Hong Kong Med J 2016 Apr;22(2):106–15 | Epub 4 Dec 2015
DOI: 10.12809/hkmj144449
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Impact of skeletal-related events on survival in patients with metastatic prostate cancer prescribed androgen deprivation therapy
KW Wong, MB, ChB1;
WK Ma, FHKAM (Surgery)1;
CW Wong, FHKAM (Surgery)2;
MH Wong, FHKAM (Surgery)3;
CF Tsang, MB, BS1;
HL Tsu, FHKAM (Surgery)1;
KL Ho, FHKAM (Surgery)4;
MK Yiu, FHKAM (Surgery)1
1 Division of Urology, Department of Surgery, The University of Hong Kong, Pokfulam, Hong Kong
2 Baptist Hospital, Kowloon Tong, Hong Kong
3 Department of Surgery, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
4 Private practice, Hong Kong
Corresponding author: Dr MK Yiu (yiumk2@ha.org.hk)
Abstract
Objective: To investigate the impact of skeletal-related
events on survival in patients with metastatic
prostate cancer prescribed long-term androgen
deprivation therapy.
Methods: This historical cohort study was conducted in two hospitals in Hong Kong. Patients who were diagnosed with metastatic prostate cancer and prescribed androgen deprivation therapy between January 2006 and
December 2011 were included. Details of skeletal-related events and mortality were examined.
Results: The median follow-up was 28 (range, 1-97)
months. Of 119 patients, 52 (43.7%) developed
skeletal-related events throughout the study, and
the majority received bone irradiation for pain
control. The median actuarial overall survival and
cancer-specific survival for patients with skeletal-related
events were significantly shorter than those
without skeletal-related events (23 vs 48 months,
P=0.003 and 26 vs 97 months, P<0.001, respectively).
Multivariate analysis revealed that the adjusted
hazard ratio of presence of skeletal-related events
on overall and cancer-specific survival was 2.73
(95% confidence interval, 1.46-5.10; P=0.002) and
3.92 (95% confidence interval, 1.87-8.23; P<0.001),
respectively. A prostate-specific antigen nadir of
>4 ng/mL was an independent poor prognostic
factor for overall and cancer-specific survival after
development of skeletal-related events (hazard
ratio=10.42; 95% confidence interval, 2.10-51.66 and
hazard ratio=10.54; 95% confidence interval, 1.94-57.28, respectively).
Conclusions: Skeletal-related events were common
in men with metastatic prostate cancer. This is the first
reported study to show that a skeletal-related event
is an independent prognostic factor in overall and
cancer-specific survival in patients with metastatic
prostate cancer prescribed androgen deprivation
therapy. A prostate-specific antigen nadir of >4
ng/mL is an independent poor prognostic factor
for overall and cancer-specific survival following
development of skeletal-related events.
New knowledge added by this study
- Skeletal-related events (SREs) in patients with metastatic prostate cancer significantly worsen their prognosis.
- The prevalence of SREs in patients with metastatic prostate cancer is high.
- Medications such as bisphosphonate therapy and receptor activator for nuclear factor κB ligand inhibitor should be considered to prevent SREs in patients with metastatic prostate cancer.
Introduction
Prostate cancer is the most common cancer
diagnosed in men in developed countries. In the
United States, there were 240 890 estimated new
cases in 2011, accounting for 29% of all new cancers
in men and over 33 000 deaths.1 According to the
Hong Kong Cancer Registry in 2012, prostate cancer
was the third most common cancer in men.2
The overall incidence of advanced-stage
prostate cancer has declined in recent years, probably
due to early detection and treatment following
application of prostate-specific antigen (PSA) for
prostate cancer screening.3 Nonetheless it has been
shown that approximately 4% of patients present
with metastatic disease at the time of diagnosis1 and
5% present with localised or regional disease that
eventually metastasises.4
Bone is the major metastatic site of prostate
cancer, and has been observed in 90% of patients
during autopsy.4 Common sites of metastases include
the vertebrae, pelvis, long bones, ribs, and skull.
Bone metastases cause major morbidity in patients
with prostate cancer. They weaken the structural
integrity of bone, leading to an increased risk for
skeletal-related events (SREs) such as pathological
fracture, spinal cord compression, and severe bone
pain requiring palliative radiotherapy or surgery to
bone.5 6
The prognosis of localised and regional
prostate cancer is excellent while that of metastatic
prostate cancer is poor. The 5-year survival rate in
patients with metastatic disease has been reported
to be as low as 30%1 with a mean survival of 24 to 48
months.7 8
Evidence of the importance of SREs for
survival in metastatic prostate cancer is limited.
Oefelein et al9 evaluated men with prostate cancer
who were prescribed androgen deprivation therapy
(ADT) regardless of staging. The relative risk of
skeletal fracture for mortality was 7.4. In another
retrospective study, patients with bone metastasis
from different primary tumours were analysed.10
In the subgroup analysis, pathological fracture
increased risk of death by 20% in patients with
prostate cancer although the authors failed to
demonstrate statistical significance.10 A population-based
cohort study demonstrated that mortality in
men with metastatic prostate cancer and SREs were
approximately twice that of patients with no SREs.11
Treatments for prostate cancer were, however, not
recorded or analysed in the study.11
The aim of this study was to investigate the
impact of SREs on survival, specifically in patients
with carcinoma of the prostate with bone metastasis
prescribed long-term ADT. Prognostic factors of
survival in patients with SREs were also investigated.
Methods
The study period was between 1 January 2006 and
31 December 2011. Patients who were diagnosed
with prostate cancer and bone metastasis and who
underwent either bilateral orchiectomy or were
prescribed a first dose of luteinising hormone
releasing hormone analogue (LHRHa) injection
during the study period at either Queen Mary
Hospital or Tung Wah Hospital in Hong Kong were included.
Patients were followed up until death or the last
follow-up taken on 31 March 2014.
