DOI: 10.12809/hkmj154783
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
COMMENTARY
NUDT15 variant and thiopurine-induced
leukopenia in Hong Kong
Felix CK Wong, MB, BS, MResMed1; Alex WK Leung, MB, ChB, FHKAM (Paediatrics)2; Jeffrey SS Kwok, FHKCPath, FHKAM (Pathology)1; Michael HM Chan, FHKCPath, FHKAM (Pathology)1;
CK Li, MD, FHKAM (Paediatrics)2; YP Yuen, FHKCPath, FHKAM (Pathology)1
1 Department of Chemical Pathology, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong
2 Department of Paediatrics, Prince of Wales Hospital, The Chinese University of Hong Kong,
Shatin, Hong Kong
Corresponding author: Dr YP Yuen (lizyuenyp@cuhk.edu.hk)
Thiopurines, including azathioprine and
6-mercaptopurine (6-MP), are widely used in the
treatment of autoimmune diseases and cancers, as well
as prevention of rejection in organ transplantation.
Azathioprine is a pro-drug that is converted to 6-MP,
and subsequently undergoes extensive metabolism
to the formation of 6-thioguanine nucleotides
(6-TGNs). Such 6-TGNs exert their therapeutic
effect by inducing apoptosis of T lymphocytes.1
Myelosuppression, manifesting as a reduction in
one or more of the haematopoietic lineages (most
commonly leukopenia), is a serious adverse drug
reaction related to the excessive generation of 6-TGNs.2 Thiopurine S-methyltransferase (TPMT)
diverts 6-MP from the formation of 6-TGNs
by converting 6-MP into inactive metabolites.
Thus, TPMT deficiency plays a causal role in the
pathogenesis of thiopurine-induced leukopenia
by shunting thiopurine metabolites towards the
formation of excessive 6-TGNs. Genetic variants
present in the TPMT gene result in TPMT deficiency
and the trait is inherited in an autosomal co-dominant
manner. TPMT*1 represents the wild-type allele
with normal TPMT activity while *2, *3A, *3B, *3C,
and *8 account for approximately 95% of all TPMT
variants known to result in TPMT deficiency.3 With
the conventional dose of thiopurines, individuals
who have inherited two copies of the inactive TPMT
allele (homozygous deficient) experience severe
myelosuppression. A significant proportion (30%-60%) of individuals who have inherited one copy of
the inactive TPMT allele (heterozygous deficient)
develop moderate-to-severe myelosuppression.
Those who carry two wild-type TPMT alleles have
the least myelosuppression. Prospective TPMT
genotyping has been recommended by the US Food
and Drug Administration.4 5 In addition, guidelines
on TPMT genotype–based dosage recommendations
are currently available that include a reduced
thiopurine starting dose or use of an alternative
non-thiopurine treatment in individuals who carry
defective TPMT allele(s).6 7
In Hong Kong, many patients are prescribed
thiopurine without prospective TPMT genotyping,
largely because of the low frequency of TPMT
variants in the Asian, including Chinese, population.
The predominant TPMT variant in the Asian
population is *3C (all other variants being
exceedingly rare), with an allele frequency of 2.3%,
in contrast to the higher allele frequency of TPMT
variants in the Caucasian population (5.3% for all
TPMT variants detected in one study).8 Nevertheless,
thiopurine-induced myelosuppression is more
common in the Asian than Caucasian population.9 10 11
Prospective TPMT genotyping can only identify
a minor proportion of Asian patients who are at
risk of thiopurine-induced myelosuppression.
Moreover, the majority of Asian patients who are
referred for TPMT genotyping after the occurrence
of myelosuppression (called retrospective TPMT
genotyping) do not carry any defective TPMT variant
both in published studies10 12 13 or in the experience
of the authors’ laboratory that has provided a TPMT
genotyping service since 2013 (Table). There are
clearly additional genetic and/or non-genetic factors
that contribute to an increased risk of thiopurine-induced
myelosuppression in Asians.
