Hong Kong Med J 2016 Feb;22(1):70–7 | Epub 8 Jan 2016
DOI: 10.12809/hkmj154685
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE CME
Alternatives to colonoscopy for population-wide colorectal cancer screening
William CY Leung1;
Dominic CC Foo, MRCSEd, FHKAM (Surgery)2;
TT Chan1;
MF Chiang1;
Allan HK Lam1;
Heywood HW Chan1;
Chris CL Cheung1
1 Li Ka Shing Faculty of Medicine, The University of Hong Kong, Pokfulam, Hong Kong
2 Department of Surgery, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Pokfulam, Hong Kong
Corresponding author: Dr William CY Leung (cywleung.hku@gmail.com)
Abstract
Colorectal cancer is one of the top three cancers
in the world in terms of incidence. Colonoscopy,
which many regard as the gold standard in
diagnosis of colonic polyps and neoplasm, is
costly, invasive and labour-intensive, and deemed
an unsuitable population-wide index screening
tool. Alternative modalities, including guaiac and
immunohistochemical faecal occult blood tests,
computed tomographic colonography, colon capsule
endoscopy, flexible sigmoidoscopy, and double-contrast
barium enema are available. The procedures,
test characteristics, and their implications are
reviewed. Immunohistochemical faecal occult blood
testing appears to be the most suitable population-wide
screening test for an average-risk population,
with flexible sigmoidoscopy as an alternative.
More evidence is needed to determine the role of
computed tomographic colonography and colon
capsule endoscopy in colorectal cancer screening.
Introduction
Colorectal cancer (CRC) became the second and
third most common cancer in women and men in
2012.1 Most cases of CRC arise from adenoma,
the process known as the adenoma-carcinoma
sequence, and are therefore amenable to screening
and early treatment.2 3 4 Ecological studies have shown
that 2.6% to 5.6% of advanced adenoma progress to
CRC annually.5
Colonoscopy remains the gold standard for
diagnosis,6 and has even been used as a primary
screening method in some countries (eg the US).
Nonetheless its use in most countries as an index
tool for mass screening of an average-risk population
is impractical due to its cost, invasiveness, and need
for expertise (ie endoscopists).
In this study, we reviewed the literature about
the procedures, test characteristics, and implications
of the following alternative screening modalities:
guaiac faecal occult blood testing (gFOBT),
immunohistochemical faecal occult blood testing
(iFOBT), computed tomographic colonoscopy
(CTC), colon capsule endoscopy (CCE), flexible
sigmoidoscopy (FS), and double-contrast barium
enema (DCBE).
Guaiac faecal occult blood testing
The gFOBT offers the strongest evidence as a suitable
screening tool for CRC. Its mechanism involves
detection of haemoglobin in the stool. The test is not
specific for human haemoglobin however, and false-positive
results can arise due to plant peroxidases
and heme in red meat. False negatives can occur
when stool contains certain chemicals, eg vitamin
C. It also detects bleeding from the gastro-intestinal
(GI) tract other than the colon and rectum. Two or
more samples are usually required.
Four large-scale randomised controlled trials
(RCTs) of gFOBT with long-term follow-up have been
conducted; they include Minnesota study in the
US,7 Nottingham trial in the UK,8 Göteborg study in Sweden,9 and Funen study in Denmark.10 A total of 328 767 individuals, aged 45 to 80 years, were involved.
The results consistently showed reduction in CRC
mortality by 12% to 33%, after up to 30 years of
follow-up.7 8 9 10 The results are summarised in Table 1.