Diagnosis of carcinoma of the prostate was
made following transrectal ultrasound-guided
prostate biopsy, incidental histological findings
of transurethral resection of a prostate specimen,
biochemical diagnosis of PSA of >100 ng/mL, or
other histological evidence such as bone biopsy in
patients who presented with pathological fracture.
Presence of bone metastasis was confirmed either
by bone scan or by cross-sectional imaging such as
computed tomography (CT) or magnetic resonance
imaging (MRI). Patients who had evidence of bone
metastases at four or more sites or visceral metastasis
were regarded as having high-volume disease.
Patients with medical castration were prescribed
regular LHRHa injection every 3 months. Patients
with underlying metabolic bone disease were
excluded from study. In this study, SRE was defined
in patients who developed pathological fractures,
cord compression related to bone metastasis, and/or those who received irradiation or prophylactic
surgery to bone metastasis.6 7 Castration-resistant
prostate cancer (CRPC) was diagnosed when there
were at least two consecutive rises in PSA, at least 1
week apart, with PSA of >2 ng/mL.
Data were collected from the electronic clinical
management system database in the government
health care system. Patients who underwent bilateral
orchiectomy or received the first dose of LHRHa
within the study period were shortlisted, reviewed,
and then recruited as eligible patients according to
the inclusion criteria. Data were collected from in-patient
and out-patient records and included age
at diagnosis; performance status; any bone pain at
diagnosis; imaging such as bone scan, CT, and MRI;
volume of metastasis; details of ADT and SREs;
history of metabolic bone disease; CRPC status;
treatment received for prostate cancer; and date and
causes of death. Two authors (KW Wong and CF
Tsang) abstracted the data and were not blinded to
the outcomes.
Data were analysed using the Statistical Package
for the Social Sciences (Windows version 21.0; SPSS
Inc, Chicago [IL], US). The primary outcome was
survival time, calculated from the date of start of
ADT until death or the last follow-up. Survival was
described with Kaplan-Meier curves. Univariate and
multivariate analyses were performed with Cox
regression model to predict prognostic factors for
survival. The dependent variables were time to death
(overall and cancer-specific), defined as the time
from the start of ADT to death. Prognostic variables
significant in the univariate analyses were entered
into the multivariate Cox regression models.
Results
A total of 119 eligible patients were identified within
the study period. The mean age at prostate cancer
diagnosis was 75 (range, 49-94) years. Initial ADT
was by bilateral orchiectomy or LHRHa injection in
58 and 61 patients, respectively, with seven patients
subsequently switched from injection to bilateral
orchiectomy. The median time of follow-up was 28
(range, 1-97) months. No patient was lost to follow-up
during the study.
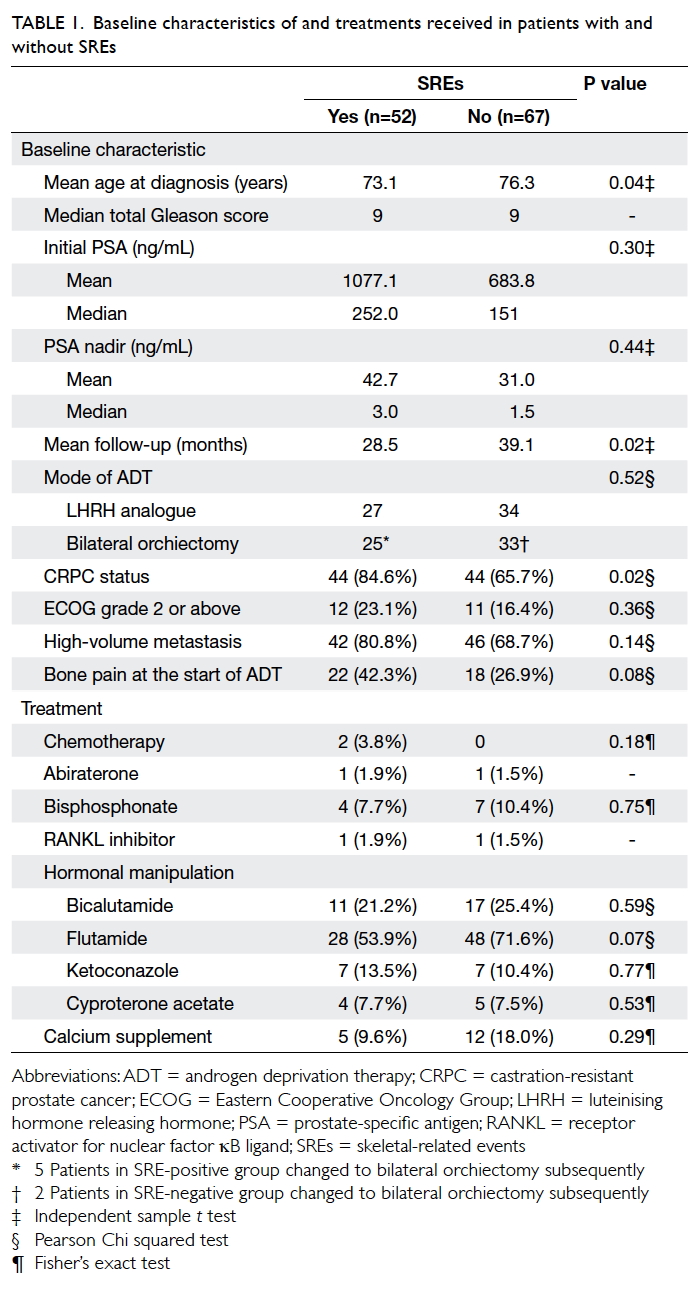
The baseline characteristics of patients are
summarised in Table 1. When stratified according to the presence of SREs, the two groups did not differ significantly in total Gleason score of prostate
cancer, PSA level at the time of diagnosis, PSA nadir,
Eastern Cooperative Oncology Group (ECOG)
performance status, volume of metastasis, presence
of bone pain at the start of ADT, or mode of ADT.
