Hong Kong Med J 2016 Oct;22(5):472–7 | Epub 26 Aug 2016
DOI: 10.12809/hkmj164897
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Management of health care workers following occupational exposure to hepatitis B, hepatitis C, and human immunodeficiency virus
Winnie WY Sin, MB, ChB, FHKAM (Medicine);
Ada WC Lin, MB, BS, FHKAM (Medicine);
Kenny CW Chan, MB, BS, FHKAM (Medicine);
KH Wong, MB, BS, FHKAM (Medicine)
Special Preventive Programme, Centre for Health Protection, Department of Health, Kowloon Bay Health Centre, Hong Kong
Corresponding author: Dr Kenny CW Chan (kcwchan@dh.gov.hk)
Abstract
Introduction: Needlestick injury or mucosal contact
with blood or body fluids is well recognised in the
health care setting. This study aimed to describe
the post-exposure management and outcome in
health care workers following exposure to hepatitis
B, hepatitis C, or human immunodeficiency virus
(HIV) during needlestick injury or mucosal contact.
Methods: This case series study was conducted
in a public clinic in Hong Kong. All health care
workers with a needlestick injury or mucosal contact
with blood or body fluids who were referred to the
Therapeutic Prevention Clinic of Department of
Health from 1999 to 2013 were included.
Results: A total of 1525 health care workers were
referred to the Therapeutic Prevention Clinic
following occupational exposure. Most sustained a
percutaneous injury (89%), in particular during post-procedure
cleaning or tidying up. Gloves were worn
in 62.7% of instances. The source patient could be
identified in 83.7% of cases, but the infection status
was usually unknown, with baseline positivity rates
of hepatitis B, hepatitis C, and HIV of all identified
sources, as reported by the injured, being 7.4%, 1.6%,
and 3.3%, respectively. Post-exposure prophylaxis
of HIV was prescribed to 48 health care workers, of
whom 14 (38.9%) had been exposed to known HIV-infected blood or body fluids. The majority (89.6%)
received HIV post-exposure prophylaxis within 24
hours of exposure. Drug-related adverse events were
encountered by 88.6%. The completion rate of post-exposure
prophylaxis was 73.1%. After a follow-up
period of 6 months (or 1 year for those who had taken
HIV post-exposure prophylaxis), no hepatitis B,
hepatitis C, or HIV seroconversions were detected.
Conclusions: Percutaneous injury in the health
care setting is not uncommon but post-exposure
prophylaxis of HIV is infrequently indicated. There
was no hepatitis B, hepatitis C, and HIV transmission
via sharps or mucosal injury in this cohort of health
care workers.
New knowledge added by this study
- The risk of hepatitis B (HBV), hepatitis C (HCV), and human immunodeficiency virus (HIV) transmission following occupational sharps or mucosal injury in Hong Kong is small.
- Meticulous adherence to infection control procedures and timely post-exposure management prevents HBV, HCV, and HIV infection following occupational exposure to blood and body fluids.
Introduction
Needlestick injury or mucosal contact with blood
or body fluids is well recognised in the health care
setting. These incidents pose a small but definite risk
for health care workers of acquiring blood-borne
viruses, notably hepatitis B virus (HBV), hepatitis
C virus (HCV), and human immunodeficiency
virus (HIV). The estimated risk of contracting HBV
infection through occupational exposure to known
infected blood via needlestick injury varies from 18%
to 30%, while that for HCV infection is 1.8% (range,
0%-7%).1 The risk of HIV transmission following
percutaneous or mucosal exposure to HIV-contaminated
blood is 0.3% and 0.09%, respectively.1
The risk is further affected by the type of exposure,
body fluid involved, and infectivity of the source.
In Hong Kong, injured health care workers
usually receive initial first aid and immediate
management in the Accident and Emergency
Department. They are then referred to designated
clinics for specific post-exposure management.
