Hong Kong Med J 2016 Jun;22(3):242–8 | Epub 6 May 2016
DOI: 10.12809/hkmj154588
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
The effect of anticonvulsant use on bone mineral density in non-ambulatory children with cerebral palsy
SW Cheng, DPD (Cardiff), MRCPCH;
CH Ko, FHKAM (Paediatrics), FRCP (Glasg);
CY Lee, FHKAM (Paediatrics), FRCP (Edin)
Department of Paediatrics and Adolescent Medicine, Caritas Medical Centre, Shamshuipo, Hong Kong
Corresponding author: Dr SW Cheng (shirley.s.cheng@gmail.com)
Abstract
Introduction: Studies showed that use of
anticonvulsants (antiepileptic drugs) might be
associated with reduced bone mineral density. The
primary objective of this study was to evaluate the
effect of anticonvulsants on bone mineral density in
non-ambulatory children with cerebral palsy. The
secondary objective was to identify their risk factors
for low bone mineral density.
Methods: This case series with internal comparisons
was conducted in a paediatric residential
rehabilitation centre in Hong Kong. Overall, 32
patients were enrolled. The study group comprised
18 patients (6 males, 12 females) aged 5.0 to 19.5
years (mean ± standard deviation, 13.8 ± 4.7 years);
all were prescribed anticonvulsant therapy for more
than 2 years. The comparison group comprised
14 patients (6 males, 8 females) aged 7.0 to 19.1
years (mean, 16.4 ± 3.0 years) who were concomitant non-ambulatory residents
with cerebral palsy and were not prescribed any
anticonvulsant therapy prior to study recruitment.
Patients underwent a physical examination, blood
tests, nutritional assessment, and dual-energy X-ray
absorptiometry scan of the total body less head. Z-scores
were calculated.
Results: There was no significant difference in Z-scores
of total body less head between groups. Among children with low bone mineral density
(Z-scores ≤–2.0) and normal bone mineral density,
multivariate analysis revealed that higher weight-for-age
Z-score (adjusted odds ratio=0.015) and
presence of puberty (adjusted odds ratio=0.027)
were independent factors for bone mineral density
improvement. Hosmer-Lemeshow goodness of fit test
(P=0.315) was not significant. Nagelkerke R2 was
0.677, signifying a relatively well-fitting model.
Conclusion: There was no evidence that
anticonvulsant therapy has any detrimental effect
on bone mineral density in non-ambulatory children
with cerebral palsy. A low weight-for-age Z-score
was associated with low bone mineral density. Early
nutritional intervention to optimise body weight
may help to increase bone mineral density.
New knowledge added by this study
- Anticonvulsant use shows no significant and detrimental impact on bone mineral density (BMD) of non-ambulatory cerebral palsy children.
- A low weight-for-age Z-score was found to be a significant independent risk factor predictive of low BMD.
- Optimal pharmacological treatment for epilepsy control can be pursued without jeopardising bone mineralisation in non-ambulatory cerebral palsy children.
- Optimising nutritional status in this group of children is important in improving bone mineralisation and thus decreasing pathological fracture.