Patients with SREs were slightly younger at the time
of diagnosis (73.1 vs 76.3 years; P=0.04) and had a
shorter mean follow-up time (28.5 vs 39.1 months;
P=0.02). More patients with SREs developed CRPC
when compared with those who did not have SREs
(84.6% vs 65.7%; P=0.02).
The treatment received by patients with
and without SREs were compared (Table 1). Only treatments received prior to development
of SREs were included in Table 1 to analyse whether the baseline characteristics of treatment
differed before the development of SREs. The proportion of patients
prescribed chemotherapy, bicalutamide, flutamide,
ketoconazole, cyproterone acetate, and calcium
supplement was statistically similar for the two
groups. Only two patients in each group received
abiraterone and denosumab therapy. No patient
received sipuleucel-T, cabazitaxel, enzalutamide,
radium-223, or other novel treatment for prostate
cancer throughout the study period.
Incidence of skeletal-related events
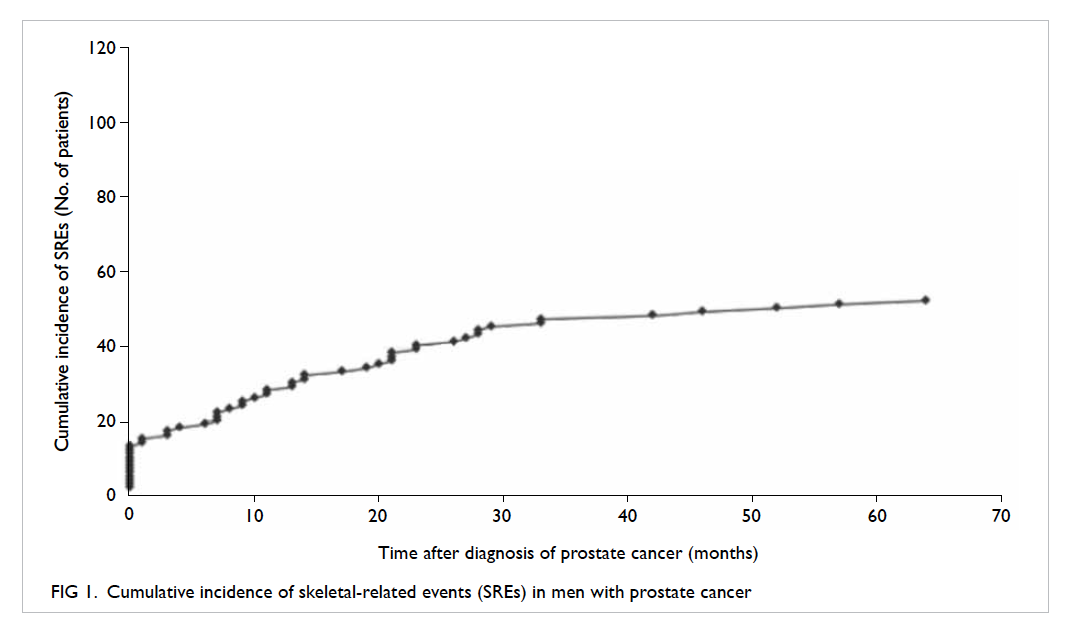
Of 119 patients, 52 (43.7%) developed SREs. A total
of 69 SREs were recorded—36 (69.2%) patients had
one SRE, 15 (28.8%) patients had two SREs, and one
patient had three SREs. Irradiation to bone for pain
control accounted for 47 (68.1%) events; 14 (20.3%)
events were cord compression and there were eight
(11.6%) events of pathological fractures without cord