Currently, aside from staff of the Hospital Authority
who are managed at two designated clinics post-exposure,
all other health care workers from
private hospitals, and government or private clinics
and laboratories are referred to the Therapeutic
Prevention Clinic (TPC) of the Integrated Treatment
Centre, Department of Health. Since its launch in
mid-1999, the TPC has provided comprehensive
post-exposure management to people with
documented percutaneous, mucosal, or breached
skin exposure to blood or body fluids in accordance
with the local guidelines set out by the Scientific
Committee on AIDS and STI, and Infection Control
Branch of Centre for Health Protection, Department
of Health.2 The present study describes the characteristics
and outcome of health care workers who attended the
TPC from mid-1999 to 2013 following occupational
exposure to blood or body fluids.
Methods
The study included all health care workers seen in
the TPC from July 1999 to December 2013 following
occupational exposure to blood or body fluids, who
attended following secondary referral by an accident
and emergency department of a public hospital.
Using two standard questionnaires (Appendices 1 and 2), data were collected by the attending nurse and
doctor during a face-to-face interview with each
health care worker on the following: demography
and occupation of the exposed client, type and
pattern of exposure, post-exposure management,
and clinical outcome.
Appendix 1. TPC First Consultation Assessment Form
Appendix 2. Therapeutic Prevention Clinic (TPC) human immunodeficiency virus (HIV) Post-exposure Prophylaxis Registry Form (to be completed on completion or cessation of post-exposure prophylaxis)
Details of the exposure, including type of
exposure and the situation in which it occurred, were
noted. The number of risk factors (see definitions
below) for HIV transmission was counted for each
exposure and further classified as high risk or low
risk. Where known and reported by the injured
party, hepatitis B surface antigen (HBsAg), HCV, and
HIV status of the source were recorded.
The timing of the first medical consultation
in the accident and emergency department, any
prescription of HIV post-exposure prophylaxis
(PEP), and the time since injury were noted.
Exposed health care workers who received HIV PEP
were reviewed at clinic visits every 2 weeks until
completion of the 4-week course of treatment, and
any treatment-related adverse effects were reported.
Blood was obtained as appropriate at these visits for
measurement of complete blood count, renal and
liver function, and amylase, creatine kinase, fasting
lipid, and glucose levels.
Apart from HIV PEP–related side-effects
(reported and rated by patients as mild, moderate, or
severe), the rate of completion of PEP, and number of
HBV, HCV, and HIV seroconversions following the
incident was also recorded. The HBsAg, anti-HBs,
anti-HCV, and anti-HIV were checked at baseline
and 6 months post-exposure to determine whether
seroconversion had occurred. Those exposed to a
known HCV-infected source or a source known to
be an injecting drug user had additional blood tests
6 weeks post-exposure for liver function, anti-HCV,
and HCV RNA. Additional HIV antibody testing at 3
and 12 months post-exposure was arranged for those
who received HIV PEP. For those who contracted
HCV infection from a source co-infected with HCV
and HIV, further HIV testing was performed at 1 year
post-exposure to detect delayed seroconversion.
Definitions
Health care workers included doctors and medical
students, dentists and dental workers, nurses,
midwives, inoculators, laboratory workers,
phlebotomists, ward or clinic attendants, and
workmen. Staff working in non–health care
institutions (eg elderly home, hostels, and sheltered
workshops) were excluded. Five factors were classified
as high-risk exposure: (i) deep percutaneous injury,
(ii) procedures involving a device placed in a blood
vessel, (iii) use of a hollow-bore needle, (iv) device that
was visibly contaminated with blood, and (iv) source
person with acquired immunodeficiency syndrome
(AIDS).3 Another five factors were classified as low-risk
exposure: (i) moderate percutaneous injury,
(ii) mucosal contact, (iii) contact with deep body
fluids other than blood, (iv) source person was HIV-infected
but not or not sure about the stage of AIDS,
and (v) any other reason contributing to increased
risk according to clinical judgement.