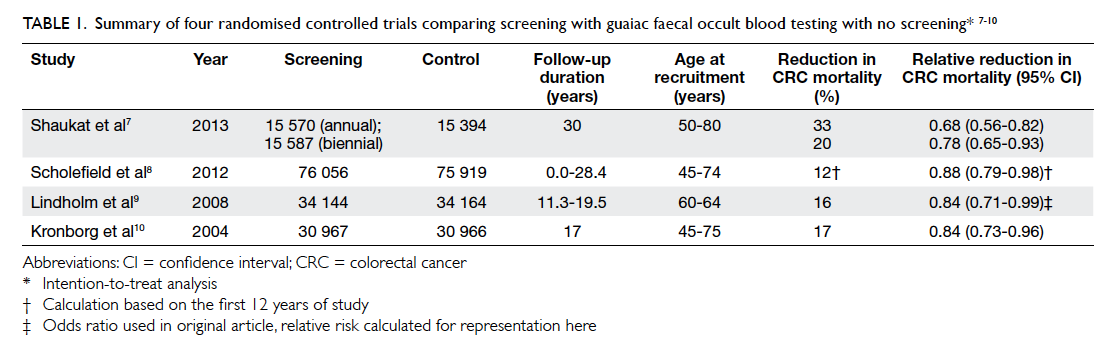
Table 1. Summary of four randomised controlled trials comparing screening with guaiac faecal occult blood testing with no screening7 8 9 10
In screening for significant or advanced
adenoma, test sensitivity was 23.8%,11 and specificity
was 97.7% to 99.0% with positive predictive values
(PPVs) of 39.0% to 55.3%. The detection rate in
intention-to-screen (ITS) analysis was 0.6% and
that in per protocol (PP) analysis was 1.2%. The
NNScreen, or the number of average-risk individuals
needed to recruit in a screening programme to
detect one advanced adenoma, was 84 to 181.12 13 The NNScope, or the number of colonoscopies
needed to diagnose an advanced adenoma after
screening revealed a likely significant lesion, was
2.2.12 Although NNScreen is useful in assessing each
modality individually, NNScope of a test provides
additional information about the role of gFOBT in
a screening programme to select patients for further
diagnostic colonoscopy. For CRC, the sensitivity was
54.2%, and specificity ranged from 96.9% to 98.1%
with a PPV of 5.2% to 13.6%. Detection rate in ITS
analysis was 0.1%, while that in PP analysis was 0.2%.
The NNScreen was 392 to 936 and the NNScope was
10.3.11 12 13 14
The Funen study10 showed the CRC mortality
dropped from 18% to 11% after five screening
rounds, as a result of decreased compliance. Similar
findings were echoed in the Tenerife study in Spain.11
Immunochemical faecal occult blood testing
The iFOBT employs an antibody-based assay, detecting
globin or early degradation products of human
haemoglobin.15 The antibodies used are human-specific,
thus the number of false positives due to
non-human blood is minimised. As globin is more
rapidly degraded than heme throughout the GI tract,
less upper GI tract bleeding is detected. It requires
no dietary restrictions16 and has a participation rate
of 38.9% to 71.9%.17 18 19 The results can be qualitative
or quantitative.20 Sampling technique, distribution
of blood in faeces, and sample instability make true
quantification difficult, however.15 Adjustment of
performance parameters is possible by altering the
cut-off values. It is generally agreed that a cut-off
of 75 ng/mL provides a balance between higher
detection rate and lower NNScope.12 15 18 21 22 It should also be noted though that different brands of iFOBT
kits may yield different results even when the same
cut-off is used.
The iFOBT on one or two consecutive faecal
samples is recommended. A study showed that 1-day
sampling had a higher miss rate for CRC compared
with 2-day sampling.23 Another study showed that
performing iFOBT at 1-, 2-, or 3-year intervals
did not yield significantly different results in terms
of advanced adenoma detection, but compliance
decreased with frequent screening.24
The stability of the iFOBT kit is temperature-dependent,
making results vulnerable to changes in
environmental temperature and the sample return
time.20 In moderate climates, the sample return
time should not exceed 7 days.25 Manufacturers
are developing buffer solutions to overcome this
problem.15
A potential disadvantage of iFOBT is its
decreased sensitivity to proximal colonic lesions.
A German study showed a sensitivity of 33% and
20% for left- and right-sided lesions, respectively.