compression. No patient underwent prophylactic
surgery for bone metastasis. With regard to timing
of SRE development, 13 (10.9%) patients had SRE
as the initial presentation of metastatic prostate
cancer. The overall cumulative incidence of SREs at
1 year and 5 years of diagnosis was 23.5% and 42.9%,
respectively (Fig 1).
Castration-resistant status and survival
The median time required to develop CRPC status
from the start of ADT was 9 months (Fig 2a). When stratified according to the presence of SREs, the
median time to CRPC status from ADT initiation
was significantly shorter in patients with SREs than
in those without (6 vs 12 months, log-rank test,
P=0.001; Fig 2b).
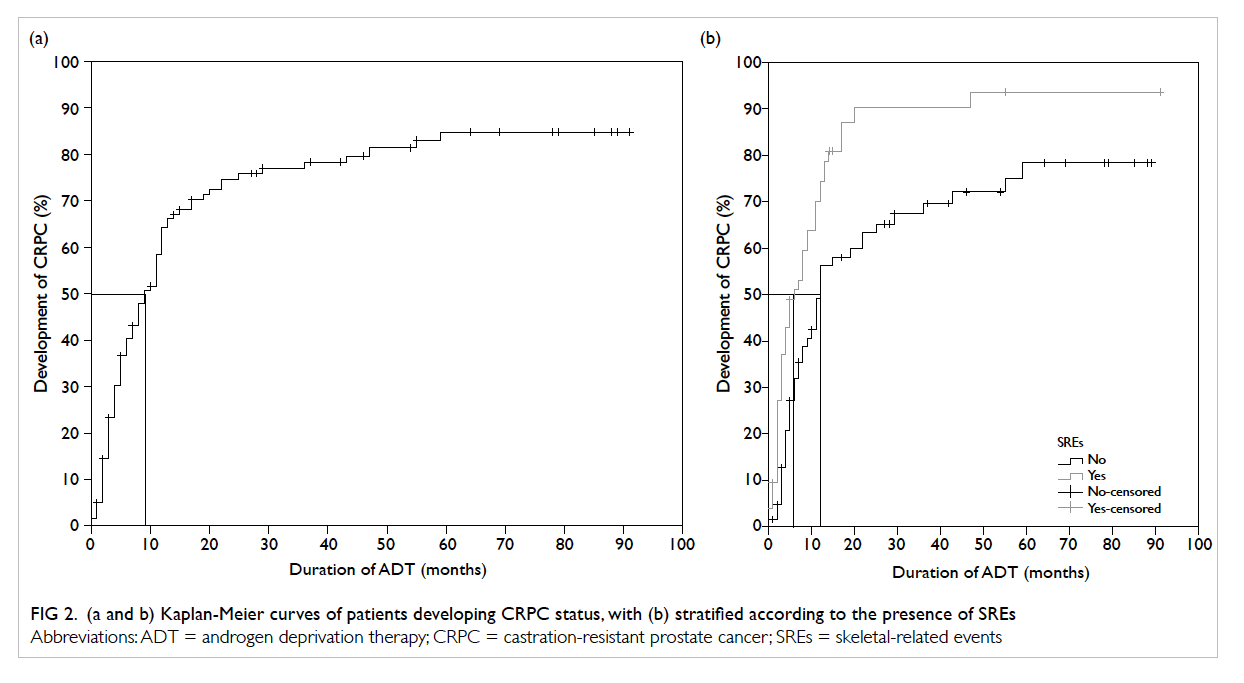
Figure 2. (a and b) Kaplan-Meier curves of patients developing CRPC status, with (b) stratified according to the presence of SREs
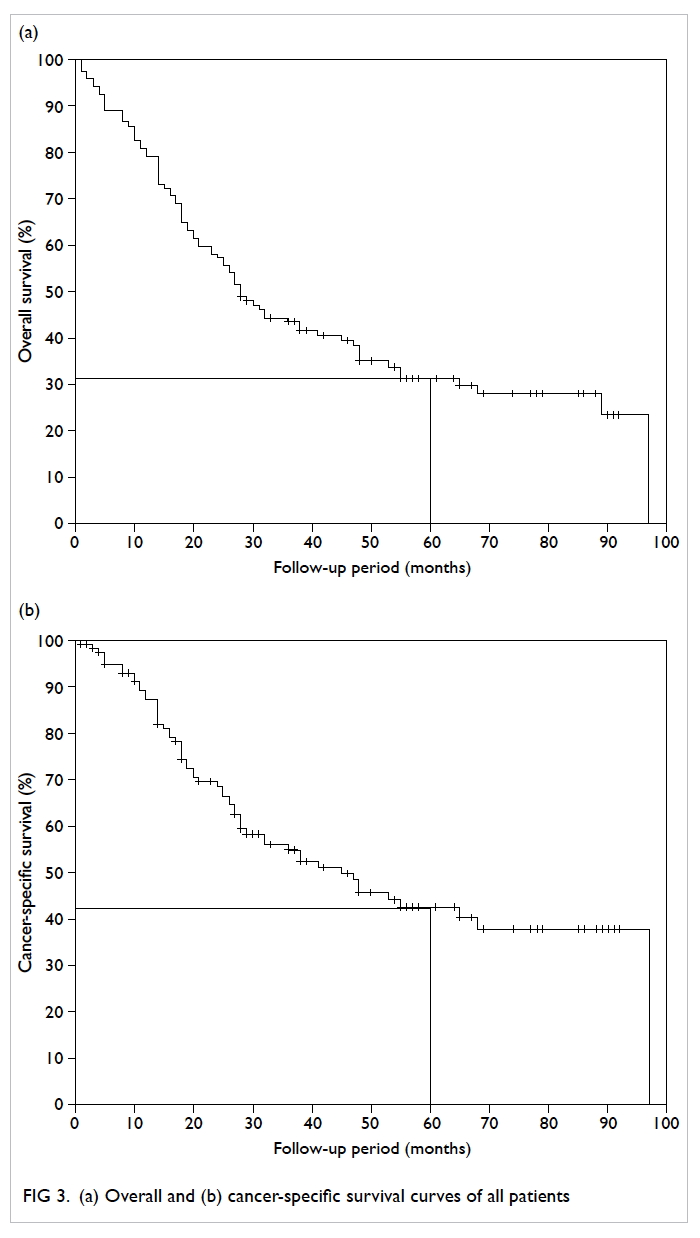
The actuarial overall survival (OS) and cancer-specific
survival (CSS) curves are shown in Figure 3. The 5-year actuarial OS and CSS was 32% and 43%, respectively. Among men without SREs, 38 (56.7%) patients died, compared with 44 (84.6%) patients
in the SRE group. When stratified according to presence of SREs (Fig 4), the median OS and CSS for patients with SREs were significantly shorter than
that for patients without SREs (log-rank test: 23 vs
48 months, P=0.003 and 26 vs 97 months, P<0.001,
respectively).