Introduction
Osteoporosis is a skeletal disorder characterised by
low bone mass and micro-architectural deterioration
of bone tissue that results in compromised bone
strength and a predisposition to fracture.1 Childhood
and adolescence are critical periods for bone
mineralisation. Peak bone mineral density (BMD)
achieved by early adulthood determines the risk of
pathological fracture. Bone mass can be objectively
measured by BMD and is correlated with risk of
osteoporotic fracture.2
Dual-energy X-ray absorptiometry (DXA)
scanning enables cost-effective quantitative
measurement of BMD. Such methodology is the gold
standard because it can detect bone mineral loss
of 2% to 5%. Abnormal homeostasis of vitamin D,
calcium, and phosphorous may lead to imbalanced
osteoclastic and osteoblastic dynamics that will
result in osteopenia and even osteoporosis. There
have been reports from large- and small-scale studies
of problems in bone mineralisation and vitamin D or
calcium metabolism in patients with cerebral palsy
(CP).3 4 Dietary restrictions, oromotor dysfunction, malabsorption, and limited sunlight exposure may
lead to poor nutrition, low calcium intake, or altered
vitamin D metabolism. These in turn contribute
to poor mineralisation. Limited weight-bearing
during the period of skeletal growth may also
lead to reduced BMD. Chronic therapy with antiepileptic
drugs (AEDs) may cause hypocalcaemia,
hypophosphataemia, raised serum alkaline
phosphatase, elevated serum parathyroid hormone,
reduced biologically active vitamin D metabolites,
rickets, and osteomalacia. The mechanism is unclear,
however.5
A study revealed that AED use was associated
with reduced BMD in 35 pairs of twin adults, and the
effect was more marked in those prescribed enzyme-inducing
AEDs, for example, phenytoin and
phenobarbitone.6 A decrease in BMD (in g/cm2) of
6.4% and 4.6% at the lumbar spine and femoral neck
respectively in their twin control pair was detected.
For valproate, the effect was not consistently
demonstrated.7 8
Chronic treatment with AEDs was observed to
be significantly correlated with a lower BMD in 96
ambulatory children and young adults with epilepsy
with a mean Z-score of -1.23 compared with 0.16 of a
control group.9 In our retrospective study of 109 CP
children in 2006, however, we could not demonstrate
any association of AEDs with increased fracture
rate in non-ambulatory CP children.10 In children
with CP and a Gross Motor Function Classification System (GMFCS)
level of 4 to 5 (bed-bound), the possible
impact of anticonvulsants on BMD was not clearly
demonstrated.
The primary objective of this study was
to evaluate the effect of AEDs on BMD in non-ambulatory
children with CP. The secondary
objective was to identify the risk factors for low
BMD in this group of children.
Methods
Subjects
Patients at the Developmental Disabilities Unit
(DDU) of Caritas Medical Centre, Hong Kong were
enrolled in the study from 1 October 2012 to 30
September 2013. The DDU is the largest long-term
care facility for children with severe developmental
disabilities and special health care needs in Hong
Kong. Among the 100 residents, over 90% were non-ambulatory
and over 50% had CP.
The inclusion criteria in this study were: (1)
children and adolescents aged between 5 and 19
years; (2) GMFCS level 4 or 5; (3) the presence of
spastic or mixed spastic dyskinetic CP; and (4)
prescribed AED for more than 2 years.
The control group without anticonvulsant
therapy comprised concomitant non-ambulatory
DDU residents with CP who were not prescribed
AEDs for more than 2 years prior to recruitment to
the study.
The exclusion criteria included (1) GMFCS
level of 1 to 3; (2) patients with underlying hepatic
or renal disease; (3) presence of disease primarily
involving bone metabolism or a family history of
bone metabolic disorders; (4) known thyroid or
parathyroid disease; (5) history of pathological
long bone fracture; (6) history of chronic diarrhoea
or malabsorption; (7) long-term intake of the
following medications: non-physiological dose of
glucocorticoid, anabolic steroid, vitamin A, non-steroidal
anti-inflammatory drugs, bisphosphonates,
thiazide diuretics, or calcitonin.
Sample size estimation
Coppola et al9 reported a mean BMD Z-score
of -1.23 and 0.16 in 96 ambulatory
epileptic patients and 63 controls, respectively. In 2012, the same
group showed the mean Z-score to be -1.69 (n=47)
and -0.83 (n=40) respectively in mentally retarded
children with CP, with and without epilepsy.11
Based on these findings, we proposed a difference
in Z-score of ≥1 to indicate a clinically significant
reduction in BMD. In order to detect a Z-score
difference of 1 with power of 0.8, 16 subjects were
recruited in each group.12
Data collection and determination of bone mineral density
Upon entry, data were collected for age, gender,
body weight, body height, body mass index (BMI),
presence of contractures, skinfold thickness (triceps,
lower biceps, and subscapular), and pubertal stage
according to Tanner’s classification. Weight-for-age
Z-score was calculated based on referenced
data from a 1993 territory-wide growth survey of
Hong Kong children.13 Blood taking was performed
on the same day of DXA scan to evaluate calcium,
phosphorous, alkaline phosphatase, transaminase,
total protein, urea, creatinine, serum osmolality, and
total cell count. A spot urine calcium-to-creatinine
ratio (mmol/mmol) and urine calcium-to-osmolality
ratio (mg/L:mosmo/kg) was also determined on the
same day. Current dietary information was reviewed
and daily calcium and vitamin D content were
calculated by a dietitian.