Nonetheless the results were statistically
insignificant,26 and were contradictory to another
Dutch trial.27
The positivity rate of iFOBT ranges from 5.5%
to 11.0%.12 13 17 19 28 29 The sensitivity and specificity for
CRC ranges from 53.3% to 94.1% and 87.5% to 96.9%,
respectively.18 28 30 31 The PPV ranges from 5.2% to
12.8% at a cut-off value of 75 ng/mL.12 13 17 18 19 28 29 30 31 32 The NNScreen and NNScope ranges from 213 to 936 and
9.8 to 17.3, respectively.12 13 17 19 28 29 30 32 These results are summarised in Table 2. For advanced adenoma,
the sensitivity and specificity ranges from 33.9% to 41.3% and 91.4% to 97.3%, respectively.18 28 31 The
PPV ranges from 49.0% to 51.8%.12 13 The NNScope and NNScreen ranges from 2.2 to 2.4 and 88.0 to
135.6, respectively (single sample).29
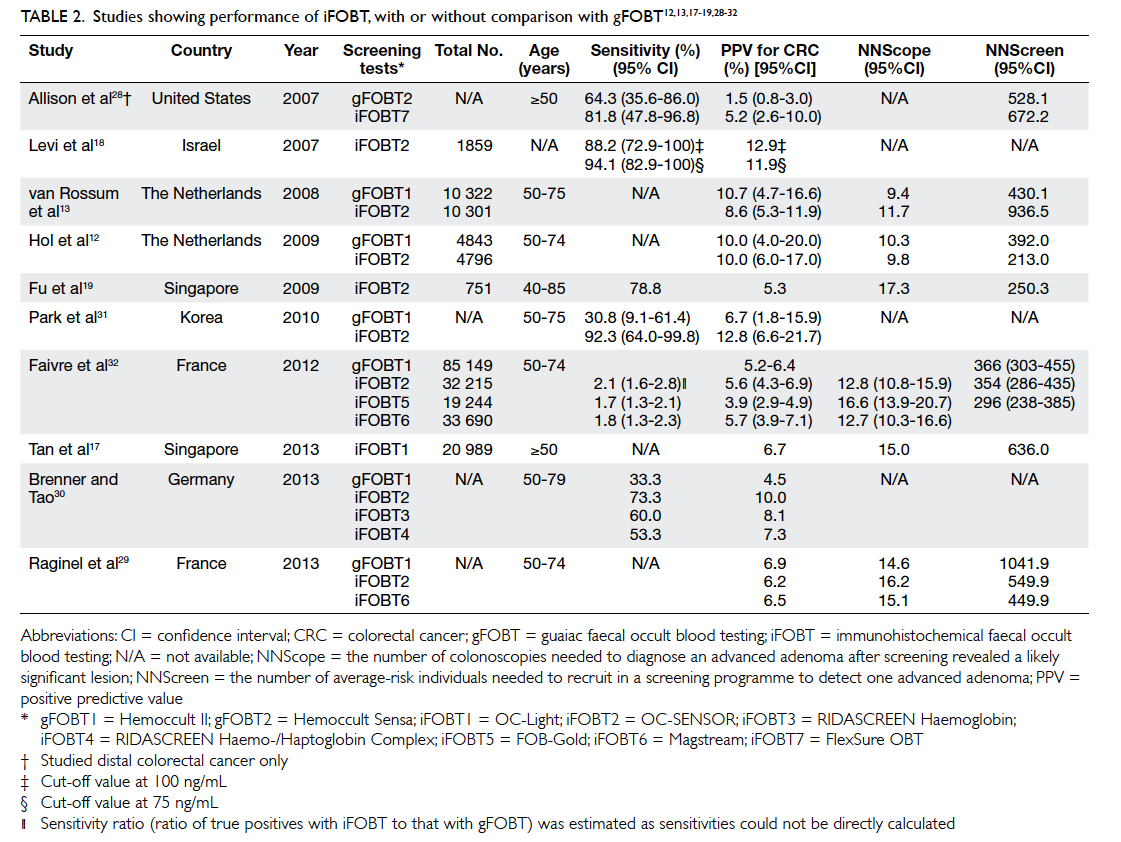
Table 2. Studies showing performance of iFOBT, with or without comparison with gFOBT12 13 17 18 19 28 29 30 31 32
Compared with gFOBT, studies in the literature
showed superior results for iFOBT that generally had
a higher positivity rate, often 2 times higher than that
of gFOBT.13 28 The detection rate for CRC in a study by Faivre et al32 was 1.6 to 2.1 times higher than in
gFOBT. This was echoed by another large-scale RCT
which showed a significantly higher detection rate
using iFOBT.13 Studies showed the detection rate
for advanced adenoma using iFOBT to be at least
double that of gFOBT.12 29 In the study by Faivre et al,32 iFOBT was 1.7 to 2.1 times more sensitive than
gFOBT for CRC.32 A study by Brenner and Tao30 showed significantly higher PPV for iFOBTs than gFOBTs
(7.3%-10.0% vs 4.5%). In two comparative studies,
the NNScreen of iFOBT was about half that of
gFOBT29 32; iFOBT also had a 13.0% to 15.0% higher participation rate than gFOBT.13 15 16 20 33
The iFOBT is more costly than its guaiac-based
counterpart,20 but modelling studies showed
that it is more cost-effective.34 35 36 37 This is largely
explained by the higher participation rate, detection
rate, sensitivity and PPV, and with lower NNScope
and NNScreen. There is a general consensus that it
should replace gFOBT.16 20 38
Computed tomographic colonography
The CTC was first described in 1994.39 It provides
a non-invasive structural assessment of the colon.