Results
From July 1999 to December 2013, 1525 health
care workers (75-168 per year) with occupational
exposure to HBV, HCV, or HIV were referred to the
TPC (Fig). Females constituted 77% of all attendees.
The median age was 33 years (range, 17-73 years).
The majority came from the dental profession
(36.8%) and nursing profession (33.4%), followed by
ward/clinic ancillary staff (11.6%) and the medical
profession (4.7%).
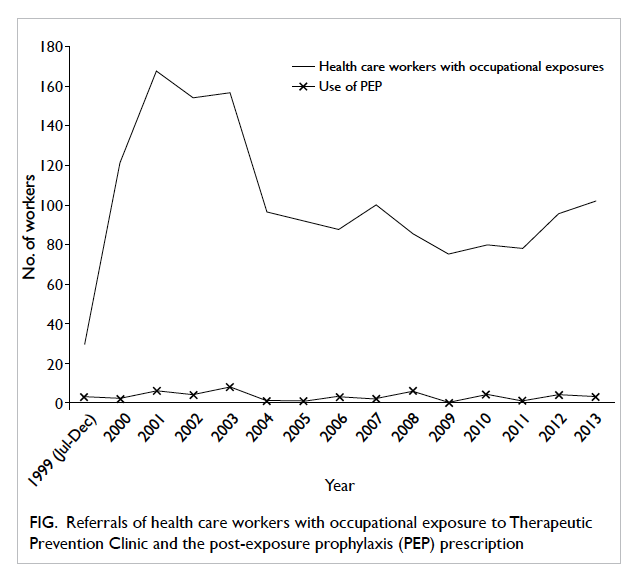
Figure. Referrals of health care workers with occupational exposure to Therapeutic Prevention Clinic and the post-exposure prophylaxis (PEP) prescription
Type and pattern of exposure
The majority of exposures occurred in a public clinic
or laboratory (n=519, 34.0%), followed by public
hospital (n=432, 28.3%), private clinic or laboratory
(n=185, 12.1%), and private hospital (n=23, 1.5%).
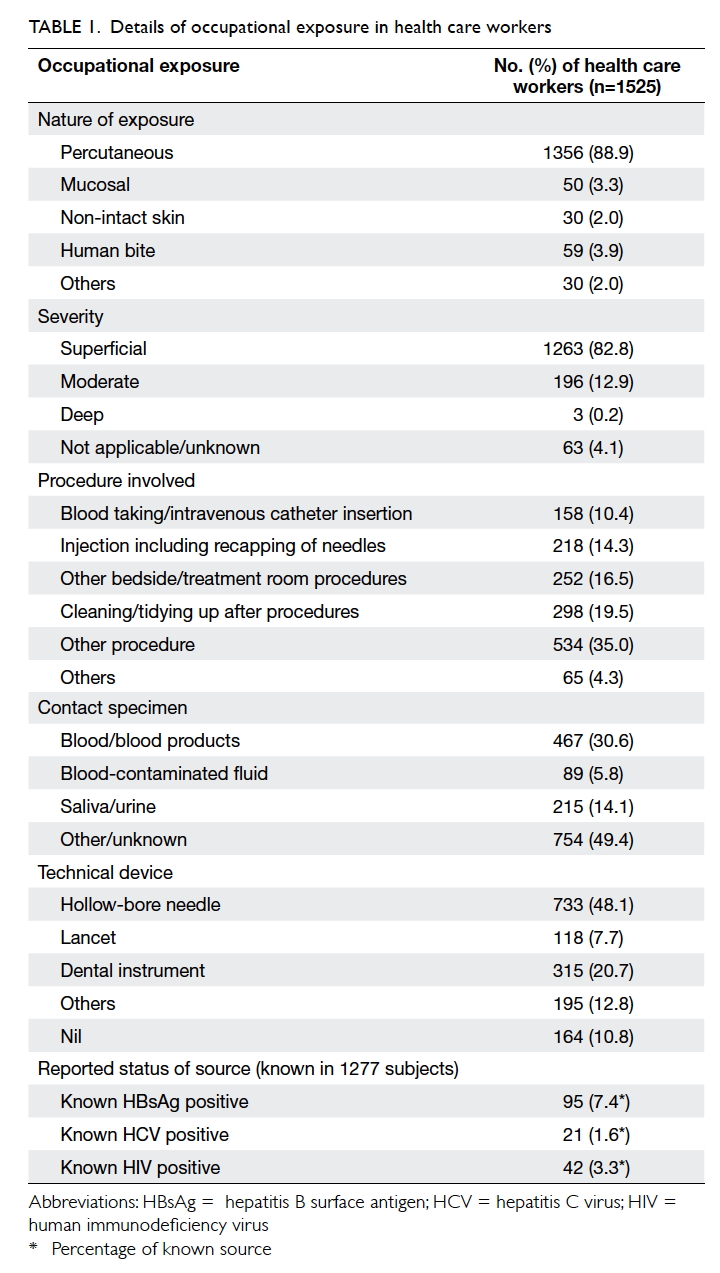
Most were a percutaneous injury (88.9%). Mucosal
contact, breached skin contact, and human bite
were infrequent (Table 1). Approximately 60% of the incidents occurred in one of the four situations:
(a) cleaning/tidying up after procedures (the most
common), (b) other bedside/treatment room
procedures, (c) injection, including recapping of