In this study, BMD was determined by DXA of
total body less head (TBLH) using a Lunar Prodigy
Advance DXA bone densitometer (GE Healthcare,
Madison [WI], US) with the latest Chinese children
total BMD database installed.14 15 All subjects had BMD measured by the same validated densitometer
to eliminate measurement error. Individual BMD
value was expressed as g/cm2 and Z-score. Z-score
is the number of standard deviations of the patient’s
BMD above or below the average age- and sex-matched
reference value. For the paediatric age-group,
the current definition for osteoporosis includes BMD
Z-score of ≤–2.0 adjusted for age, gender, and body
size plus a clinically significant fracture defined in
the International Society for Clinical Densitometry
(ISCD) 2007 official positions.16 The head is
disproportionately large in young children and may
mask deficits at other skeletal sites, thus TBLH
(subtotal/TBLH) BMD, which was recommended
by ISCD 2007 official positions for the evaluation
of child’s bone health, was used in this study. For
children with CP, TBLH BMD is more feasible and
provides a holistic picture for BMD estimation.
Statistical analyses
Effect of antiepileptic drugs on bone mineral density in non-ambulatory children with cerebral palsy
In bivariate analysis, normally and non-normally
distributed data were analysed by independent
sample t tests and Mann-Whitney U tests,
respectively. Categorical data were compared using
Chi squared and Fisher’s exact tests. Clinically
relevant covariates (presence of puberty, daily
calcium intake, and number of AED use) and the
covariate (weight-for-age Z-score) approaching
statistical significance (P<0.05) in bivariate analysis
were entered into multivariable analysis (Enter
Method). Statistical significance was defined as two-tailed
probability below 0.05.
Risk factors for low bone mineral density in non-ambulatory children with cerebral palsy
Data of TBLH were split into low-BMD group versus
normal-BMD group—those with TBLH Z-score of ≤–2.0 were considered to have low BMD. Baseline
characteristics of the low- and normal-BMD groups
were compared. Multivariable logistic regression
analysis was used to examine the independent effect
of puberty, weight-for-age Z-score (gender adjusted),
number of AEDs, and daily calcium intake on TBLH
Z-score.
All statistical analyses were performed using
the Statistical Package for the Social Sciences
(Windows version 13.0; SPSS Inc, Chicago [IL], US).
The study was approved by the Ethics Committee
of Kowloon West Cluster of Hospital Authority,
Hong Kong. Informed consent was obtained from
respective parents or the legal guardians from the
Social Welfare Department, Government of the
Hong Kong Special Administrative Region.
Results
Effect of antiepileptic drugs on bone mineral density in non-ambulatory children with cerebral palsy
The subjects were enrolled from 1 October 2012 to 30
September 2013 in DDU. Sixty-two children with CP
aged 5 to 19 years were identified. Informed consent
was obtained from a legal guardian for 44 patients
of whom 12 were excluded from study—four had a
history of fracture, two had severe deformity making
DXA technically difficult, one had poorly controlled
asthma and required long-term steroid therapy, and
five had deranged liver or renal function. Of the
remaining 32 children and adolescents, 18 (6 males, 12 females) aged 5.0 to 19.5 (mean ± standard deviation, 13.8 ± 4.7) years comprised the study group. The control group
consisted of 14 children and adolescents (6 males, 8
females) aged 7.0 to 19.1 (mean, 16.4 ± 3.0) years.
