Hong Kong Med J 2016 Jun;22(3):256–62 | Epub 6 May 2016
DOI: 10.12809/hkmj154736
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Colorectal endoscopic submucosal dissection at
a low-volume centre: tips and tricks, and learning
curve in a district hospital in Hong Kong
Deon HM Chong, FRCSEd;
CM Poon, FRCSEd;
HT Leong, FRCSEd
Department of Surgery, North District Hospital, Sheung Shui, Hong Kong
Corresponding author: Dr HT Leong (lamyn@ha.org.hk)
Abstract
Introduction: Colorectal endoscopic submucosal
dissection is not a widely adopted procedure due to
its technical difficulties. This study aimed to share
the experience in setting up this novel procedure and
to report the learning curve for such a procedure at a
low-volume district hospital in Hong Kong.
Methods: This case series comprised 71 colorectal
endoscopic submucosal dissections that were
performed by a single endoscopist without
experience in gastric or colorectal endoscopic
submucosal dissection. Lesion characteristics,
procedure time per unit area of tumour, en-bloc
resection rate, R0 resection rate, complications, and
length of stay were recorded prospectively. Results
were compared for two consecutive periods to study
the learning curve.
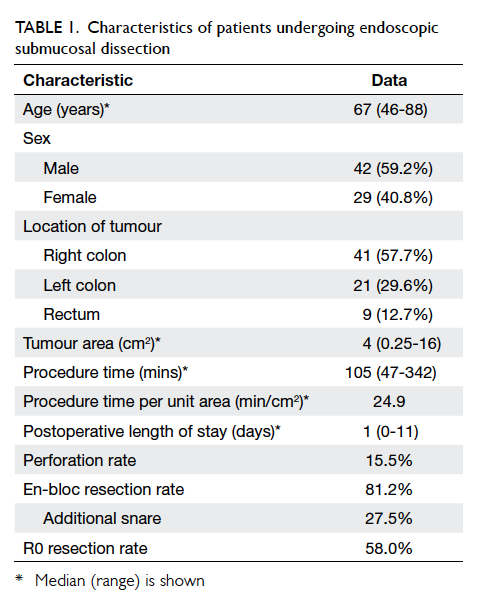
Results: Overall, 41 (57.7%) tumours were located
in the right colon, 21 (29.6%) in the left colon, and nine
(12.7%) in the rectum. The median tumour area was
4 cm2 (range, 0.25-16 cm2). The median operating
time was 105 (range, 47-342) minutes. The median
procedure time per unit area of tumour was 24.9
min/cm2. There was one instance of intra-operative
bleeding that required conversion to laparoscopic
colectomy. There was no postoperative haemorrhage.
The overall perforation rate was 15.5%, in which one
required conversion to laparoscopic colectomy. The
overall morbidity rate was 16.9% and there was no
mortality. The median hospital stay was 1 day (range,
0-11 days). The overall en-bloc resection rate and R0
resection rate was 81.2% and 58.0%, respectively.
Comparison of the two study periods revealed that
procedure time per unit area of tumour decreased
significantly from 31.5 min/cm2 to 21.5 min/cm2
(P=0.032). The en-bloc resection rate improved
from 78.8% to 83.3% (P=0.15). The R0 resection rate
improved significantly from 39.4% to 75.0% (P<0.01).
Conclusion: Untutored colorectal endoscopic
submucosal dissection is feasible with acceptable
clinical outcomes at a low-volume district hospital
in Hong Kong.
New knowledge added by this study
- Untutored colorectal endoscopic submucosal dissection (ESD) has an acceptable clinical outcome after 35 procedures at a low-volume centre.
- ESD can be safely performed at a low-volume centre.
- ESD can be started at the colorectum instead of the stomach.
Introduction
For many years, conventional endoscopic mucosal
resection (EMR) and surgery were the only options
for treating a large (>20 mm) sessile or flat colorectal
lesion. Conventional EMR, however, often results in
piecemeal removal and there is a significant local
recurrence rate ranging from 7.4% to 17%.1 2 3 Full
histological evaluation is also difficult.
