14:145-61.
25. Tompkins DA, Campbell CM. Opioid-induced
hyperalgesia: clinically relevant or extraneous research
phenomenon? Curr Pain Headache Rep 2011;15:129-36. Crossref
26. Pain & Policy Studies Group. Global opioid consumption,
2013. Available from: http://www.painpolicy.wisc.edu/global. Accessed 1 Aug 2015.
27. Pain & Policy Studies Group. Opioid consumption maps—Morphine equivalence (ME), mg/capita, 2014. Available
from: https://ppsg.medicine.wisc.edu. Accessed 11 Oct
2015.
28. Pain & Policy Studies Group. Consumption data at-a-glance:
Hong Kong SAR, 2012. Available from: http://www.painpolicy.wisc.edu/country/profile/hong-kong-sar.
Accessed 1 Aug 2015.
29. United Nations Office on Drugs and Crime. World Drug
Report 2008. Available from: http://www.unodc.org/unodc/en/data-and-analysis/WDR-2008.html. Accessed 7
Jan 2016.
30. Drug Office, Department of Health, The Government of
Hong Kong Special Administrative Region. Registered pharmaceutical products. Available from: http://www.drugoffice.gov.hk/eps/do/en/consumer/reg_pharm_products/index.html. Accessed 11 Oct 2015.
31. Ho KY, Chua NH, George JM, et al. Evidence-based
guidelines on the use of opioids in chronic non-cancer
pain—a consensus statement by the Pain Association
of Singapore Task Force. Ann Acad Med Singapore
2013;42:138-52.
32. Manchikanti L, Abdi S, Atluri S, et al. American Society
of Interventional Pain Physicians (ASIPP) guidelines for
responsible opioid prescribing in chronic non-cancer pain:
Part 2—guidance. Pain Physician 2012;15(3 Suppl):67S-116S.
33. Canadian guideline for safe and effective use of opioids for
chronic non-cancer pain. Canada: National Opioid Use
Guideline Group (NOUGG); 2010. Available from: http://nationalpaincentre.mcmaster.ca/opioid/. Accessed 9 Aug
2016.
34. American College of Occupational and Environmental
Medicine. Guidelines for chronic use of opioids. Available
from: http://www.acoem.org/Guidelines_Opioids.aspx.
Accessed 25 Nov 2014.
35. Australian and New Zealand College of Anaesthetists
Faculty of Pain Medicine. Recommendations regarding
the use of opioid analgesics in patients with chronic
non-cancer pain. Available from: http://www.fpm.anzca.edu.au/resources/professional-documents/documents/PM1%202010.pdf. Accessed 25 Nov 2014.
36. British Pain Society. Opioids aware: A structured approach
to prescribing. Faculty of Pain Medicine, Royal College of Anaesthetists. Available from: https://www.rcoa.ac.uk/faculty-of-pain-medicine/opioids-aware/structured-approach-to-prescribing. Accessed 9 Aug 2016.
37. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for
the use of chronic opioid therapy in chronic noncancer pain. J Pain
2009;10:113-30. Crossref
38. Cheung CW, Qiu Q, Choi SW, Moore B, Goucke R, Irwin
M. Chronic opioid therapy for chronic non-cancer pain:
a review and comparison of treatment guidelines. Pain
Physician 2014;17:401-14.
39. Von Korff M, Saunders K, Thomas Ray G, et al. De facto
long-term opioid therapy for noncancer pain. Clin J Pain
2008;24:521-7. Crossref
40. International Association for the Study of Pain. Pain
treatment services. Available from: http://www.iasppain.org/Education/Content.aspx?ItemNumber=1381.
Accessed 9 Sep 2015.
41. Gatchel RJ, McGeary DD, McGeary CA, Lippe B.
Interdisciplinary chronic pain management: past, present,
and future. Am Psychol 2014;69:119-30. Crossref
42. Jeffrey MM, Butler M, Stark A, Kane RL. Multidisciplinary
pain programs for chronic noncancer pain: Technical brief
No. 8. AHRQ Publication No. 11-EHC064-EF. Rockville,
MD: Agency for Healthcare Research and Quality; Sep
2011. Available from: http://www.ncbi.nlm.nih.gov/books/NBK82511/pdf/Bookshelf_NBK82511.pdf. Accessed 9
Aug 2016.