needles, or (d) blood taking/intravenous catheter
insertion. The contact specimen was blood or blood
products, blood-contaminated fluid, and saliva
or urine in 30.6%, 5.8%, and 14.1% of the cases,
respectively. The technical device involved was a
hollow-bore needle in 48.1%, dental instrument in
20.7%, and lancet in 7.7%. More than 80% considered
the injury superficial.
High-risk and low-risk factors were noted in
869 (57%) and 166 (11%) exposures, respectively.
Blood taking/intravenous catheter insertion carried
the highest risk among all the procedures, with a
mean risk factor of 1.29 (Table 2). Gloves were used in 956 (62.7%) exposures, goggles/mask in 50 (3.3%),
and gown/apron in 55 (3.6%). Nonetheless, 101
(6.6%) health care workers indicated that they did
not use any personal protective equipment during
the exposure.
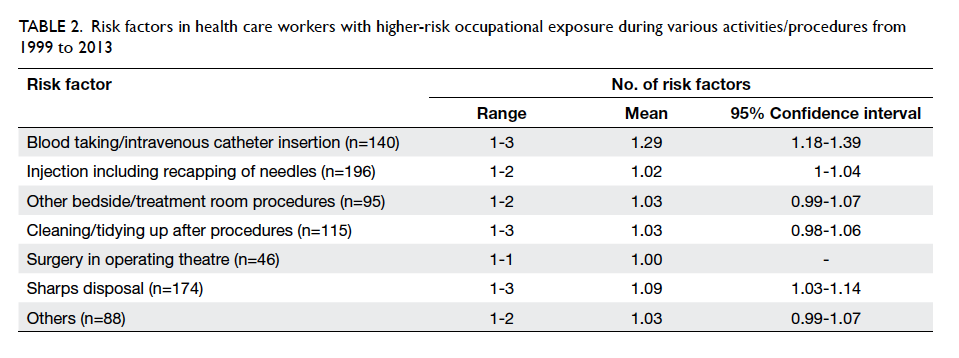
Table 2. Risk factors in health care workers with higher-risk occupational exposure during various activities/procedures from 1999 to 2013
The source patient could be identified in 1277
(83.7%) cases but the infectious status was unknown
in most. The baseline known positivity rate for HBV,
HCV, and HIV of all identified sources was 7.4%,
1.6%, and 3.3%, respectively (Table 1).