Endoscopic submucosal dissection (ESD),
pioneered in Japan for treating early upper
gastro-intestinal malignancy, was introduced in
the late 1990s by Yamamoto et al4 and Fujishiro et
al5 to treat colorectal lesions. The technique has
an advantage over EMR in that its effectiveness
is not limited by size or shape of the lesion. In the
past decade, colorectal ESD has been shown to
be superior to EMR, in terms of higher en-bloc
resection rate and lower recurrence rate.6 Colorectal
ESD can be applied not only to adenoma, but also
to intramucosal carcinoma and low-risk submucosal
carcinoma, as defined by the Paris classification7
and the Japanese Society for Cancer of the Colon
and Rectum.8 Recently, a large-scale multicentre study has shown that ESD alone is adequate in the
management of patients with low-risk submucosal
carcinoma and achieves an excellent 5-year
recurrence-free survival of 98% and 5-year overall
survival of 94%.9
Despite the growing evidence to support
the use of colorectal ESD, it is not established as a
standard treatment outside Japan. The drawbacks of
colorectal ESD include longer operating time6 and
higher complication rates, especially perforation.
Although the perforation rate of ESD is much
higher than that of EMR, most ESD perforations
can be treated conservatively by clip closure
during endoscopy.10 11 In a multicentre study of iatrogenic perforations in Japan, the respective EMR and ESD perforation rate was 0.58% and 14% in 15 160 therapeutic colonoscopies.12 Endoscopic
clipping failed in 43.5% of ESD perforations and
surgical intervention was necessary.12
Perhaps one of the major hurdles to its general
application is that it is a technically demanding
procedure that is difficult to set up at a low-volume
district hospital. We would like to share
our experience of applying this novel technique in a
district hospital in Hong Kong.
Methods
Case selection
North District Hospital was a district hospital
serving 700 000 population with a case volume of 15
to 20 cases of ESD per year. Since the introduction of
the ESD technique at the hospital in 2009, all lateral
spreading tumours larger than 2 cm or those unable
to be resected en bloc by conventional polypectomy
were referred to a single endoscopist to determine
the appropriateness of ESD. Colonoscopy was
repeated by a single endoscopist to determine the
location, size, and nature of each tumour by white-light and narrow band imaging (NBI). Benign
polyps not amendable to removal by EMR were
triaged to ESD. Target biopsy was performed on
Sano III lesions that were triaged to conventional
laparoscopic colectomy. No tumours were excluded
based on location.
Preoperative evaluation of the depth of invasion
Evaluation of the depth of invasion is important
to determine the treatment strategy. To predict
the depth of invasion, we used NBI colonoscopy,
based on Sano’s capillary pattern classification. The
underlying principle is that angiogenesis is critical
for transition of a premalignant lesion to a malignant
one and the microcapillary pattern changes in this
process. Sano et al13 focused on this microcapillary
difference based on their histopathological findings
and devised three classifications: types I, II, and III.
Type III was further subdivided into IIIA and IIIB.14
The diagnostic accuracy of NBI colonoscopy
in differentiating a neoplastic from non-neoplastic
lesion is superior to conventional colonoscopy
and equivalent to chromoendoscopy using indigocarmine.15 For estimation of the depth of invasion, the sensitivity, specificity, and diagnostic accuracy
of capillary pattern type III for differentiating
pM-ca (intramucosal) or pSM1 (superficial) from pSM2-3 (deep) was 84.8%, 88.7% and
87.7%, respectively.14 We preferred NBI colonoscopy
because it is fast and easy to use, without the need to
spray dye as in chromoendoscopy.
Preparation
The procedure was performed in the operating
theatre with the patient under conscious sedation.
All patients were assessed by an anaesthetist in a
preoperation clinic.
Patients were instructed to eat a low-residue
diet 2 days before the procedure and a fluid diet
on the day before ESD. Bowel preparation with
4 L polyethylene glycol solution was given on the
day before ESD. Prophylactic antibiotics were not
prescribed.
Setting
All procedures were performed in the operating
theatre. This ensured that all equipment was on
hand should conversion to an open procedure be
required, for example, if there was full-thickness
perforation that could not be closed endoscopically.
Endoscopic system
In our hospital, ESD was performed using a single-channel colonoscope (CF-H180AL; Olympus,
Tokyo, Japan). This colonoscope was a high-density
television compatible with a wide angle of 170°,
3.7-mm instrument channel, and auxiliary water
jet. A short transparent hood was fitted to the tip of
the endoscope so that the whole ring could be seen
in endoscopic view. Carbon dioxide was used for
insufflation to decrease patient discomfort. We used
a high-frequency electrosurgical generator (ESG-100; Olympus, Tokyo, Japan) with a peristaltic pump
(AFU-100; Olympus, Tokyo, Japan). The energy
setting used for incision and dissection was “forced
coagulation 2” 30W, whereas “soft coagulation”
100W was used for haemostasis.