43. Gatchel RJ, Okifuji A. Evidence-based scientific data
documenting the treatment and cost-effectiveness of
comprehensive pain programs for chronic nonmalignant
pain. J Pain 2006;7:779-93. Crossref
44. Guzmán J, Esmail R, Karjalainen K, Malmivaara A, Irvin E,
Bombardier C. Multidisciplinary rehabilitation for chronic
low back pain: systematic review. BMJ 2001;322:1511-6. Crossref
45. Turk DC. Clinical effectiveness and cost-effectiveness
of treatments for patients with chronic pain. Clin J Pain
2002;18:355-65. Crossref
46. Federation of State Medical Boards. Model policy on the
use of opioid analgesics in the treatment of chronic pain,
July 2013. Available from: http://www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/pain_policy_july2013.pdf.
Accessed 9 Aug 2016.
47. Agency Medical Directors’ Group. AMDG 2015
Interagency guidelines on prescribing opioids for pain.
Available from: http://www.agencymeddirectors.wa.gov/guidelines.asp. Accessed 11 Aug 2015.
48. Turk DC, Dworkin RH, Revicki D, et al. Identifying
important outcome domains for chronic pain clinical
trials: an IMMPACT survey of people with pain. Pain
2008;137:276-85. Crossref
49. Lee D, Armaghani S, Archer KR, et al. Preoperative opioid
use as a predictor of adverse postoperative self-reported
outcomes in patients undergoing spine surgery. J Bone
Joint Surg Am 2014;96:e89. Crossref
50. Armaghani SJ, Lee DS, Bible JE, et al. Preoperative
opioid use and its association with perioperative opioid
demand and postoperative opioid independence in
patients undergoing spine surgery. Spine (Phila Pa 1976)
2014;39:E1524-30. Crossref
51. Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS.
What percentage of chronic nonmalignant pain patients
exposed to chronic opioid analgesic therapy develop
abuse/addiction and/or aberrant drug-related behaviors?
A structured evidence-based review. Pain Med 2008;9:444-59. Crossref
52. Morasco BJ, Gritzner S, Lewis L, Oldham R, Turk DC,
Dobscha SK. Systematic review of prevalence, correlates,
and treatment outcomes for chronic non-cancer pain
in patients with comorbid substance use disorder. Pain
2011;152:488-97. Crossref
53. Højsted J, Sjøgren P. Addiction to opioids in chronic pain patients: a literature review. Eur J Pain 2007;11:490-518. Crossref
54. Atluri S, Akbik H, Sudarshan G. Prevention of opioid abuse
in chronic non-cancer pain: an algorithmic, evidence based
approach. Pain Physician 2012;15(3 Suppl):ES177-89.
55. Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M.
Risk factors for clinically recognized opioid abuse and
dependence among veterans using opioids for chronic
non-cancer pain. Pain 2007;129:355-62. Crossref
56. Turk DC, Swanson KS, Gatchel RJ. Predicting opioid
misuse by chronic pain patients: a systematic review and
literature synthesis. Clin J Pain 2008;24:497-508. Crossref
57. Webster LR, Webster RM. Predicting aberrant behaviors
in opioid-treated patients: preliminary validation of the
Opioid Risk Tool. Pain Med 2005;6:432-42. Crossref
58. Arnold RM, Han PK, Seltzer D. Opioid contracts in
chronic nonmalignant pain management: objectives and
uncertainties. Am J Med 2006;119:292-6. Crossref
59. Geppetti P, Benemei S. Pain treatment with opioids:
achieving the minimal effective and the minimal interacting
dose. Clin Drug Investig 2009;29 Suppl 1:3S-16S. Crossref
60. Department of Health, Government of Western Australia.
Quick clinical guideline for the use of opioids in chronic
non-malignant pain. Available from: http://www.hnehealth.nsw.gov.au/Pain/Pages/Health-professional-resources.aspx. Accessed 28 Jul 2015.
61. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM.