Care and clinical outcome
Nearly half of the injured health care workers
attended a medical consultation within 2 hours
(n=720, 47.2%) and another 552 (36.2%) attended between 2 and 12 hours following
exposure. The median time between exposure and
medical consultation was 2.0 hours.
During the study period, 48 (3.1%) health
care workers received HIV PEP for occupational
exposure, ranging from zero to eight per year (Fig). One third
received PEP within 2 hours of exposure, and the
majority (89.6%) within 24 hours. The median time
to PEP was 4.0 hours post-exposure (interquartile
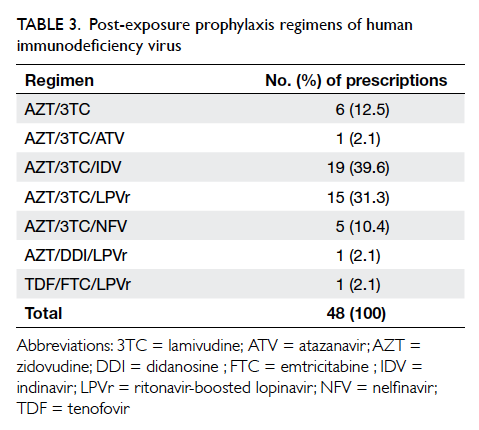
range, 2.0-8.1 hours). A three-drug regimen was
prescribed in 85.7% of cases. The most common
regimen was zidovudine/lamivudine/indinavir
(39.6%), followed by zidovudine/lamivudine/ritonavir-boosted lopinavir (31.3%), and zidovudine/lamivudine (12.5%) [Table 3]. Upon consultation and risk assessment at the TPC, 36 (75%) workers
had treatment continued from the accident and
emergency department. Among them, the source
was confirmed to be HIV-positive in 14 (38.9%) cases.
Of the 35 clients with known outcome, drug-related
adverse events were seen in 31 (88.6%) health care
workers; more than half (n=18, 58.1%) of which were
considered to be moderate or severe. Treatment-related
side-effects led to early termination of PEP
in eight (22.9%) health care workers. Excluding nine
clients in whom prophylaxis was stopped when the
source was established to be HIV-negative, 19 (73.1%)
clients were able to complete the 28-day course of
PEP. Of the 14 clients who sustained injury from an
HIV-infected source patient, all received PEP but
two did not complete the course; the completion rate
was 85.7%.
At baseline, none of the injured health care
workers tested positive for HCV or HIV, while 49
(3.2% of all health care workers seen in TPC) tested
HBsAg-positive. Almost half of the health care
workers (n=732, 48.0%) were immune to HBV (anti-HBs positive). After follow-up of 6 months (1 year
for those who took PEP), no case of HBV, HCV, or
HIV seroconversion was detected in this cohort.
Discussion
Health care workers may be exposed to blood-borne
viruses when they handle sharps and body fluids.
Thus, adherence to standard precautions of infection
control is an integral component of occupational
health and safety for health care workers. In this
cohort, percutaneous injury with sharps during
cleaning or tidying up after procedures remained
the most common mechanism of injury. Many of
these incidents could have been prevented by safer
practice, for instance, by not recapping needles or by
disposing needles directly into a sharps box after use.
The use of gloves as part of standard precautions was
suboptimal and greater emphasis on the importance
of wearing the appropriate personal protective
equipment should be made during staff training
at induction and on refresher courses. Technical
devices with safety needleless features may reduce
sharps injuries. Improvement in the system (eg
by placing a sharps box near the work area) or the
workflow to minimise distraction may also help
compliance with infection control measures.