Cutting devices
In our initial practice, we used the Flex Knife (KD-630L; Olympus, Tokyo, Japan) to perform the procedure. It had a loop-shaped tip that allowed
easy control in any direction, as it was soft and
flexible. Nonetheless, we found it difficult to
adjust the length of the tip and there was frequent
accumulation of debris around the tip. We then
changed to Dual Knife (KD-650L; Olympus, Tokyo,
Japan) with a fixed length (1.5 mm) and hence a
more stable knife movement. More recently, we
have used Flush Knife BT (DK2618JB/DK2618JN;
Fujifilm, Saitama, Japan) for dissection. It has a
ball tip of fixed length that touches a wider part of
tissue and enhances haemostasis. The knife has a
water jet channel and achieves two purposes: (1) it
can wash away any tissue that accumulates around
the tip, thereby maintaining the sharpness of the
knife; and (2) submucosal normal saline injection
can be performed without the need to change the
instrument for further hyaluronate injection.
Injecting agent
We used a mixture of 10% sodium hyaluronate (LG
Chemical, South Korea) and 1:200 000 adrenaline
saline at a ratio of 1:1.5. This solution was chosen for
three reasons: the addition of adrenaline can produce
a haemostasis effect; dilution of sodium hyaluronate
made it less viscous and thus easier to inject; and
it reduced the amount used of the relatively more
expensive sodium hyaluronate.
Endoscopist
All ESD procedures were performed by a single
experienced endoscopist who had performed more
than 500 therapeutic colonoscopic procedures and
more than 200 laparoscopic colectomies. The ESD
procedure was implemented by the endoscopist
following completion of training on an animal model
in the Second Master Workshop on Novel Endoscopic
Technology & Endoscopic Submucosal Dissection
in 2009 at Prince of Wales Hospital in Hong Kong,
which was organised by the Department of Surgery,
The Chinese University of Hong Kong (http://www.surgery.cuhk.edu.hk/events/2009-07-22-ESD.pdf). The workshop included both lecture sessions and hands-on sessions to perform ESD in a pig. The
endoscopist had no experience in gastric or
colorectal ESD. He received further overseas training
in ESD in 2011 at Osaka Medical Center for Cancer
and Cardiovascular Diseases in Japan as a clinical
observer with hands-on animal model training.
Procedure
With the patient initially lying in the left lateral
position, a full colonoscopy was first performed to
confirm and locate the site of pathology. Patients
were then re-positioned such that the lesion was
at an anti-gravitational position in the endoscopic
view at 6 o’clock. This could be easily achieved by
seeing the injected water pooling opposite to the
lesion. In this position, the gravitational force aided
in retracting the lesion away from the submucosal
plane during dissection. We then injected 1:100 000
adrenaline saline at 1 cm distal to the lesion, aiming
at the submucosal layer. This could be ascertained by
seeing the formation of a dwell. With the injecting
needle still in situ, the solution was then changed to the
mixture of adrenaline saline and sodium hyaluronate
to provide a precipitous elevation of sufficient height.
After elevating the lesion, a mucosal incision was
made proximal to the lesion. The mucosal incision
was started at the proximal two thirds of the lesion.
After mucosal incision, the submucosal plane was
dissected with the submucosa dissected away from
the muscle layer. Care was taken to manipulate the
dissection plan parallel to the intestinal wall to prevent
perforation. When a more than 1-mm diameter vessel
was detected, it was coagulated using haemostasis
forceps (Radial Jaw 4; Boston Scientific, US). When
the flap was sufficient for retraction, the mucosal
incision was completed. In case of perforation, the
defect was closed with endoscopic clipping (EZ Clip;
Olympus, Tokyo, Japan). The resected area was not
closed as healing usually occurred in a few weeks
without complications.16
Histological assessment
All specimens were pinned on a piece of foam
and fixed in formalin. Histological type, depth of
invasion, as well as lateral and vertical resection
margins were recorded. En-bloc resection was
defined as one-piece resection of an entire lesion as
observed endoscopically. R0 resection was defined
as clear lateral and vertical resection margin.
Post–endoscopic submucosal dissection
All patients were allowed to resume a full diet on the
same day. We performed no routine blood tests or
imaging and patients were discharged the next day if
there were no signs of perforation or haemorrhage.