Compared with conventional colonoscopy, CTC is
sedation-free and has an extremely low risk of bowel
perforation (0.005%-0.059%).40 41 Furthermore, assessment of the extra-colonic organs can be
performed at the same time.42 A lower volume bowel
preparation may be used43 and the radiation risk is
negligible.41 Its main disadvantage is that biopsy is
not possible, and the patient may require a second
procedure with another bowel preparation, thus
imposing additional costs and discomfort to the
patient. Its role in CRC screening remains debatable.
The American Cancer Society supports screening
with CTC every 5 years.44 Other guidelines including
the National Institutes of Health Asia Pacific
Consensus Recommendations do not support its use,
however, stating its lack of evidence as a screening
technique in an average-risk population.45 46
Studies of CTC in the literature use detection of
polyps in general as the end-point. Data for detection
of invasive carcinoma as well as reduction in CRC
mortality were not available. Different studies use
either ‘per patient’ or ‘per polyp’ for analysis. Two
large US trials supported CTC as a screening tool
in asymptomatic average-risk populations. Per-patient analyses demonstrated a sensitivity of 78.0%
to 93.8%, and specificity of 79.6% to 96.0%, respectively.47 48
Meta-analyses in 2011 and 2014 reviewed 15
trials,49 50 including the two aforementioned studies. All trials focused on a population aged over 50 years
with average risk. Martín-López et al49 showed an
overall per-patient sensitivity and specificity for
CTC of 66.8% and 80.3%, which was lower than that
of colonoscopy of 92.5% and 73.2%, respectively.
The sensitivity and specificity were higher for larger
polyps. For polyps larger than 1 cm, the sensitivity
was 91.2% and specificity 87.3%. Another meta-analysis
reported sensitivities for ≥6-mm and ≥10-mm polyps as 75.9% and 83.3% and specificities as
94.6% and 98.7%, respectively.50
Estimation of the cost-effectiveness remains
complicated. Based on a systematic review of 16
studies,51 the cost-effectiveness of CTC remains
controversial. There is generally a stronger preference
for CTC over colonoscopy in asymptomatic
individuals,52 although some may hold an opposite
opinion due to more pain and discomfort in CTC.53
The use of ‘low-prep’ or laxative-free CTC is being
further investigated.43
The CTC can detect asymptomatic polyps and
has the potential to prevent them from progressing
to advanced adenoma and CRC. These polyps may
not be detected by gFOBT or iFOBT until they result
in microscopic haemorrhage in the lower GI tract.
This is an advantage of CTC compared with gFOBT
and iFOBT. The role of CTC in reducing CRC
mortality remains uncertain, however.
Colon capsule endoscopy
The CCE makes use of a double-headed capsule with
a wide viewing angle, visualising the colon beyond
the haustral folds.54 Its sensitivity and specificity
for significant polyps has been reported to be 83%
and 89%, respectively.55 56 57 The European Society of
Gastrointestinal Endoscopy recommends CCE as
an alternative screening method for average-risk
individuals.58 In February 2014, it also received the US
Food and Drug Administration clearance for use in
patients following incomplete colonoscopy. It is also
proven to be beneficial when the patient is unwilling
or is unable to undergo colonoscopy.59 60 With its presumed increased uptake, it is a promising new
CRC screening modality.61 The newest generation of
CCE has improved resolution by adapting its frame
rate to the speed of capsule movement. Some newer
capsules also have four cameras to provide a 360-degree view.62
Despite its promising role in screening, some
disadvantages of CCE have limited its use thus far.
Strict bowel preparation, diet restrictions, and use
of suppositories and prokinetics may be needed
to ensure a smooth and quick journey of the
capsule through the bowel, while minimising the
interference of debris when identifying lesions.63
Potential complications include capsule impaction
and retention (1.4%64) that may require endoscopic
or surgical removal. It is also not recommended in
pregnancy or with implanted electromedical devices
such as pacemakers.62 The cost of CCE is much
higher than that of colonoscopy,65 and includes the
reading of the captured video footage. There is also
no current evidence to prove the mortality benefit of
CCE use in CRC.