Once exposure occurs, PEP is the last defence
against HBV and HIV. For HBV infection, PEP with
hepatitis B immunoglobulin followed by hepatitis
B vaccination has long been the standard practice
in Hong Kong. For HIV infection, the efficacy of
PEP in health care workers following occupational
exposure was demonstrated by a historic landmark
overseas case-control study.3 Prescription of
zidovudine achieved an 81% reduction in risk of HIV
seroconversion following percutaneous exposure
to HIV-infected blood.3 Local and international
guidelines now recommend a combination of three
antiretroviral drugs for PEP.2 4 5 6 In this cohort,
although more than half of the exposures had higher
risk factors for HIV acquisition, it was uncommon
for the source patients to have known HIV infection
(2.8% of these exposures). Thus, in accordance
with the local guideline, PEP was not commonly
prescribed. Nevertheless, PEP was prescribed in
all 14 exposures to a known HIV-positive source and
in other 34 exposures after risk assessment.
Our experience is comparable with the health care
service in the UK and US. In the UK, 78% of health
care workers exposed to an HIV-infected source
patient were prescribed PEP.7 In a report from the
US, only 68% of health care workers with such
exposure took PEP.8 For HCV, PEP with antiviral
therapy is not recommended according to the latest
guidelines from American Association for the Study
of Liver Diseases/Infectious Diseases Society of
America.9 In case seroconversion occurs and early
treatment is considered desirable, these patients with
acute hepatitis C can be treated with direct-acting
antivirals using the same regimen recommended for
chronic hepatitis C.
If indicated, HIV PEP should be taken as
early as possible after exposure to achieve maximal
effect. Initiation of PEP after 72 hours of exposure
was shown to be ineffective in animal studies.10 The
timing of PEP initiation in our cohort appeared to
be less prompt (33.3% within 2 hours compared with
more than 60% and 80% within 3 hours in the UK and
US, respectively). Overall, however, 89.6% managed
to start PEP within 24 hours, in line with experience
in the UK or US. Health care workers should be
reminded about post-exposure management and
the need for timely medical assessment following
occupational exposure. In the accident and
emergency department, priority assessment should
be given to health care workers exposed to blood-borne
viruses. The median duration of PEP intake of
28 days was in line with the local guidelines. With
the availability of newer drugs with fewer toxicities,
the tolerance and compliance rate should improve.
Finally, using the estimated risk of HIV
transmission with percutaneous injury of 0.3%, we
would expect four HIV seroconversions in 1356
percutaneous exposures in TPC if all were exposed
to HIV-infected blood. Because in most of these
exposures the source HIV status was unknown and
likely negative in this region of overall low HIV
prevalence (approximately 0.1%11), the actual risk
of HIV transmission was much lower in the health
care setting of Hong Kong. This finding is confirmed by the fact that no HIV seroconversion occurred in this cohort. In addition, those with exposure of the highest
risk received HIV PEP. In the UK, there were 4381
significant occupational exposures from 2002 to 2011,
of which 1336 were exposures to HIV-infected blood
or body fluid. No HIV seroconversions occurred
among these exposures.7 In the US, there has been
one confirmed case of occupational transmission of
HIV in health care workers since 1999.12 Similarly,
the local prevalence of HCV infection is low
(<0.1% in new blood donors13), partly explaining
the absence of HCV transmission in this cohort. In
contrast, there were 20 cases of HCV seroconversion
in health care workers reported between 1997 and
2011 in the UK.7 Hepatitis B is considered to be
endemic in Hong Kong, with HBsAg positivity of
1.1% in new blood donors and 6.5% in antenatal
women in 2013.13 Nonetheless, the HBV vaccination
programme in health care workers coupled with
HBV PEP has proven successful in preventing
HBV transmission to health care workers. With
concerted efforts in infection control and timely PEP,
transmission of blood-borne viruses via sharps and
mucosal injury in the health care setting is largely
preventable.
There are several limitations to our study.
First, data were collected from a single centre and
based on secondary referral. We did not have data
for other health care workers who had occupational
exposure but who were not referred to the TPC for
post-exposure management, or who were referred
but did not attend. Thus, we were not able to draw
any general conclusions on the true magnitude of
the problem. Second, details of the exposure and
the infection status of the source were self-reported
by the exposed client and prone to bias and under-reporting.