Clinical importance of changes in chronic pain intensity
measured on an 11-point numerical pain rating scale. Pain
2001;94:149-58. Crossref
62. Fishbain DA, Cutler RB, Rosomoff HL, Rosomoff RS. Are
opioid-dependent/tolerant patients impaired in driving-related
skills? A structured evidence-based review. J Pain
Symptom Manage 2003;25:559-77. Crossref
63. Engeland A, Skurtveit S, Mørland J. Risk of road traffic
accidents associated with the prescription of drugs: a
registry-based cohort study. Ann Epidemiol 2007;17:597-602. Crossref
64. Hadi I, da Silva O, Natale R, Boyd D, Morley-Forster PK.
Opioids in the parturient with chronic nonmalignant pain:
a retrospective review. J Opioid Manag 2006;2:31-4.
65. Broussard CS, Rasmussen SA, Reefhuis J, et al. Maternal
treatment with opioid analgesics and risk for birth defects.
Am J Obstet Gynecol 2011;204:314.e1-11. Crossref
66. Fajemirokun-Odudeyi O, Sinha C, Tutty S, et al. Pregnancy
outcome in women who use opiates. Eur J Obstet Gynecol
Reprod Biol 2006;126:170-5. Crossref
67. Passik SD, Kirsh KL, Whitcomb L, et al. A new tool to
assess and document pain outcomes in chronic pain
patients receiving opioid therapy. Clin Ther 2004;26:552-61. Crossref
68. Gourlay DL, Heit HA, Caplan YH. Urine drug testing in
clinical practice: the art and science of patient care. 6th ed. Baltimore, MD: John Hopkins University School
of Medicine; Aug 2015. Available from: http://www.udtmonograph6.com/view-monograph.html. Accessed 23 Aug
2016.
69. Peppin JF, Passik SD, Couto JE, et al. Recommendations for
urine drug monitoring as a component of opioid therapy in
the treatment of chronic pain. Pain Med 2012;13:886-96. Crossref
70. Fine PG, Portenoy RK, Ad Hoc Expert Panel on Evidence
Review and Guidelines for Opioid Rotation. Establishing
“best practices” for opioid rotation: conclusions of an
expert panel. J Pain Symptom Manage 2009;38:418-25. Crossref
71. Quigley C. Opioid switching to improve pain relief
and drug tolerability. Cochrane Database Syst Rev
2004;(3):CD004847. Crossref
72. Mercadante S, Bruera E. Opioid switching: a systematic
and critical review. Cancer Treat Rev 2006;32:304-15. Crossref
73. Pasternak GW. Molecular biology of opioid analgesia. J
Pain Symptom Manage 2005;29(5 Suppl):2S-9S. Crossref
74. Knotkova H, Fine PG, Portenoy RK. Opioid rotation: the
science and the limitations of the equianalgesic dose table.
J Pain Symptom Manage 2009;38:426-39. Crossref
75. Nielsen S, Degenhardt L, Hoban B, Gisev N. Comparing
opioids: a guide to estimating oral morphine equivalents
(OME) in research. Technical Report No. 329. Sydney:
National Drug and Alcohol Research Centre, University
of New South Wales; 2014. Available from: https://ndarc.med.unsw.edu.au/resource/comparing-opioids-guide-estimating-oral-morphine-equivalents-ome-research.
Accessed 24 Mar 2016.
76. UK Medicines Information (UKMi). Medicines Q&As:
Q&A 42.7: What are the equivalent doses of oral morphine
to other oral opioids when used as analgesics in adult
palliative care? Available from: http://www.ukmi.nhs.uk/activities/medicinesQAs/default.asp. Accessed 24 Mar
2016.
77. Lexicomp. Drug information handbook: a clinically
relevant resource for all healthcare professionals. 23rd ed.
Ohio: Lexicomp; 2014.
78. Mercadante S, Caraceni A. Conversion ratios for opioid
switching in the treatment of cancer pain: a systematic
review. Palliat Med 2011;25:504-15. Crossref
79. Benyamin R, Trescot AM, Datta S, et al. Opioid
complications and side effects. Pain Physician 2008;11(2
Suppl):105S-120S.
80. Sehgal N, Manchikanti L, Smith HS. Prescription opioid
abuse in chronic pain: a review of opioid abuse predictors
and strategies to curb opioid abuse. Pain Physician
2012;15(3 Suppl):ES67-92.
81. Weissman DE, Haddox JD. Opioid pseudoaddiction—an iatrogenic syndrome. Pain 1989;36:363-6. Crossref