Flexible sigmoidoscopy
The FS examines the distal 40 to 60 cm of the lower GI
tract. Full colonoscopy can be performed when there
are positive findings. Compared with colonoscopy,
it requires a simpler bowel preparation and dietary
restriction is not necessary.66
In two large-scale RCTs that involved 170 432
and 55 736 individuals, in PP analysis, there was a
43.0% reduction in CRC mortality and improved
hazard ratio of 0.41.67 68 This was echoed by another RCT that involved 77 445 patients and showed a 21%
reduction in the incidence of both proximal and distal
cancer and a 50% reduction in mortality from distal
cancer.69 The PPV was 91.9% for any adenoma.70 The positivity rate for adenoma was 17.3%.71 Most studies
were in individuals aged ≥5070 71 72 or ≥55 years.68 69 73
The sensitivity of FS depends on the adequacy
of mucosal inspection and is operator-dependent.73
Studies have shown inadequate screening in up
to 91.7% of cases, ie <50 cm depth of insertion.73
The technique had relatively low and fluctuating
participation rates (20.9%-63.0%).70 71 A 35.3% decrease in adherence from baseline to subsequent
study was observed.69
The impact of FS as a screening tool is well
established in the literature and accepted in various
screening protocols.44 46 This technique should be included as an alternative choice for a population-wide
screening programme, and the shortage of
endoscopists could be partially addressed by training
specialised nurses in the procedure.74
Combining flexible sigmoidoscopy with guaiac and immunohistochemical faecal occult blood testing
Flexible sigmoidoscopy cannot replace the role of
colonoscopy in individuals with a positive faecal
occult blood test.72 In a non-randomised trial,
the detection rate of combined gFOBT and FS for
cancer was higher than that of gFOBT alone (1.5 vs
0.7 per 1000), but was not superior to FS alone (1.5
vs 5.2 per 1000).70 Results were similar for advanced
neoplasia.
Double-contrast barium enema
The DCBE involves an X-ray study of the colon
and rectum following injection of air and barium
transrectally. Once regarded as a routine screening
tool, its role has diminished since the introduction
of other screening modalities. While it was the safest
screening method next to FOBT with a perforation
rate of 1 in 25 000,75 the sensitivity for polyps of
≥10 mm was only 48%, rendering it suboptimal for
screening.76 77
Combining double-contrast barium enema with flexible sigmoidoscopy
When DCBE was combined with FS, they had
the same sensitivity for cancer as colonoscopy
(96.7%).78 Two RCTs in the 1990s reported a lower
detection rate for small polyps for FS plus DCBE
when compared with colonoscopy.79 80 Nonetheless the detection rate for cancers and large polyps was
comparable.79 Sensitivity analyses in both studies
revealed that in screening, FS plus DCBE was less
cost-effective than colonoscopy.
Current guidelines
The Asia Pacific Consensus Recommendations in
2015 suggested the use of iFOBT over gFOBT, and
FS and colonoscopy were deemed effective.46 On the
contrary, CTC and CCE were not recommended
for screening. In the US, surveillance programme
guidelines from the American Cancer Society
provided two sets of test options for asymptomatic
adults aged ≥50 years.44 For adenomatous polyps
and cancer, FS, DCBE, or CTC every 5 years, or
colonoscopy every 10 years was recommended.
For cancer alone, annual gFOBT or iFOBT testing
was recommended. The American College of
Gastroenterology supported replacement of
gFOBT by iFOBT as a first-line screening test.81 The
National Health Service in the UK recommends
screening for average-risk men and women aged 60
to 74 years with FOBT every 2 years.82 The European
Union did not offer a comprehensive system, with
a recommendation of FOBT for men and women
aged 50 to 74 years.83 The Australian government
encouraged biennial iFOBT for an asymptomatic
population aged >50 years.84
There is no formal consensus on a CRC
screening programme in Hong Kong. The Hong
Kong Cancer Fund, a cancer support organisation,
recommends screening of the average-risk
population aged ≥50 years, with either FOBT every
year, FS or DCBE every 5 years, or colonoscopy every
10 years.85
Discussion
Colonoscopy remains the gold standard
diagnostic tool for CRC, but its costs, discomfort,
inconvenience, and potential complications render
it impractical as the first-line investigation in a
population-wide CRC screening programme for
average-risk individuals. Multiple alternative tools
have since been developed, aimed at minimising
discomfort and inconvenience and thus achieving
better compliance, while at the same time not
jeopardising the screening effectiveness. While it is
not possible for these tools to replace colonoscopy
for diagnosis, they may assume an essential role in a
screening programme as an index investigation for
risk stratification, thus selecting patients to undergo
further diagnostic colonoscopy.