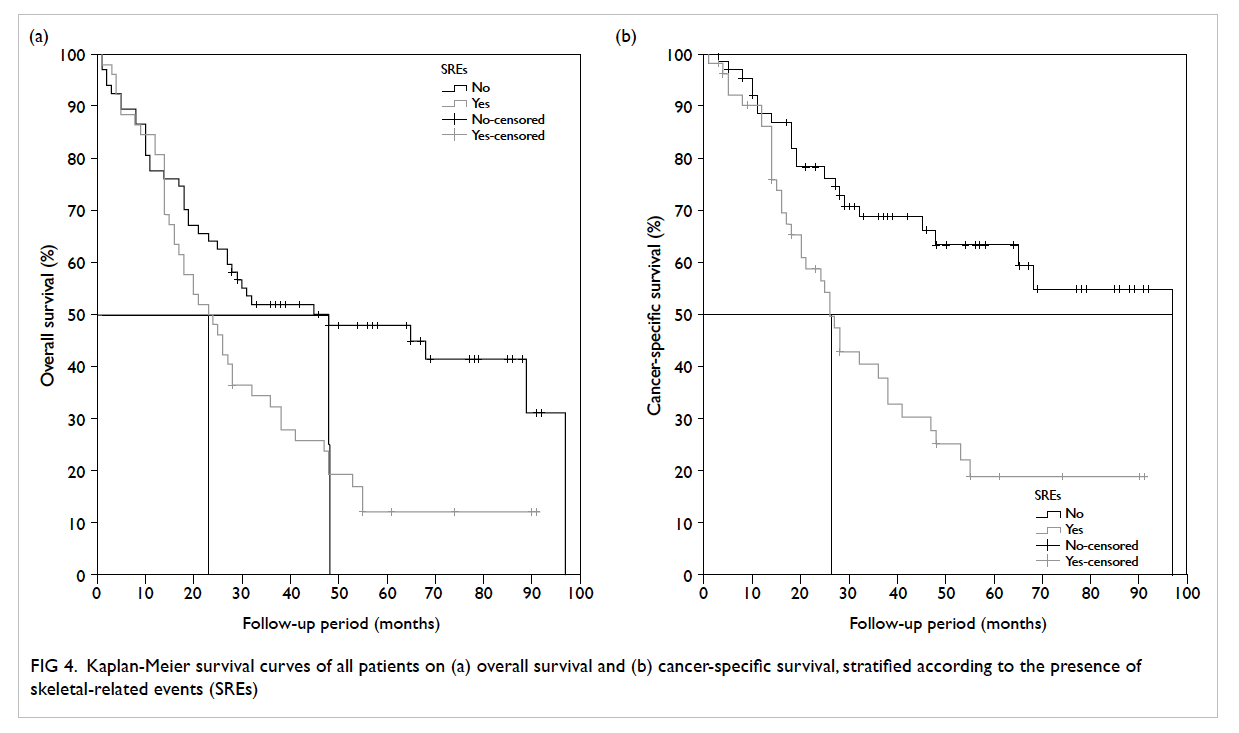
Figure 4. Kaplan-Meier survival curves of all patients on (a) overall survival and (b) cancer-specific survival, stratified according to the presence of skeletal-related events (SREs)
Risk factors for survival
Various possible factors that could affect survival
were analysed (Table 2a). All treatments for prostate cancer received both before and after SRE were
included. Univariate analysis revealed that in terms
of OS, presence of SREs (P=0.003), PSA nadir of >4
ng/mL (P<0.001), ECOG grade 2 or above (P=0.01),
and calcium supplement (P=0.03) were significant
risk factors. On multivariate analysis, only the
presence of SREs and PSA nadir of >4 ng/mL
remained statistically significant, with hazard ratio
(HR) of 2.73 (95% confidence interval [CI], 1.46-5.10; P=0.002) and 3.01 (95% CI, 1.54-5.90; P=0.001),
respectively. In terms of CSS, presence of SREs
(P<0.001), PSA nadir of >4 ng/mL (P=0.004), and
ketoconazole therapy (P=0.05) remained significant risk
factors on both univariate and multivariate analyses.
The HR for the presence of SREs, PSA nadir of >4
ng/mL, and ketoconazole therapy was 3.92 (95% CI, 1.87-8.23; P<0.001), 2.98 (95% CI, 1.43-6.23;
P=0.004), and 2.10 (95% CI, 1.01-4.38; P=0.05), respectively.
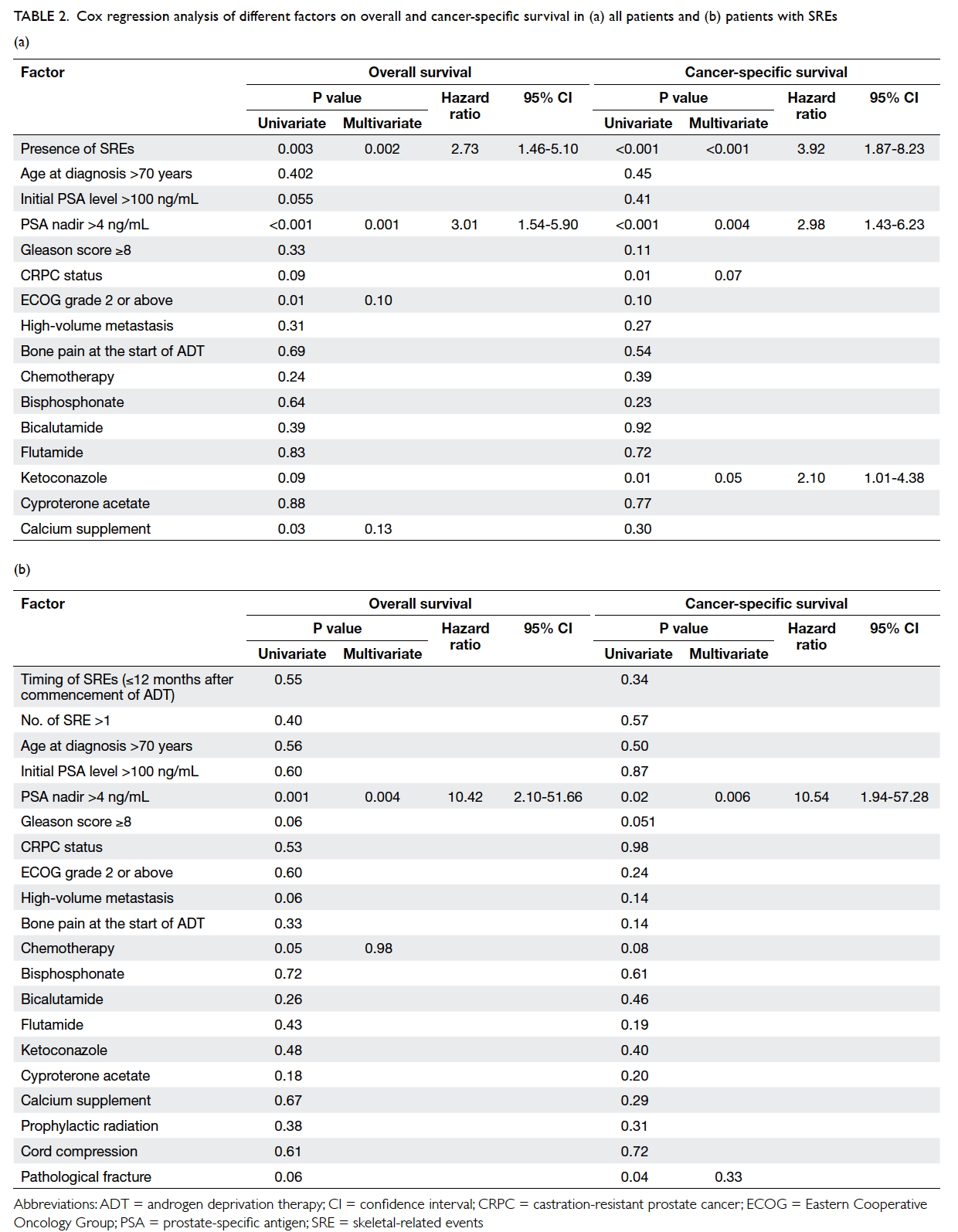
Table 2. Cox regression analysis of different factors on overall and cancer-specific survival in (a) all patients and (b) patients with SREs
The median survival period after occurrence
of SRE was 11.5 months. A post-hoc analysis for OS
and CSS after SRE revealed PSA nadir of >4 ng/mL
as the only independent predictor for survival after
SRE in both univariate and multivariate analyses,
with HR of 10.42 (95% CI, 2.10-51.66; P=0.004) and
10.54 (95% CI, 1.94-57.28; P=0.006), respectively
(Table 2b).