Postoperative haemorrhage was defined as clinical
evidence of bleeding manifested by melena or
haematochezia that required endoscopic haemostasis
within 0 to 14 days of the procedure.11
Follow-up
All patients were followed up in clinic 2 weeks
later to review the pathology report. Additional
surgery would be offered in case of carcinoma with
one of the following criteria: (1) margin involved;
(2) >1 mm submucosal invasion; (3) positive
lymphovascular permeation; (4) poorly differentiated
adenocarcinoma, signet ring cell carcinoma, or
mucinous carcinoma; or (5) high-grade tumour
budding.17 Surveillance colonoscopy was performed
1 year after ESD.
Statistical analysis
All continuous variables were described as median
and range. To study the learning curve, all patients
were grouped chronologically into two periods:
group 1 with cases 1 to 35; and group 2 with cases
36 to 71. Comparisons between non-parametric data
were done with Mann-Whitney U test, while Chi
squared test was used for categorical variables. A P
value of <0.05 was considered statistically significant.
Results
From March 2009 to December 2013, a total of 71
ESDs were performed. Characteristics of the patients
are shown in Table 1.
There was one conversion to laparoscopic
colectomy due to intra-operative bleeding that could
not be controlled endoscopically (40 mm x 15 mm
tumour at the transverse colon). For perforation,
there were 11 (15.5%) perforations: eight in the colon
and three in the rectum, all were noticed during the
ESD. Endoscopic clipping was successful in 10 of the
perforations. The median number of clips used was
2 (range, 1-6). One perforation required conversion
to laparoscopic colectomy (30 mm x 5 mm tumour
at the sigmoid colon). Both laparoscopic operations
were uneventful and patients were discharged
without any surgical complications. The overall
morbidity rate was 16.9% including bleeding and
perforation and there was no mortality. The median
postoperative stay was 1 day (range, 0-11 days).
Of the 69 patients who completed the
endoscopic procedure, en-bloc resection was
successful in 56 (81.2%) patients, of whom 19
(27.5%) required additional snare (SD-210U-25,
Snare Master; Olympus, Tokyo, Japan) to complete
the en-bloc resection. Conversion to piecemeal
resection by snare occurred in 14 (20.3%) patients.
In these 69 patients, the resection margin
was unclear in 15 patients as it was too close to the
cauterised edge. These, together with piecemeal
resection, were classified as R1 (29 patients in total).
R0 resection was achieved in 40 (58.0%) patients. The
histopathological diagnosis was tubular adenoma
for 26 (36.6%) tumours, tubulovillous adenoma for
28 (39.4%), villous adenoma for two (2.8%), serrated
adenoma for six (8.5%), carcinoid for one (1.4%) with
involved margin, intramucosal carcinoma for two
(2.8%), and carcinoma with submucosal invasion for
six (8.5%). The ESD procedure was considered curative
for the two patients with intramucosal carcinoma.
One patient who had submucosal carcinoma
refused further treatment because of a subsequent
diagnosis of primary lung cancer. All other patients
with submucosal carcinoma had curative interval
laparoscopic surgeries. There was no residual tumour
and no lymph node involvement found in the surgical
specimen for any of these patients. The patient with
rectal carcinoid also underwent subsequent interval
laparoscopic total mesorectal excision: the pathology
was well-differentiated carcinoid with invasion to
the muscularis propria. There was one lymph node
metastasis out of 11 lymph nodes retrieved.
Recurrence and surveillance colonoscopy
After excluding the six interval surgeries and
two conversions to laparoscopic colectomy, the
remaining 63 patients were offered colonoscopy
surveillance of whom four refused and one defaulted
from follow-up. Until August 2014, 51 patients had
undergone surveillance colonoscopy and seven
were awaiting colonoscopy. Recurrence of polyp
occurred in seven (13.7%) out of 51 patients: three
recurrences occurred after piecemeal resection,
another three recurrences occurred after additional
snare to complete the en-bloc resection. All three
cases had uncertain margin due to proximity to the
cauterised edge. In one patient, recurrence occurred
after successful en-bloc resection by ESD, in which
the deep margin was clear but the circumferential
margin was not certain.