Among the 18 subjects in the study group, eight
were prescribed one AED and 10 were prescribed
two or more. Sodium valproate was prescribed to
eight children, carbamazepine to three, topiramate to
three, phenobarbitone to five, phenytoin to one, and
lamotrigine to four. In view of the limited population
size, the impact of individual anticonvulsants was
not analysed.
The two groups showed no significant
difference in age, gender, BMI, weight-for-age Z-score,
CP type, daily calcium intake, or daily vitamin
D intake (Table 1).
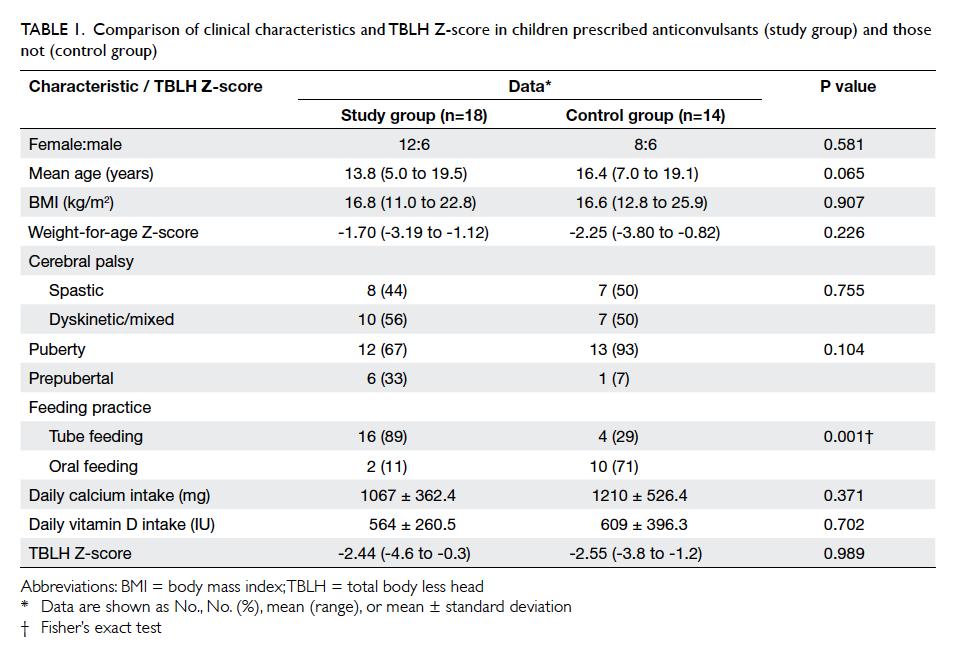
Table 1. Comparison of clinical characteristics and TBLH Z-score in children prescribed anticonvulsants (study group) and those not (control group)
Twelve (67%) patients in the study group were
in puberty, compared with 13 (93%) patients in the
control group (P=0.104). Sixteen (89%) patients in
the study group were tube feeders compared with
four (29%) in the control group (P=0.001). Among
the oral feeders, all were reported by their caretaker
to have a satisfactory dietary intake. Mean weight-for-age
Z-score showed no significant difference
between the study and control groups (P=0.226)
[Table 1]. There was also no significant difference in
the TBLH Z-scores (P=0.989; Table 1) or biochemical
parameters of bone metabolism between the two
groups (Table 2).