Discussion
The importance of SREs in survival of patients with
prostate cancer with different disease stage and
treatments was studied10 11 12 but not specifically in
patients with metastatic prostate cancer prescribed
ADT. This group of patients was selected because
patients with metastatic prostate cancer are at risk of
developing SREs.11 In addition, ADT is the standard
first-line treatment for metastatic prostate cancer.12
It has been proven to provide a clear benefit in terms
of preventing SREs.13 Focusing on patients who are
prescribed ADT can ensure that the effect of ADT in
preventing SREs is balanced out during analysis. A
study by Oefelein et al9 showed that skeletal fractures
negatively correlate with OS in men with prostate
cancer prescribed ADT, but it included patients with
localised disease and all kinds of fracture including
osteoporotic fractures. Berruti et al4 reported the
incidence of skeletal complications in patients with
CRPC and bone metastasis, but failed to demonstrate
any difference in survival between patients with and
without skeletal complications. Multivariate analysis
was not performed on survival either. Daniell et al14 15 reported eight fractures in 49 patients with prostate cancer at various times following orchiectomy but
did not take into account the preventive effect of
ADT in SREs.13 In our study, patients with SREs
had much worse OS and CSS when compared with
those without SREs (23 vs 48 months and 26 vs 97
months, respectively), and remained significantly
so after multivariate Cox regression analysis. To
our knowledge, this is the first reported study to
investigate the impact of SREs on survival in this
homogeneous group of patients.
The baseline characteristics were similar
between patients with or without SREs except that
those with SREs were slightly younger at diagnosis
(73.1 vs 76.3 years; P=0.04). This, however, does
not affect data interpretation since age at diagnosis
was not a significant factor in subsequent analyses
for both OS and CSS. In our targeted group of
patients with metastatic prostate cancer prescribed
ADT, the presence of SREs was first shown to be an
independent predictive factor for OS and CSS with
a notable HR of 2.73 and 3.92 respectively, taking
into account baseline cancer characteristics, ECOG
performance status, development of CRPC status,
and different treatments received. In addition, PSA
nadir was found to be another predictive factor
for OS and CSS. This finding has been reported in
previous studies16 although most included patients
who were heterogeneous in terms of clinical stage
of prostate cancer. Kitagawa et al16 showed that
PSA nadir of >4 ng/mL was associated with HR of
5.22 (95% CI, 2.757-9.89; P<0.001) in OS in patients
with prostate cancer. The cohort, however, included
patients with either locally advanced non-metastatic
disease or metastatic disease. Park et al17 reported
that a higher PSA nadir level correlated with shorter
CSS. Similar to the previous study,16 patients with
lymph node metastasis were also included. In
another retrospective study,18 a high PSA nadir level
was shown to be associated with shorter OS in a
homogeneous group of patients with metastatic
prostate cancer prescribed ADT. Nonetheless only
87 patients were included in the study. In our study,
in patients who developed SREs, PSA nadir was the
only predictive factor for both OS and CSS with HR
of 10.42 and 10.54, respectively. This is previously
unreported.
Various treatments have been proven to improve
OS in patients with metastatic prostate cancer,
including docetaxel,19 cabazitaxel,20 abiraterone,21 22 23
sipuleucel-T,24 and enzalutamide.25 26 Various bone-modulating
agents have also been studied for patients
with bone metastasis. Bisphosphonate therapy has
been shown to improve bone mineral density and
quality of life,27 28 and reduce the incidence of SREs in patients with metastatic CRPC in a randomised
controlled trial (RCT), although there was no proven
survival benefit.6 The receptor activator for nuclear
factor κB ligand (RANKL) inhibitor denosumab is
another bone-modulating agent proven to reduce
the incidence of SREs in metastatic CRPC patients
but also without survival benefit.29 30 Radium-223,
a bone-seeking calcium-mimicking alpha emitter,
was shown in a RCT31 to not only delay first
symptomatic SRE, but also improve OS. Therefore,
when investigating the incidence of SRE and survival
in these groups of patients, the aforementioned
treatments have to be taken into account. In our
study, treatments received by patients without SREs
and in patients prior to development of SREs were
statistically similar (Table 1). The number of patients prescribed chemotherapy or novel hormonal agents
was relatively small in our series. Sipuleucel-T,
enzalutamide, and radium-223 were not available
in this locality during the study period. Denosumab
and abiraterone therapies were used by only two
patients in each group as these medications were
not subsidised by the local government and were
not affordable for many patients. Docetaxel has
been shown to improve bone pain and OS in a phase
III RCT.19 After development of SREs, six more
patients received chemotherapy in our series. All
but one patient received docetaxel. The remaining
patient received estramustine and etoposide before
development of SREs. The fact that all patients
prescribed docetaxel were in the SRE group suggests
that its potential benefit in improving OS has been
offset by SREs and so this is not a confounding factor
in our study.