Conclusions
Percutaneous injury with sharps during cleaning or
tidying up after procedures was the most common
cause of occupational exposure to blood or body
fluids in this cohort of health care workers. The
majority of source patients were not confirmed HIV-positive
and HIV PEP was not generally indicated.
Prescriptions of HIV PEP were appropriate and
timely in most cases. There were no HIV, HBV, and
HCV seroconversions in health care workers who
attended the TPC following sharps or mucosal injury
from mid-1999 to 2013.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Pruss-Ustun A, Rapiti E, Hutin Y. Sharps injuries: Global
burden of disease from sharps injuries to health-care
workers (World Health Organization Environmental
Burden of Disease Series, No. 3). Available from: http://www.who.int/quantifying_ehimpacts/publications/en/sharps.pdf?ua=1. Accessed 2 Feb 2016.
2. Scientific Committee on AIDS and STI (SCAS), and
Infection Control Branch, Centre for Health Protection,
Department of Health. Recommendations on the
management and postexposure prophylaxis of needlestick
injury or mucosal contact to HBV, HCV and HIV. Hong
Kong: Department of Health; 2014.
3. Cardo DM, Culver DH, Ciesielski CA, et al. A case-control
study of HIV seroconversion in health care workers after
percutaneous exposure. Centers for Disease Control and
Prevention Needlestick Surveillance Group. N Engl J Med
1997;337:1485-90. Crossref
4. Kuhar DT, Henderson DK, Struble KA, et al. Updated
US Public Health Service guidelines for the management
of occupational exposures to human immunodeficiency
virus and recommendations for postexposure prophylaxis.
Infect Control Hosp Epidemiol 2013;34:875-92. Crossref
5. UK Department of Health. HIV post-exposure prophylaxis:
guidance from the UK Chief Medical Officers’ Expert
Advisory Group on AIDS. 19 September 2008 (last updated
29 April 2015).
6. WHO Guidelines Approved by the Guidelines Review
Committee. Guidelines on Post-Exposure Prophylaxis
for HIV and the Use of Co-Trimoxazole Prophylaxis for
HIV-Related Infections Among Adults, Adolescents and
Children: Recommendations for a Public Health Approach:
December 2014 supplement to the 2013 consolidated
guidelines on the use of antiretroviral drugs for treating
and preventing HIV infection. Geneva: World Health
Organization; December 2014.
7. Eye of the Needle. United Kingdom surveillance of
significant occupational exposures to bloodborne viruses
in healthcare workers. London: Health Protection Agency;
December 2012.
8. US Department of Health and Human Services, Centers for
Disease Control and Prevention. The National Surveillance
System for Healthcare Workers (NaSH): Summary report
for blood and body fluid exposure data collected from
participating healthcare facilities (June 1995 through
December 2007).
9. American Association for the Study of Liver Diseases/Infectious Diseases Society of America. HCV guidance:
recommendations for testing, managing, and treating
hepatitis C (updated 24 February 2016). Available from:
http://www.hcvguidelines.org. Accessed 5 May 2016.
10. Tsai CC, Emau P, Follis KE, et al. Effectiveness of
postinoculation (R)-9-(2-phosphonylmethoxypropyl)
adenine treatment for prevention of persistent simian
immunodeficiency virus SIVmne infection depends
critically on timing of initiation and duration of treatment.
J Virol 1998;72:4265-73.
11. HIV surveillance report—2014 update. Department
of Health, The Government of the Hong Kong Special
Administrative Region; December 2015.
12. Joyce MP, Kuhar D, Brooks JT, Occupationally acquired
HIV infection among health care workers—United States,
1985-2013. MMWR Morb Mortal Wkly Rep 2015;63:1245-6.
13. Surveillance of viral hepatitis in Hong Kong—2014 update.
Department of Health, The Government of the Hong Kong
Special Administrative Region; December 2015.