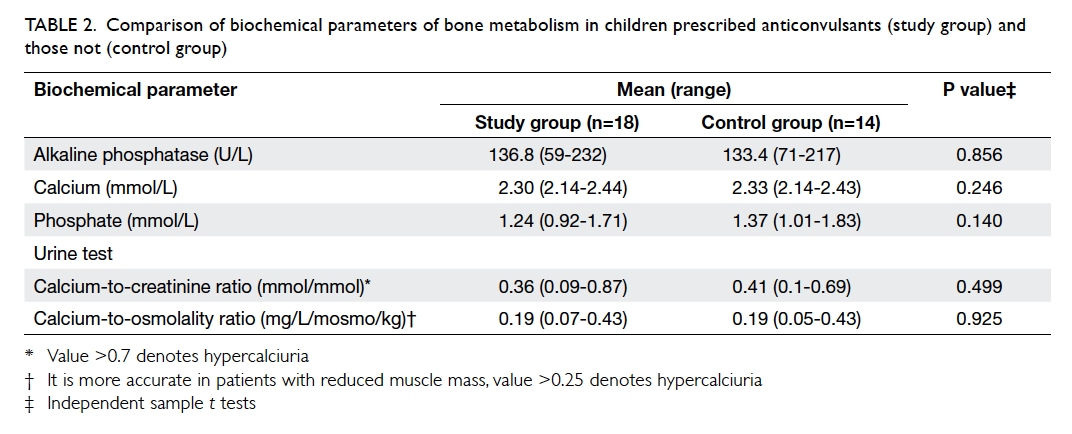
Table 2. Comparison of biochemical parameters of bone metabolism in children prescribed anticonvulsants (study group) and those not (control group)
Low BMD was defined as TBLH Z-score of
≤–2.0 and was evident in 18 (56%) patients. When
comparing groups with low and normal BMD, there
was no significant difference on univariate analysis
in gender, age, CP, BMI, feeding mode, use of AEDs,
puberty, or daily calcium and vitamin D intake
(Table 3). Weight-for-age Z-score was significantly
lower (-2.57 vs -1.12; P=0.001) in the low BMD
group (Table 3).
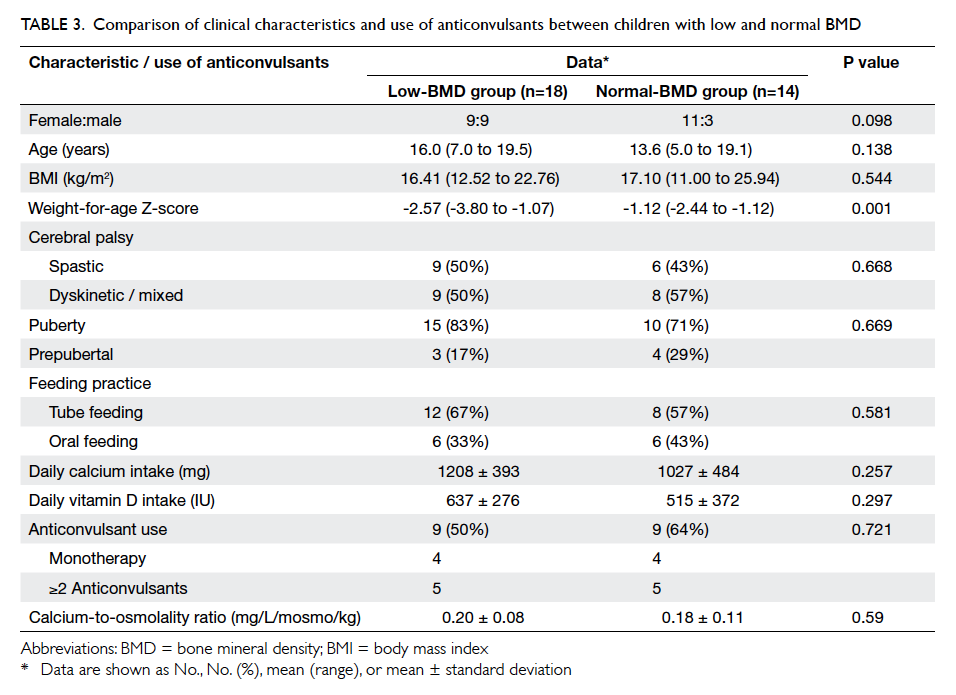
Table 3. Comparison of clinical characteristics and use of anticonvulsants between children with low and normal BMD
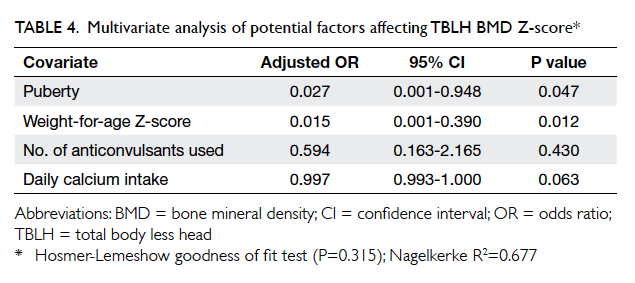
When covariates including weight-for-age
Z-score, presence of puberty, daily calcium intake,
and number of AEDs used were analysed by
multiple logistic regression, higher weight-for-age Z-score
(adjusted odds ratio [OR]=0.015; 95% confidence
interval [CI], 0.001-0.390) and presence of puberty
(adjusted OR=0.027; 95% CI, 0.001-0.948) remained
significant independent predictors for occurrence
of a relatively normal BMD (ie TBLH Z-score >2.0). Hosmer-Lemeshow goodness of fit test (P=0.315) was non-significant,
indicating a model prediction that
was not significantly different to observed values.