Learning curve between the two chronological groups
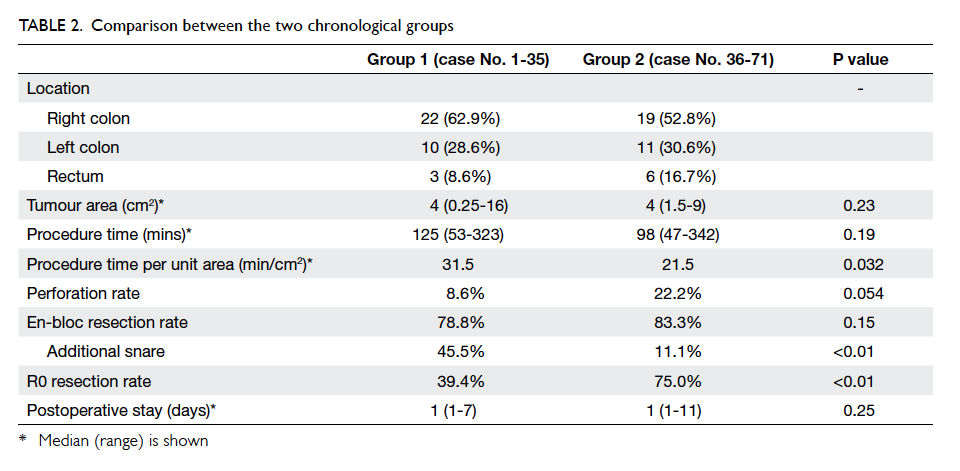
All patients were grouped chronologically into two
periods: group 1 with cases 1 to 35; and group 2 with
cases 36 to 71. The comparison between the two
groups is shown in Table 2. The median procedure
time per unit area of tumour improved significantly
from 31.5 min/cm2 to 21.5 min/cm2 (P=0.032).
There were three (8.6%) perforations in group
1; one of them required conversion to laparoscopic
colectomy. There were eight (22.2%) perforations
in group 2; all managed successfully by endoscopic
clipping. There was no significant difference in
perforation rate (P=0.054). The intra-operative
bleeding that required a conversion to laparoscopic
colectomy also belonged to group 1.
For the 33 patients in group 1 who completed
the endoscopic procedure, en-bloc resection was
successful in 26 (78.8%), while 30 (83.3%) out of 36
patients in group 2 had successful en-bloc resection.
This trend of improvement, however, did not reach
statistical significance (P=0.15). Among these
successful en-bloc resections, 15 (57.7%) out of 26
patients in group 1 and four (13.3%) out of 30 patients
in group 2 required additional snare to complete the
en-bloc resection (P<0.01).
R0 resection rate had improved significantly in
group 2 despite a lower rate of snare application: 13
(39.4%) of 33 patients in group 1 had R0 resection,
whereas in group 2, 27 (75.0%) of 36 patients had R0
resection (P<0.01).
The median postoperative stay was 1 day in
both groups and was not significantly different
(P=0.25). The median postoperative stay in patients
with perforation in group 1 and group 2 was 1 day
(range, 1-7 days) and 2 days (range, 1-11 days),
respectively (P=0.73).
Discussion
Colorectal ESD has been shown to be feasible and
safe when performed at expert centres in Japan.
In a recent prospective multicentre cohort study
involving 1111 patients in Japan, the reported
en-bloc resection rate was 88% and R0 resection rate
was 89%.11 The total perforation rate was only 5.3%
and postoperative bleeding was 1.5%.11 Nonetheless,
these excellent results are largely reported from
Japan. Colorectal ESD is technically difficult, as the
lumen is narrow and angulated and the very thin
wall presents a high risk of perforation. In Japan,
endoscopists are first required to gain experience
in gastric ESD, which is technically less demanding,
before they move on to colorectal ESD.18 This is not
possible in western and Asian countries outside
Japan, where early gastric cancer is much less
prevalent. In a recent review of 82 rectosigmoid
ESD from a tertiary centre in Germany, the en-bloc
resection rate and R0 resection rate was only 81.6%
and 69.7%, respectively.19 This reflects the difficulty
in generalising this novel procedure in other counties
outside Japan, and an even greater challenge to low-volume
district centres that lack local expertise.