These screening modalities differ in their
development. Both gFOBT and FS are time-honoured,
heavily researched, and proven to reduce
CRC mortality. Large amounts of research data are
emerging in support of newer options such as iFOBT
and CTC. While comparison of gFOBT and iFOBT
is easily achievable, direct comparison of CTC
and iFOBT is more difficult as there are different
‘performance’ parameters.
The technique iFOBT is evolved from gFOBT
and shares a similar mechanism. While gFOBT has
been well proven by long-duration RCTs to reduce
CRC mortality, it has been postulated that iFOBT
may achieve the same effect. For a population-wide
screening programme to be successful, the test
has to be acceptable to asymptomatic individuals.
This eventually determines the penetration and
compliance with the programme. Compared with
gFOBT, iFOBT undeniably has a higher participation
rate,13 20 33 and even more so compared with FS.70 71 In a population-wide screening programme with iFOBT,
implementation could be achieved in a relatively
short period of time as it could be performed by
primary care physicians and nurses. Installation
of sophisticated hardware is not required. Given a
positivity rate of 5.5% to 11.0%,12 13 17 19 28 29 however,
it would have a significant impact on health care
services. A major increase in the number of referrals
for colonoscopy would be anticipated and thus
require a corresponding increase in the availability
of endoscopy centres and endoscopists.
Test characteristics are not the only factor
that dictates the success of a screening programme;
compliance plays a crucial role. Studies have shown
that those who communicate well with their health
care providers are more likely to adhere to a screening
programme.86 When implementing a population-wide
programme, recruiting primary care physicians
to promote CRC screening and perform office-based
iFOBT would be logical and is feasible.
Conclusion
Each CRC screening modality has its own niche,
providing unique prognostic benefits but with their
own shortcomings. Based on the available evidence
to date, feasibility, and participant acceptance,
iFOBT appears to be the most suitable CRC index
screening tool for the average-risk population, with
FS as an alternative.
References
1. Bernard WS, Christopher PW. World Cancer Report 2014:
Colorectal cancer. Lyon, France: International Agency for
Research on Cancer, WHO; 2014: 392-402.
2. Jass JR. Classification of colorectal cancer based on
correlation of clinical, morphological and molecular
features. Histopathology 2007;50:113-30. Crossref
3. Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic
alterations during colorectal-tumor development. N Engl
J Med 1988;319:525-32. Crossref
4. Snover DC. Update on the serrated pathway to colorectal
carcinoma. Hum Pathol 2011;42:1-10. Crossref
5. Brenner H, Hoffmeister M, Stegmaier C, Brenner G,
Altenhofen L, Haug U. Risk of progression of advanced
adenomas to colorectal cancer by age and sex: estimates
based on 840,149 screening colonoscopies. Gut
2007;56:1585-9. Crossref
6. Waldmann E, Regula J, Ferlitsch M. How can screening
colonoscopy be optimized? Dig Dis 2015;33:19-27. Crossref
7. Shaukat A, Mongin SJ, Geisser MS, et al. Long-term
mortality after screening for colorectal cancer. N Engl J
Med 2013;369:1106-14. Crossref
8. Scholefield JH, Moss SM, Mangham CM, Whynes DK,
Hardcastle JD. Nottingham trial of faecal occult blood
testing for colorectal cancer: a 20-year follow-up. Gut
2012;61:1036-40. Crossref
9. Lindholm E, Brevinge H, Haglind E. Survival benefit in a
randomized clinical trial of faecal occult blood screening
for colorectal cancer. Br J Surg 2008;95:1029-36. Crossref
10. Kronborg O, Jørgensen OD, Fenger C, Rasmussen M.
Randomized study of biennial screening with a faecal
occult blood test: results after nine screening rounds.
Scand J Gastroenterol 2004;39:846-51. Crossref
11. Parra-Blanco A, Gimeno-García AZ, Quintero E, et al.
Diagnostic accuracy of immunochemical versus guaiac
faecal occult blood tests for colorectal cancer screening. J
Gastroenterol 2010;45:703-12. Crossref
12. Hol L, Wilschut JA, van Ballegooijen M, et al. Screening
for colorectal cancer: random comparison of guaiac and
immunochemical faecal occult blood testing at different
cut-off levels. Br J Cancer 2009;100:1103-10. Crossref
13. van Rossum LG, van Rijn AF, Laheij RJ, et al. Random
comparison of guaiac and immunochemical fecal occult
blood tests for colorectal cancer in a screening population.