Nagelkerke R2 was 0.677, again signifying a relatively
well-fitting model (Table 4).
Discussion
In the present study, DXA evaluation in non-ambulatory
children with CP, with or without
AED use, revealed a low BMD in 56% of those
who underwent DXA. This is concordant with the
findings in previous studies4 17 18 19 with prevalence of
low BMD ranging from 27% to 77%. Low BMD is the
proximate cause of low-energy fracture in this group
of children. Other factors such as stiff joints, poor
balance leading to falls, and violent seizures may
also contribute. Nearly 20% of such children have
sustained a femoral fracture at some point.20
The impact of AED on BMD was unclear in
non-ambulatory children with CP. The physician
often faces a dilemma in optimising anticonvulsant
treatment in this group of children who are already
at high risk of developing osteoporotic long bone
fracture.
Early studies revealed that treatment with
enzyme-inducing anticonvulsants—for example,
phenytoin and phenobarbitone—may induce
catabolism of 25-hydroxyvitamin D, leading to
rickets.21 22 Recent research on the effect of AED on BMD is inconclusive, and the results are often
confounded by methodological flaws. In a cross-sectional
study, Farhat et al5 measured the BMD in
71 ambulatory subjects who were prescribed AED
for more than 6 months. Reduced BMD was found in
adults (n=42) but not children (n=29). There was no
control group and the findings were only compared
with paediatric data from the manufacturer,
rendering it difficult to detect any small but significant
difference secondary to AED treatment. Ecevit et al23
measured femoral neck BMD in 31 healthy children
and 33 subjects with idiopathic epilepsy treated with
either carbamazepine (n=17) or valproate (n=16).
Valproate but not carbamazepine monotherapy was
associated with a significant reduction in BMD. In
this study, however, more children had attained
puberty in the control group (n=13, 93%) than in the
AED group (n=12, 67%), which may have confounded
the results. Coppola et al9 compared the BMD of 96
children with epilepsy alone or in association with
CP and/or mental retardation against a control group
of 63 healthy ambulatory subjects. While univariate
analysis illustrated a reduced BMD Z-score in the
AED group, no difference was found after adjustment
for ambulatory status, mental retardation, lack of
physical activity, and BMI in multivariate analysis.
In this study, we focused on non-ambulatory
children with CP and severe mental retardation. This
is the population most vulnerable to pathological
long bone fracture, with a lifetime risk estimated
to be 20%. This group of children is also prone to
develop refractory epilepsy that requires multiple
AEDs at high doses. By recruiting concomitant
residents from the same facility as the control group,
we minimised the confounding effect of factors that
may contribute to low BMD, namely ambulatory
status, physical activity, and nutritional status. Our
findings support that long-term use of AED in this
group of children does not have any significant
adverse effect on BMD. Optimal pharmacological
treatment can be pursued without jeopardising bone
mineralisation.
In multivariable analysis, there was a significant
association of BMD Z-score with the presence of
puberty. This may be due to the physiology and time
frame for bone mineralisation that surges in puberty
and reaches a peak at 20 years of age. Variation in
time of starting puberty was a confounding factor as
BMD Z-score was adjusted for gender and age only.