The low case volume and the absence of expertise
in western countries leads to the development of
untutored colorectal ESD when it is impossible to
have a step-up approach in ESD training starting
from the stomach before proceeding to colon. The
reported learning curve for untutored colorectal
ESD has an acceptable outcome, however. Berr et al20 reported a case series of 48 colorectal ESDs with 76% en-bloc resection, 14% perforation, and 4%
requirement for surgical intervention. This compared
favourably with results in Japan.11 Another learning
curve study of colorectal ESD found procedure time
could be significantly shorter after 25 procedures.21
With a similar situation in Hong Kong, this
study showed that untutored colorectal ESD is
safe and feasible. Results demonstrated obvious
improvement after 35 procedures, as evidenced
by the significant reduction in combined ESD and
EMR with snare from 45.5% to 11.1%. Procedure
time per unit area of tumour as well as R0 resection
rate also significantly improved after 35 cases.
Although there were more perforations in group
2, it did not determine adverse outcome. None of
the perforations in group 2 required conversion to
laparoscopic colectomy. There was no significant
impact on hospital stay. The higher perforation
rate in group 2 may reflect a second learning curve
to perform a complete ESD procedure without
the assistance of a hybrid technique to achieve a
reasonable R0 resection rate. A perforation rate
comparable with the Japanese series11 is expected in
the third tranche of 35 patients.
Contrary to perforations in traditional
therapeutic colonoscopies, all perforations in
ESD were only 1 to 2 mm in size and were noticed
intra-operatively, thus immediate endoscopic
repair was possible. No patient required surgical
intervention solely for treatment of perforation.
The only conversion to laparoscopic colectomy in
the initial learning curve aimed to offer one-stop
treatment rather than treating perforations. In a
multicentre review of colonoscopic perforations by
Teoh et al,22 43 (0.113%) perforations were found
in 37 971 colonoscopies. Only seven (43.8%) out
of 16 therapeutic colonoscopic perforations were
noticed during the endoscopic procedure. The mean
size of perforation was 0.98 cm. The overall 30-day
morbidity and mortality rate was 48.7% and 25.6%,
respectively and the stoma rate was 38.5%. This
showed clearly that surgical outcome was much
worse in conventional colonoscopic perforations
compared with perforations in ESD, despite a much
higher perforation rate of 15.7% in ESD group.22
After implementation of the ESD service, the
following were noted:
(1) Venue of procedure—operating theatre was chosen instead of an endoscopic unit to enable conversion to conventional laparoscopic colectomy without the need to change location as well as the ready availability of an anaesthetist to give conscious sedation.
(2) Mode of anaesthesia—in the first few patients, we performed ESD under general anaesthesia for patient comfort and in the event conversion to laparoscopic colectomy was necessary. Positioning of patients was clumsy particularly when a prone position was needed to perform the procedure. Subsequently conscious sedation by an anaesthetist was used instead. Patients could follow instructions for positioning and deeper sedation could be achieved if necessary. There were no complaints from patients about any discomfort during the procedure.
(3) Choice of injecting agents—albumin 20% (Albumex 20; CSL, Australia) was used as submucosal injecting agent in the first few patients when sodium hyaluronate was not available. Albumin 20% has both a good cushioning effect without any inflammatory effect and the cost was much cheaper at HK$2.7/mL compared with commercially available sodium hyaluronate at HK$68/mL.23 Yet sodium hyaluronate has the longest lasting cushioning effect among all injecting agents. We recommend its use whenever available.
(4) A mixture of adrenaline saline and sodium hyaluronate was favourable for the assistant to inject and a lesser volume of sodium hyaluronate was required. Moreover, there were no instances of postoperative haemorrhage, although it was difficult to conclude whether this was due to the addition of adrenaline saline.
(5) Endoscopic technique—in our initial practice, we performed a full circumferential mucosal incision before submucosal dissection. We noticed it was technically more difficult compared with a two-third circumferential incision, because firstly, the submucosal elevation was lost quickly due to faster leakage of injecting agent, and secondly, it was difficult to retract the lesion at the end of dissection, and we had to complete the en-bloc resection with snare. After changing to two-third circumferential incision in the second period of the study, the need for additional snare to complete the en-bloc resection was significantly decreased (from 57.7% to 13.3%).
(6) Postoperative management—it was feasible and safe to resume diet immediately after the procedure and discharge patients the day following ESD without the need for routine blood taking or imaging. In one study from Japan, abdominal computed tomography was performed on day 1 and blood tests were carried out for 2 consecutive days. Oral intake was gradually stepped up and patients were discharged 5 days after ESD.10 In contrast, we allowed full diet on the same day after ESD and did not perform computed tomography or blood tests routinely. Our overall median postoperative stay was 1 day. Further development of ESD as a day procedure can be explored.