Gastroenterology 2008;135:82-90. Crossref
14. Dancourt V, Lejeune C, Lepage C, Gailliard MC, Meny
B, Faivre J. Immunochemical faecal occult blood tests
are superior to guaiac-based tests for the detection of
colorectal neoplasms. Eur J Cancer 2008;44:2254-8. Crossref
15. Duffy MJ, van Rossum LG, van Turenhout ST, et al. Use
of faecal markers in screening for colorectal neoplasia: a
European group on tumor markers position paper. Int J
Cancer 2011;128:3-11. Crossref
16. Leggett BA, Hewett DG. Colorectal cancer screening.
Intern Med J 2015;45:6-15. Crossref
17. Tan WS, Tang CL, Koo WH. Opportunistic screening
for colorectal neoplasia in Singapore using faecal
immunochemical occult blood test. Singapore Med J
2013;54:220-3. Crossref
18. Levi Z, Rozen P, Hazazi R, et al. A quantitative
immunochemical fecal occult blood test for colorectal
neoplasia. Ann Intern Med 2007;146:244-55. Crossref
19. Fu WP, Kam MH, Ling WM, Ong SF, Suzannah N, Eu
KW. Screening for colorectal cancer using a quantitative
immunochemical faecal occult blood test: a feasibility study
in an Asian population. Tech Coloproctol 2009;13:225-30. Crossref
20. Kuipers EJ, Rösch T, Bretthauer M. Colorectal cancer
screening—optimizing current strategies and new
directions. Nat Rev Clin Oncol 2013;10:130-42. Crossref
21. Guittet L, Bouvier V, Mariotte N, et al. Performance of
immunochemical faecal occult blood test in colorectal
cancer screening in average-risk population according to
positivity threshold and number of samples. Int J Cancer
2009;125:1127-33. Crossref
22. Grazzini G, Visioli CB, Zorzi M, et al. Immunochemical
faecal occult blood test: number of samples and positivity
cutoff. What is the best strategy for colorectal cancer
screening? Br J Cancer 2009;100:259-65. Crossref
23. Faivre J, Dancourt V, Manfredi S, et al. Positivity rates and
performances of immunochemical faecal occult blood
tests at different cut-off levels within a colorectal cancer
screening programme. Dig Liver Dis 2012;44:700-4. Crossref
24. van Roon AH, Goede SL, van Ballegooijen M, et al. Random
comparison of repeated faecal immunochemical testing at
different intervals for population-based colorectal cancer
screening. Gut 2013;62:409-15. Crossref
25. van Roon AH, Hol L, van Vuuren AJ, et al. Are fecal
immunochemical test characteristics influenced by
sample return time? A population-based colorectal cancer
screening trial. Am J Gastroenterol 2012;107:99-107. Crossref
26. Haug U, Kuntz KM, Knudsen AB, Hundt S, Brenner H.
Sensitivity of immunochemical faecal occult blood testing
for detecting left- vs right-sided colorectal neoplasia. Br J
Cancer 2011;104:1779-85. Crossref
27. de Wijkerslooth TR, Stoop EM, Bossuyt PM, et al.
Immunochemical fecal occult blood testing is equally
sensitive for proximal and distal advanced neoplasia. Am
J Gastroenterol 2012;107:1570-8. Crossref
28. Allison JE, Sakoda LC, Levin TR, et al. Screening for
colorectal neoplasms with new fecal occult blood tests:
Update on performance characteristics. J Natl Cancer Inst
2007;99:1462-70. Crossref
29. Raginel T, Puvinel J, Ferrand O, et al. A population-based
comparison of immunochemical fecal occult blood
tests for colorectal cancer screening. Gastroenterology
2013;144:918-25. Crossref
30. Brenner H, Tao S. Superior diagnostic performance of
faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood
test among 2235 participants of screening colonoscopy.