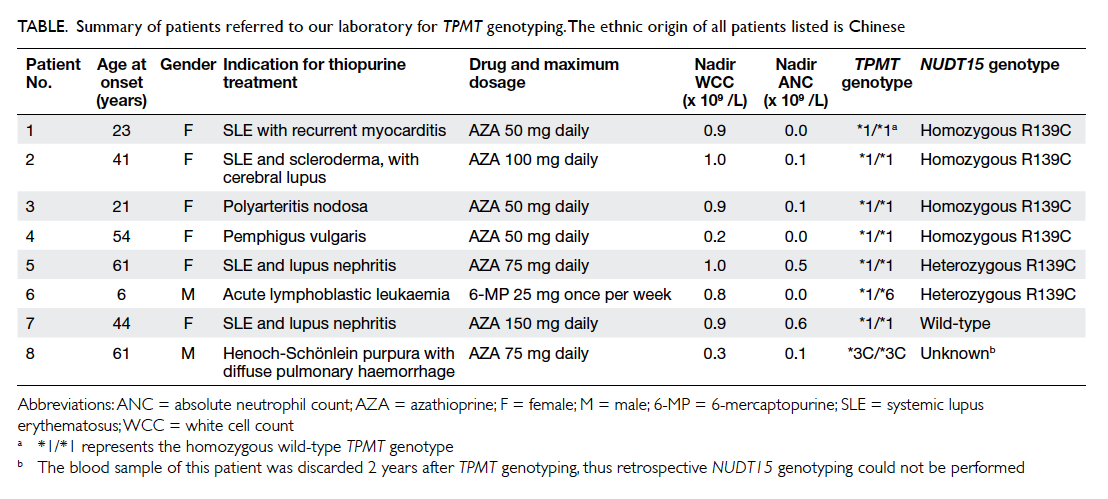
Table. Summary of patients referred to our laboratory for TPMT genotyping. The ethnic origin of all patients listed is Chinese
In 2014, the NM_018283.2:c.415C>T,
p.Arg139Cys (R139C) variant in the NUDT15 gene
(dbSNP ID: rs116855232) was found to have a strong
association with thiopurine-induced leukopenia in
a large retrospective cohort of Koreans prescribed
thiopurines for Crohn’s disease.14 Of those patients
who carried one or two NUDT15 R139C variants,
89.4% (59/66) developed leukopenia within the
first 8 weeks of thiopurine therapy (defined as early
leukopenia). In contrast, this risk allele was found in
only 6.8% (43/632) of controls who did not develop
leukopenia while on thiopurine therapy. Most
strikingly, all patients (14/14) who were homozygous
for the R139C variant developed early leukopenia. In
addition, 25.6% (45/176) and 50% (88/176) of patients
who were heterozygous for the R139C variant
developed early and late leukopenia (occurrence of
leukopenia after 8 weeks), respectively. A gene-dose
effect was also seen as the number of risk alleles
increased, demonstrated by a lower thiopurine
dose at which leukopenia occurred, a shorter time
interval from the start of treatment till occurrence
of leukopenia, and a higher grade of leukopenia.
Overall, the presence of one or two of this risk allele
had a sensitivity of 89.4% and specificity of 93.2%
for early leukopenia. The association of NUDT15
R139C with thiopurine-induced leukopenia has
subsequently been demonstrated in Japanese
patients with inflammatory bowel disease15 and
Taiwan Chinese patients with childhood acute
lymphoblastic leukaemia (ALL).16 NUDT15 R139C
is much more common than the TPMT*3C variant
in the Asian population, with an allele frequency
of 16% in Southern Han Chinese.17 Thus, NUDT15
R139C testing is of greater diagnostic value than
TPMT genotyping for prospective risk assessment of
thiopurine-induced leukopenia in the local Chinese
population. The exact role of NUDT15 R139C in
thiopurine toxicity remains unclear. NUDT15 is
a nudix hydrolase that degrades 8-oxo-dGTP and
8-oxo-dGDP in vitro, suggesting that it prevents
misincorporation of 8-oxo-2’-deoxyguanosine 5’-triphosphate (8-oxo-dGDP) into DNA in
vivo.3 18 In-vitro studies showed that treatment with
6-MP resulted in a higher percentage of apoptosis
and necrosis in cells transfected with the NUDT15
R139C construct compared with cells with the wild-type
construct.14
We performed NUDT15 R139C testing by
polymerase chain reaction and bidirectional Sanger
sequencing on all patient samples received by our
laboratory for TPMT genotyping from August 2013
to November 2015. All patients were originally
referred for retrospective TPMT genotyping.
We received no requests for prospective TPMT
genotyping during this period of time. The clinical
history and test results are summarised in the Table.
NUDT15 R139C was identified in six (85.7%) of the
seven patients referred to our laboratory for TPMT
genotyping in whom a specimen was available for
testing, while TPMT variants (*6 and *3C detected
in this patient cohort) were identified in two (25%)
of the eight patients. The TPMT*6 variant is a
rare variant with an allele frequency of 0.16% in
the Chinese population.19 Of the six patients who
were positive for the NUDT15 R139C, four were
homozygous, one was heterozygous, and one patient
was doubly heterozygous for NUDT15 R139C and
TPMT*6. The identification of double heterozygosity
is clinically relevant as one study showed that double
heterozygotes required a substantially lower dose
intensity of 6-MP in the treatment of childhood ALL
compared with those with heterozygous genotype
for only one of the two genes.20 Although limited by
the small number of cases, our results demonstrate
the relevance of NUDT15 R139C testing in local
Chinese patients who develop thiopurine-induced
leukopenia. In view of the close association of
NUDT15 R139C with early leukopenia and the
relatively high carrier rate of this variant in the local
Chinese population, prospective NUDT15 R139C
testing together with TPMT genotyping will likely
become a necessary requirement for all patients
in whom thiopurine therapy is indicated. Dose
recommendations based on combined NUDT15/TPMT genotyping results may be feasible as more
clinical data accumulate.