(1) Venue of procedure—operating theatre was chosen instead of an endoscopic unit to enable conversion to conventional laparoscopic colectomy without the need to change location as well as the ready availability of an anaesthetist to give conscious sedation.
(2) Mode of anaesthesia—in the first few patients, we performed ESD under general anaesthesia for patient comfort and in the event conversion to laparoscopic colectomy was necessary. Positioning of patients was clumsy particularly when a prone position was needed to perform the procedure. Subsequently conscious sedation by an anaesthetist was used instead. Patients could follow instructions for positioning and deeper sedation could be achieved if necessary. There were no complaints from patients about any discomfort during the procedure.
(3) Choice of injecting agents—albumin 20% (Albumex 20; CSL, Australia) was used as submucosal injecting agent in the first few patients when sodium hyaluronate was not available. Albumin 20% has both a good cushioning effect without any inflammatory effect and the cost was much cheaper at HK$2.7/mL compared with commercially available sodium hyaluronate at HK$68/mL.23 Yet sodium hyaluronate has the longest lasting cushioning effect among all injecting agents. We recommend its use whenever available.
(4) A mixture of adrenaline saline and sodium hyaluronate was favourable for the assistant to inject and a lesser volume of sodium hyaluronate was required. Moreover, there were no instances of postoperative haemorrhage, although it was difficult to conclude whether this was due to the addition of adrenaline saline.
(5) Endoscopic technique—in our initial practice, we performed a full circumferential mucosal incision before submucosal dissection. We noticed it was technically more difficult compared with a two-third circumferential incision, because firstly, the submucosal elevation was lost quickly due to faster leakage of injecting agent, and secondly, it was difficult to retract the lesion at the end of dissection, and we had to complete the en-bloc resection with snare. After changing to two-third circumferential incision in the second period of the study, the need for additional snare to complete the en-bloc resection was significantly decreased (from 57.7% to 13.3%).
(6) Postoperative management—it was feasible and safe to resume diet immediately after the procedure and discharge patients the day following ESD without the need for routine blood taking or imaging. In one study from Japan, abdominal computed tomography was performed on day 1 and blood tests were carried out for 2 consecutive days. Oral intake was gradually stepped up and patients were discharged 5 days after ESD.10 In contrast, we allowed full diet on the same day after ESD and did not perform computed tomography or blood tests routinely. Our overall median postoperative stay was 1 day. Further development of ESD as a day procedure can be explored.
There are a number of limitations in this
study. First, this was the learning curve of a single
endoscopist and 35 procedures may not be a typical
number required for such a learning curve. Second,
the inclusion criteria for colorectal ESD were less
strict than those in Japan. Lesion less than 2 cm that
could not be removed en bloc would be subjected
to ESD instead of piecemeal resection. These kinds
of lesion were expected to be easier with shorter
operating time. Third, this was a retrospective
comparison of two chronological groups which might not have been directly comparable. Lastly, the
7.9% patient default rate may lead to underestimation
of recurrence in this series.
Conclusion
Untutored colorectal ESD at a low-volume centre
was an option in the absence of enough experts to
supervise the procedure. Training on an animal
model and clinical observation of real-time
demonstrations was useful to start ESD without
supervision. A cut-off at 35 procedures showed
an acceptable R0 resection rate at a significantly
improved procedure time per unit area. There was
a second learning curve to achieve a complete ESD
procedure without EMR at a higher perforation rate.
Colorectal ESD performed by a colorectal surgeon
enables any complications to be managed by the
same operator, or any lesion unresectable by ESD to
be surgically removed. It was not necessary to first
perform gastric ESD as the start of ESD training.
When more endoscopists have gained experience
in colorectal ESD, a structured training programme
with accreditation can be established.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Tanaka S, Haruma K, Oka S, et al. Clinicopathological
features and endoscopic treatment of superficially
spreading colorectal neoplasms larger than 20 mm.