The overall prevalence of bisphosphonate
therapy was low (17%). Nine patients received
bisphosphonate therapy only after development of
SREs. In fact, in patients receiving bisphosphonate
therapy, two out of seven patients without SREs
and six out of 13 patients with SREs only received
one dose of bisphosphonate due to various
reasons, including side-effects of the medication,
affordability, and early mortality after medication.
With the heterogeneous timing of start and duration
of therapy, we cannot accurately comment on the
benefit of bisphosphonate in our series. No further
patient received RANKL inhibitor or abiraterone
after development of SREs.
Since the pre-chemotherapy era, the concept of
complete androgen blockade with classic hormonal
manipulation by both steroidal anti-androgen, such
as cyproterone acetate,32 and non-steroidal anti-androgen
(such as bicalutamide,33 flutamide,34 and
nilutamide35) has been widely adopted when patients
develop CRPC status. This practice remains in use
in this locality despite the fact that no associated
survival benefit has ever been reported12 due to the
side-effects and availabilities of aforementioned
novel treatments for CRPC. Ketoconazole, a broad-spectrum
imidazole antifungal agent, was previously
the hormonal treatment of choice after anti-androgen
withdrawal for complete androgen blockade.35
It works by preventing adrenal steroidogenesis
with inhibition of the enzyme cytochrome P450
14 alpha-demethylase.36 Bicalutamide, flutamide,
cyproterone acetate, and ketoconazole were used in
our centre for hormonal manipulation. Interestingly,
ketoconazole use appeared to have a deleterious
effect on CSS even with multivariate Cox regression
in our study. This result contradicts that of a phase
III RCT35 which showed positive PSA and objective
response but no survival benefit or harm. As our
study was retrospective in nature, the implication of
ketoconazole use is doubtful based on the results of
this study and requires further evaluation.
With a median follow-up of 28 months, the
incidence of SREs in men with metastatic prostate
cancer was high (43.7%) and is comparable with
43.6% reported from the Danish group population-based
cohort study with similar follow-up period.11
The median time to CRPC status from first ADT
was 9 months, which is 5.7 months shorter than the
control arm of the CHAARTED trial.37 This may
be explained by the fact that the CHAARTED trial
included patients prescribed ADT for less than 24
months but those with disease progression within 12
months were excluded.
We obtained local data of the natural history
of metastatic prostate cancer with or without SREs
and the impact of SREs on survival. A PSA nadir
of >4 ng/mL was an independent poor prognostic
factor for OS and CSS after development of SREs.
Its clinical use in terms of predicting prognosis
and patient counselling is highly feasible. Based
on our results, prevention of SREs in patients with
metastatic prostate cancer may translate to longer
survival. Nonetheless most bone-targeting therapies,
including bisphosphonate therapy and RANKL
inhibitors, have failed to demonstrate survival benefit
even though they prevent SREs.6 30 34 38 Radium-223
appears to hold promise as it delays symptomatic SREs by 5.8 months and improves OS by 3.8 months
in metastatic prostate cancer patients.31 Further
studies are needed in this field.
There are several limitations in this study. This
was a retrospective study with small sample size so
statistical power is limited. There are even fewer
patients in post-hoc analysis. The data collected
may not accurately reflect the condition of patients
because the follow-up protocol was not standardised.
Furthermore, the data abstraction process was not
blinded. For better presentation of data, several
factors such as PSA nadir, initial PSA, and age at
diagnosis were analysed as categorical data. This
could lead to information bias. The definition of
CRPC was less stringent than that suggested from
international guidelines12 because testosterone level
and follow-up imaging such as bone scans were
not routinely performed due to limited resources.
Potential confounding factors for survival such as
smoking and co-morbidity were also not included in
the study and may have affected the validity of the
results. The small number of patients prescribed
novel treatments or bone-modulating agents did
not allow a comprehensive understanding of their
influence on SRE occurrence. Further prospective
trials with a large cohort size are necessary.
Conclusions
Skeletal-related events were common in men with
metastatic prostate cancer and were first shown by
this study to be an independent prognostic factor
of OS and CSS in patients with metastatic prostate
cancer prescribed ADT. A PSA nadir of >4 ng/mL is
an independent poor prognostic factor for OS and
CSS following development of SREs.
References
1. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics,
2011: the impact of eliminating socioeconomic and racial
disparities on premature cancer deaths. CA Cancer J Clin
2011;61:212-36. Crossref
2. Hong Kong Cancer Registry 2012. Available from:
http://www3.ha.org.hk/cancereg/Summary%20of%20CanStat%202012.pdf. Accessed 6 Oct 2015.
3. Mullan RJ, Jacobsen SJ, Bergstralh EJ, et al. Decline in the
overall incidence of regional-distant prostate cancer in
Olmsted County, MN, 1980-2000. BJU Int 2005;95:951-5. Crossref
4. Berruti A, Dogliotti L, Bitossi R, et al. Incidence of skeletal
complications in patients with bone metastatic prostate
cancer and hormone refractory disease: predictive role
of bone resorption and formation markers evaluated at
baseline. J Urol 2000;164:1248-53. Crossref
5. Saad F, Gleason DM, Murray R, et al. A randomized,
placebo-controlled trial of zoledronic acid in patients with
hormone-refractory metastatic prostate carcinoma. J Natl
Cancer Inst 2002;94:1458-68. Crossref
6. Saad F, Gleason DM, Murray R, et al. Long-term efficacy of
zoledronic acid for the prevention of skeletal complications
in patients with metastatic hormone-refractory prostate
cancer. J Natl Cancer Inst 2004;96:879-82. Crossref
7. Galasko CS. Skeletal metastases. Clin Orthop Relat Res
1986;210:18-30. Crossref
8. Logothetis CJ, Navone NM, Lin SH. Understanding the
biology of bone metastases: key to the effective treatment
of prostate cancer. Clin Cancer Res 2008;14:1599-602. Crossref
9. Oefelein MG, Ricchiuti V, Conrad W, Resnick MI. Skeletal
fractures negatively correlate with overall survival in men
with prostate cancer. J Urol 2002;168:1005-7. Crossref
10. Saad F, Lipton A, Cook R, Chen YM, Smith M, Coleman
R. Pathologic fractures correlate with reduced survival
in patients with malignant bone disease. Cancer
2007;110:1860-7. Crossref
11. Nørgaard M, Jensen AØ, Jacobsen JB, Cetin K, Fryzek JP, Sørensen HT. Skeletal related events, bone metastasis and
survival of prostate cancer: a population based cohort
study in Denmark (1999 to 2007). J Urol 2010;184:162-7. Crossref
12. Mottet N, Bastian PJ, Bellmunt J, et al. European
Association of Urology Guidelines on Prostate Cancer
2014. Available from: http://uroweb.org/wp-content/uploads/1607-Prostate-Cancer_LRV3.pdf. Accessed 6 Oct
2015.