This study was carried out in a single institution
for disabled children in Hong Kong. Because of
the need for sedation and exposure to radiation,
guardians of children with more severe neurological
disease tended not to consent for the study.
Moreover, DXA could not be properly performed in
patients with severe deformity. These children were
not included in the study.
An inadequate number of patients in each
group may have impacted the power of this study.
Nevertheless, the result showed a strong association
for weight-for-age Z-score with TBLH BMD. We
believe the association was highly statistically
significant.
Children with CP are at risk of malnutrition
that has a significant impact on linear growth,
wound recovery, motor function, and even survival.
In addition to poor linear growth, malnourished
children with severe CP are likely to have poor
bone mineralisation leading to painful pathological
fracture. In a retrospective study of 107 CP children
(90 non-ambulatory), weight-for-age Z-score
proved to be the best predictor of BMD Z-score.24
In the present study, we demonstrated a significant
correlation of low weight-for-age Z-score with
low BMD, with a relatively well-fitting model after
adjusting for pubertal stage, calcium intake, and
AED use. This is concordant with our previous
findings in the same population in which there was
a significant protective effect of increase in weight-for-age Z-score in reducing fracture risk (adjusted
OR=0.41).10 A recent review of 32 cross-sectional,
cohort, case-control, and randomised controlled
trials suggested that reduced bone density, impaired
bone growth, and vitamin D deficiency may be
seen in children treated with anticonvulsants.25 In
our study, individual vitamin D daily intake was
calculated by dietitians. Sun exposure was limited
in the institutional setting. Thus, we assumed that
the calculated daily oral vitamin D intake correlated
well with the serum vitamin D level. Although the
daily vitamin D intake was similar in the low- and
normal-BMD groups (P=0.297) as well as the study
and control groups (P=0.702), 10 out of 32 subjects
in the study consumed less than the recommended
daily vitamin D intake (400 IU/day). To improve
BMD and decrease fracture risk, regular nutritional
assessment is necessary to identify malnourished
children. Supplementation of vitamin D and
calcium should be considered if the calculated daily
need cannot be met. A nutritional rehabilitation
programme should be implemented to optimise
weight-for-age Z-score. Early gastrostomy tube
placement should be considered in children with
poor oral feeding. In children with weight-for-age Z
score of ≤–2.0, serial BMD measurements can help to
identify severe osteoporosis before fracture occurs.
This window of time allows implementation of
intensive nutritional rehabilitation, pharmacological
intervention, and precautions in handling to prevent
fracture occurrence.
Conclusion
Use of AED in non-ambulatory children with CP is
unlikely to pose a detrimental impact on BMD. A low
weight-for-age Z-score is a significant independent
risk factor predictive of low BMD. Optimising
nutritional status in this group of children is
paramount to improving bone mineralisation and
thus decreasing pathological fracture.
Acknowledgement
The authors would like to thank Ms Geraldine Ng,
dietitian of Caritas Medical Centre, for her arduous
effort to assess dietary calcium and vitamin D intake
for our study patients.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Houlihan CM, Stevenson RD. Bone density in cerebral