Gastrointest Endosc 2001;54:62-6. Crossref
2. Walsh RM, Ackroyd FW, Shellito PC. Endoscopic resection
of large sessile colorectal polyps. Gastrointest Endosc
1992;38:303-9. Crossref
3. Uraoka T, Fujii T, Saito Y, et al. Effectiveness of glycerol
as a submucosal injection for EMR. Gastrointest Endosc
2005;61:736-40. Crossref
4. Yamamoto H, Kawata H, Sunada K, et al. Successful en-bloc
resection of large superficial tumors in the stomach and
colon using sodium hyaluronate and small-caliber-tip
transparent hood. Endoscopy 2003;35:690-4. Crossref
5. Fujishiro M, Yahagi N, Kakushima N, et al. Outcomes of
endoscopic submucosal dissection for colorectal epithelial
neoplasms in 200 consecutive cases. Clin Gastroenterol
Hepatol 2007;5:678-83. Crossref
6. Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome
of endoscopic submucosal dissection versus endoscopic
mucosal resection of large colorectal tumors as determined
by curative resection. Surg Endosc 2010;24:343-52. Crossref
7. The Paris endoscopic classification of superficial neoplastic
lesions: esophagus, stomach, and colon: November 30
to December 1, 2002. Gastrointest Endosc 2003;58(6
Suppl):S3-43.
8. Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society
for Cancer of the Colon and Rectum (JSCCR) guidelines
2010 for the treatment of colorectal cancer. Int J Clin Oncol
2012;17:1-29. Crossref
9. Yoda Y, Ikematsu H, Matsuda T, et al. A large-scale
multicenter study of long-term outcomes after endoscopic
resection for submucosal invasive colorectal cancer.
Endoscopy 2013;45:718-24. Crossref
10. Yoshida N, Wakabayashi N, Kanemasa K, et al. Endoscopic
submucosal dissection for colorectal tumors: technical
difficulties and rate of perforation. Endoscopy 2009;41:758-61. Crossref
11. Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective,
multicenter study of 1111 colorectal endoscopic
submucosal dissections (with video). Gastrointest Endosc
2010;72:1217-25. Crossref
12. Taku K, Sano Y, Fu KI, et al. Iatrogenic perforation
associated with therapeutic colonoscopy: a multicenter
study in Japan. J Gastroenterol Hepatol 2007;22:1409-14. Crossref
13. Sano Y, Muto M, Tajiri H, et al. Optical/digital
chromoendoscopy during colonoscopy using narrow-band
image system. Dig Endosc 2005;17(Suppl):S43-8. Crossref
14. Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary
pattern type IIIA/IIIB by magnifying narrow band imaging
for estimating depth of invasion of early colorectal
neoplasms. BMC Gastroenterol 2010;10:33. Crossref
15. Machida H, Sano Y, Hamamoto Y, et al. Narrow-band
imaging in the diagnosis of colorectal mucosal lesions: a
pilot study. Endoscopy 2004;36:1094-8. Crossref
16. Iguchi M, Yahagi N, Fujishiro M, et al. The healing process
of large artificial ulcers in the colorectum after endoscopic
mucosal resection [abstract]. Gastrointest Endosc
2003;57:AB226.
17. Asano M. Endoscopic submucosal dissection and surgical
treatment for gastrointestinal cancer. World J Gastrointest
Endosc 2012;4:438-47. Crossref
18. Tanaka S, Tamegai Y, Tsuda S, Saito Y, Yahagi N, Yamano
HO. Multicenter questionnaire survey on the current
situation of colorectal endoscopic submucosal dissection
in Japan. Dig Endosc 2010;22 Suppl 1:S2-8. Crossref
19. Probst A, Golger D, Anthuber M, Märkl B, Messmann H.
Endoscopic submucosal dissection in large sessile lesions
of the rectosigmoid: learning curve in a European center.
Endoscopy 2012;44:660-7. Crossref
20. Berr F, Wagner A, Kiesslich T, Friesenbichler P, Neureiter
D. Untutored learning curve to establish endoscopic
submucosal dissection on competence level. Digestion
2014;89:184-93. Crossref
21. Białek A, Pertkiewicz J, Karpińska K, Marlicz W, Bielicki
D, Starzyńska T. Treatment of large colorectal neoplasms
by endoscopic submucosal dissection: a European single-center
study. Eur J Gastroenterol Hepatol 2014;26:607-15. Crossref
22. Teoh AY, Poon CM, Lee JF, et al. Outcomes and predictors
of mortality and stoma formation in surgical management
of colonoscopic perforations: a multicenter review. Arch
Surg 2009;144:9-13. Crossref
23. ASGE Technology Committee, Kantsevoy SV,
Adler DG, Conway JD, et al. Endoscopic mucosal resection
and endoscopic submucosal dissection. Gastrointest
Endosc 2008;68:11-8. Crossref