References
1. Tiede I, Fritz G, Strand S, et al. CD28-dependent Rac1
activation is the molecular target of azathioprine in primary
human CD4+ T lymphocytes. J Clin Invest 2003;111:1133-45. Crossref
2. Lennard L, Lilleyman JS. Variable mercaptopurine
metabolism and treatment outcome in childhood
lymphoblastic leukemia. J Clin Oncol 1989;7:1816-23.
3. Roberts RL, Barclay ML. Update on thiopurine
pharmacogenetics in inflammatory bowel disease.
Pharmacogenomics 2015;16:891-903. Crossref
4. Imuran (azathioprine). FDA drug labels. Available
from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/016324s034s035lbl.pdf. Accessed 21 Dec
2015.
5. Purinethol (mercaptopurine) 50-mg scored tablets. FDA
drug labels. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/009053s032lbl.pdf.
Accessed 21 Dec 2015.
6. Relling MV, Gardner EE, Sandborn WJ, et al. Clinical
Pharmacogenetics Implementation Consortium guidelines
for thiopurine methyltransferase genotype and thiopurine
dosing. Clin Pharmacol Ther 2011;89:387-91. Crossref
7. Relling MV, Gardner EE, Sandborn WJ, et al. Clinical
pharmacogenetics implementation consortium guidelines
for thiopurine methyltransferase genotype and thiopurine
dosing: 2013 update. Clin Pharmacol Ther 2013;93:324-5. Crossref
8. Collie-Duguid ES, Pritchard SC, Powrie RH, et al. The
frequency and distribution of thiopurine methyltransferase
alleles in Caucasian and Asian populations.
Pharmacogenetics 1999;9:37-42. Crossref
9. Kim JH, Cheon JH, Hong SS, et al. Influences of thiopurine
methyltransferase genotype and activity on thiopurine-induced
leukopenia in Korean patients with inflammatory
bowel disease: a retrospective cohort study. J Clin
Gastroenterol 2010;44:e242-8. Crossref
10. Takatsu N, Matsui T, Murakami Y, et al. Adverse reactions
to azathioprine cannot be predicted by thiopurine S-methyltransferase
genotype in Japanese patients with
inflammatory bowel disease. J Gastroenterol Hepatol
2009;24:1258-64. Crossref
11. Lee HJ, Yang SK, Kim KJ, et al. The safety and efficacy of
azathioprine and 6-mercaptopurine in the treatment of
Korean patients with Crohn’s disease. Intest Res 2009;7:22-31.
12. Zhu Q, Cao Q. Thiopurine methyltransferase gene
polymorphisms and activity in Chinese patients with
inflammatory bowel disease treated with azathioprine.
Chin Med J (Engl) 2012;125:3665-70.
13. Cao Q, Zhu Q, Shang Y, Gao M, Si J. Thiopurine
methyltransferase gene polymorphisms in Chinese patients
with inflammatory bowel disease. Digestion 2009;79:58-63. Crossref
14. Yang SK, Hong M, Baek J, et al. A common missense
variant in NUDT15 confers susceptibility to thiopurine-induced
leukopenia. Nat Genet 2014;46:1017-20. Crossref
15. Tanaka Y, Kato M, Hasegawa D, et al. Susceptibility to
6-MP toxicity conferred by a NUDT15 variant in Japanese
children with acute lymphoblastic leukaemia. Br J
Haematol 2015;171:109-15. Crossref
16. Liang DC, Yang CP, Liu HC, et al. NUDT15 gene
polymorphism related to mercaptopurine intolerance
in Taiwan Chinese children with acute lymphoblastic
leukemia. Pharmacogenomics J 2015 Oct 27. Epub ahead
of print. Crossref
17. 1000 Genomes. Homo sapiens—Population genetics—rs116855232 (SNP). Available from: http://browser.1000genomes.org/Homo_sapiens/Variation/Population?db=core;r=13:48619355-48620355;v=rs116855232;vdb=variation;vf=28973268. Accessed 8 Nov 2015.
18. Takagi Y, Setoyama D, Ito R, Kamiya H, Yamagata Y,
Sekiguchi M. Human MTH3 (NUDT18) protein hydrolyzes
oxidized forms of guanosine and deoxyguanosine
diphosphates: comparison with MTH1 and MTH2. J Biol
Chem 2012;287:21541-9. Crossref
19. Kham SK, Soh CK, Liu TC, et al. Thiopurine S-methyltransferase
activity in three major Asian
populations: a population-based study in Singapore. Eur J
Clin Pharmacol 2008;64:373-9. Crossref
20. Yang JJ, Landier W, Yang W, et al. Inherited NUDT15
variant is a genetic determinant of mercaptopurine
intolerance in children with acute lymphoblastic leukemia.
J Clin Oncol 2015;33:1235-42. Crossref