palsy. Phys Med Rehabil Clin N Am 2009;20:493-508. Crossref
2. Marshall D, Johnell O, Wedel H. Meta-analysis of how well
measures of bone mineral density predict occurrence of
osteoporotic fractures. BMJ 1996;312:1254-9. Crossref
3. Lee JJ, Lyne ED, Kleerekoper M, Logan MS, Belfi RA.
Disorders of bone metabolism in severely handicapped
children and young adults. Clin Orthop Relat Res
1989;245:297-302. Crossref
4. Henderson RC, Lark RK, Gurka MJ, et al. Bone density and
metabolism in children and adolescents with moderate to
severe cerebral palsy. Pediatrics 2002;110:e5. Crossref
5. Farhat G, Yamout B, Mikati MA, et al. Effect of antiepileptic
drugs on bone density in ambulatory patients. Neurology
2002;58:1348-53. Crossref
6. Petty SJ, Paton LM, O’Brien TJ, et al. Effect of antiepileptic
medication on bone mineral measures. Neurology
2005;65:1358-65. Crossref
7. Sheth RD, Wesolowski CA, Jacob JC, et al. Effect of
carbamazepine and valproate on bone mineral density. J
Pediatr 1995;127:256-62. Crossref
8. Triantafyllou N, Lambrinoudaki I, Armeni E, et al. Effect of
long-term valproate monotherapy on bone mineral density
in adults with epilepsy. J Neurol Sci 2010;290:131-4. Crossref
9. Coppola G. Fortunato D, Auricchio G, et al. Bone mineral
density in children, adolescents, and young adults with
epilepsy. Epilepsia 2009;50:2140-6. Crossref
10. Ko CH, Tse PW, Chan AK. Risk factors of long bone
fracture in non-ambulatory cerebral palsy children. Hong
Kong Med J 2006;12:426-31.
11. Coppola G, Fortunato D, Mainolfi C, et al. Bone mineral
density in a population of children and adolescents with
cerebral palsy and mental retardation with or without
epilepsy. Epilepsia 2012;53:2172-7. Crossref
12. Portney LG, Watkins MP. Foundations of clinical research
applications to practice. 3rd ed. Upper Saddle River, NJ:
Pearson/Prentice Hall; 2009: 832-47.
13. Leung SS, Lau JT, Tse LY, Oppenheimer SJ. Weight-for-age
and weight-for-height references for Hong Kong children
from birth to 18 years. J Paediatr Child Health 1996;32:103-9. Crossref
14. Xu H, Chen JX, Gong J, et al. Normal reference for bone
density in healthy Chinese children. J Clin Densitom
2008;10:266-75. Crossref
15. Guo B, Xu Y, Gong J, Tang Y, Xu H. Age trends of bone
mineral density and percentile curves in healthy Chinese
children and adolescents. J Bone Miner Metab 2013;31:304-14. Crossref
16. Lewiecki EM, Gordon CM, Baim S, et al. International
Society for Clinical Densitometry 2007 Adult and Pediatric
Official Positions. Bone 2008;43:1115-21. Crossref
17. Hartman C, Brik R, Tamir A, Merrick J, Shamir R. Bone
quantitative ultrasound and nutritional status in severely
handicapped institutionalized children and adolescents.
Clin Nutr 2004;23:89-98. Crossref
18. King W, Levin R, Schmidt R, Oestreich A, Heubi JE.
Prevalence of reduced bone mass in children and adults
with spastic quadriplegia. Dev Med Child Neurol
2003;45:12-6. Crossref
19. Ali O, Shim M, Fowler E, Cohen P, Oppenheim W. Spinal
bone mineral density, IGF-1 and IGFBP-3 in children with
cerebral palsy. Horm Res 2007;68:316-20. Crossref
20. Sturm PF, Alman BA, Christie BL. Femur fractures in
institutionalized patients after hip spica immobilization. J
Pediatr Orthop 1993;13:246-8.
21. Crosley CJ, Chee C, Berman PH. Rickets associated with
long-term anticonvulsants therapy in a pediatric outpatient
population. Pediatrics 1975;56:52-7.
22. Keck E, Gollnick B, Reinhardt D, Karch D, Peerenboom
H, Krüskemper HL. Calcium metabolism and vitamin
D metabolite levels in children receiving anticonvulsant
drugs. Eur J Pediatr 1982;139:52-5. Crossref
23. Ecevit C, Aydoğan A, Kavakli T, Altinöz S. Effect of
carbamazepine and valproate on bone mineral density.
Pediatr Neurol 2004;31:279-82. Crossref
24. Henderson RC, Kairalla J, Abbas A, Stevenson RD.
Predicting low bone density in children and young adults
with quadriplegic cerebral palsy. Dev Med Child Neurol
2004;46:416-9. Crossref
25. Vestergaard P. Effects of antiepileptic drugs on bone health
and growth potential in children with epilepsy. Pediatr
Drugs 2015;17:141-50. Crossref