13. Nair B, Wilt T, MacDonald R, Rutks I. Early versus
deferred androgen suppression in the treatment of
advanced prostatic cancer. Cochrane Database Syst Rev
2002;(1):CD003506.
14. Daniell HW. Osteoporosis after orchiectomy for prostate
cancer. J Urol 1997;157:439-44. Crossref
15. Daniell HW, Dunn SR, Ferguson DW, Lomas G,
Niazi Z, Stratte PT. Progressive osteoporosis during
androgen deprivation therapy for prostate cancer. J Urol
2000;163:181-6. Crossref
16. Kitagawa Y, Ueno S, Izumi K, et al. Nadir prostate-specific
antigen (PSA) level and time to PSA nadir following primary
androgen deprivation therapy as independent prognostic
factors in a Japanese large-scale prospective cohort study
(J-CaP). J Cancer Res Clin Oncol 2014;140:673-9. Crossref
17. Park YH, Hwang IS, Jeong CW, Kim HH, Lee SE, Kwak
C. Prostate specific antigen half-time and prostate
specific antigen doubling time as predictors of response
to androgen deprivation therapy for metastatic prostate
cancer. J Urol 2009;181:2520-4; discussion 2525. Crossref
18. Sasaki T, Onishi T, Hoshina A. Nadir PSA level and time
to PSA nadir following primary androgen deprivation
therapy are the early survival predictors for prostate cancer
patients with bone metastasis. Prostate Cancer Prostatic
Dis 2011;14:248-52. Crossref
19. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus
prednisone or mitoxantrone plus prednisone for advanced
prostate cancer. N Engl J Med 2004;351:1502-12. Crossref
20. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone
plus cabazitaxel or mitoxantrone for metastatic castration-resistant
prostate cancer progressing after docetaxel
treatment: a randomised open-label trial. Lancet
2010;376:1147-54. Crossref
21. Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in
metastatic prostate cancer without previous chemotherapy.
N Engl J Med 2013;368:138-48. Crossref
22. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and
increased survival in metastatic prostate cancer. N Engl J
Med 2011;364:1995-2005. Crossref
23. Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate
for treatment of metastatic castration-resistant prostate
cancer: final overall survival analysis of the COU-AA-301
randomised, double-blind, placebo-controlled phase 3
study. Lancet Oncol 2012;10:983-92. Crossref
24. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T
immunotherapy for castration-resistant prostate cancer. N
Engl J Med 2010;363:411-22. Crossref
25. Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide
in metastatic prostate cancer before chemotherapy. N Engl
J Med 2014;371:424-33. Crossref
26. Scher HI, Fizazi K, Saad F, et al. Increased survival with
enzalutamide in prostate cancer after chemotherapy. N
Engl J Med 2012;367:1187-97. Crossref
27. Saad F. Maintaining bone health throughout the continuum
of care for prostate cancer. In: Progress in bone cancer
research. Hauppauge, NY: Nova Science Publishers; 2006.
28. Smith MR. Bisphosphonates to prevent osteoporosis in
men receiving androgen deprivation therapy for prostate
cancer. Drugs Aging 2003;20:175-83. Crossref
29. Smith MR, Egerdie B, Hernández Toriz N, et al. Denosumab
in men receiving androgen-deprivation therapy for
prostate cancer. N Engl J Med 2009;361:745-55. Crossref
30. Fizazi K, Carducci M, Smith M, et al. Denosumab versus
zoledronic acid for treatment of bone metastases in men
with castration-resistant prostate cancer: a randomised,
double-blind study. Lancet 2011;377:813-22. Crossref
31. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter
radium-223 and survival in metastatic prostate cancer. N
Engl J Med 2013;369:213-23. Crossref
32. Goldenberg SL, Bruchovsky N. Use of cyproterone acetate
in prostate cancer. Urol Clin North Am 1991;18:111-22.
33. Scher HI, Liebertz C, Kelly WK, et al. Bicalutamide for
advanced prostate cancer: the natural versus treated
history of disease. J Clin Oncol 1997;15:2928-38.
34. Crawford ED, Eisenberger MA, McLeod DG, et al. A
controlled trial of leuprolide with and without flutamide in
prostatic carcinoma. N Engl J Med 1989;321:419-24. Crossref
35. Small EJ, Halabi S, Dawson NA, et al. Antiandrogen
withdrawal alone or in combination with ketoconazole in
androgen-independent prostate cancer patients: a phase
III trial (CALGB 9583). J Clin Oncol 2004;22:1025-33. Crossref
36. Loose DS, Kan PB, Hirst MA, Marcus RA, Feldman D.
Ketoconazole blocks adrenal steroidogenesis by inhibiting
cytochrome P450-dependent enzymes. J Clin Invest
1983;71:1495-9. Crossref
37. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal
therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 2015;373:737-46. Crossref
38. Dole EJ, Holdsworth MT. Nilutamide: an antiandrogen
for the treatment of prostate cancer. Ann Pharmacother
1997;31:65-75.