Eur J Cancer 2013;49:3049-54. Crossref
31. Park DI, Ryu S, Kim YH, et al. Comparison of guaiac-based
and quantitative immunochemical fecal occult blood
testing in a population at average risk undergoing colorectal
cancer screening. Am J Gastroenterol 2010;105:2017-25. Crossref
32. Faivre J, Dancourt V, Denis B, et al. Comparison between
a guaiac and three immunochemical faecal occult blood
tests in screening for colorectal cancer. Eur J Cancer
2012;48:2969-76. Crossref
33. Hol L, van Leerdam ME, van Ballegooijen M, et al. Screening
for colorectal cancer: randomised trial comparing guaiac-based
and immunochemical faecal occult blood testing
and flexible sigmoidoscopy. Gut 2010;59:62-8. Crossref
34. Berchi C, Bouvier V, Réaud JM, Launoy G. Cost-effectiveness
analysis of two strategies for mass screening
for colorectal cancer in France. Health Econ 2004;13:227-38. Crossref
35. Berchi C, Guittet L, Bouvier V, Launoy G. Cost-effectiveness
analysis of the optimal threshold of an
automated immunochemical test for colorectal cancer
screening: performances of immunochemical colorectal
cancer screening. Int J Technol Assess Health Care
2010;26:48-53. Crossref
36. Grazzini G, Ciatto S, Cislaghi C, et al. Cost evaluation
in a colorectal cancer screening programme by faecal
occult blood test in the District of Florence. J Med Screen
2008;15:175-81. Crossref
37. van Rossum LG, van Rijn AF, Verbeek AL, et al.
Colorectal cancer screening comparing no screening,
immunochemical and guaiac fecal occult blood tests: a
cost-effectiveness analysis. Int J Cancer 2011;128:1908-17. Crossref
38. Lieberman D. Colorectal cancer screening: practice
guidelines. Dig Dis 2012;30 Suppl 2:34-8. Crossref
39. Vining DJ, Shifrin RY, Grishaw EK, Liu K, Gelfand DW.
Virtual colonoscopy. Radiology 1994;193(P):446.
40. Sosna J, Blachar A, Amitai M, et al. Colonic perforation at
CT colonography: assessment of risk in a multicenter large
cohort. Radiology 2006;239:457-63. Crossref
41. Berrington de Gonzalez A, Kim KP, Yee J. CT colonography:
perforation rates and potential radiation risks. Gastrointest
Endosc Clin N Am 2010;20:279-91. Crossref
42. Pickhardt PJ, Hanson ME, Vanness DJ, et al. Unsuspected
extracolonic findings at screening CT colonography:
clinical and economic impact. Radiology 2008;249:151-9. Crossref
43. Zalis ME, Blake MA, Cai W, et al. Diagnostic accuracy
of laxative-free computed tomographic colonography for
detection of adenomatous polyps in asymptomatic adults,
a prospective evaluation. Ann Intern Med 2012;156:692-702. Crossref
44. Levin B, Lieberman DA, McFarland B, et al. Screening and
surveillance for the early detection of colorectal cancer
and adenomatous polyps, 2008: a joint guideline from
the American Cancer Society, the US Multi-Society Task
Force on Colorectal Cancer, and the American College of
Radiology. Gastroenterology 2008;134:1570-95. Crossref
45. Steinwachs D, Allen JD, Barlow WE, et al. NIH state-of-the-science conference statement: Enhancing use and
quality of colorectal cancer screening. NIH Consens State
Sci Statements 2010;27:1-31.
46. Sung JJ, Ng SC, Chan FK, et al. An updated Asia Pacific
Consensus Recommendations on colorectal cancer
screening. Gut 2015;64:121-32. Crossref
47. Pickhardt PJ, Choi JR, Hwang I, et al. Computed
tomographic virtual colonoscopy to screen for colorectal
neoplasia in asymptomatic adults. N Engl J Med
2003;349:2191-200. Crossref
48. Johnson CD, Chen MH, Toledano AY, et al. Accuracy of
CT colonography for detection of large adenomas and
cancers. N Engl J Med 2008;359:1207-17. Crossref
49. Martín-López JE, Beltrán-Calvo C, Rodríguez-López
R, Molina-López T. Comparison of the accuracy of
CT colonography and colonoscopy in the diagnosis of
colorectal cancer. Colorectal Dis 2014;16:O82-9. Crossref
50. de Haan MC, van Gelder RE, Graser A, Bipat S, Stoker
J. Diagnostic value of CT-colonography as compared to
colonoscopy in an asymptomatic screening population: a
meta-analysis. Eur Radiol 2011;21:1747-63. Crossref
51. Hanly P, Skally M, Fenlon H, Sharp L. Cost-effectiveness of
computed tomography colonography in colorectal cancer
screening: a systematic review. Int J Technol Assess Health
Care 2012;28:415-23. Crossref
52. Lin OS, Kozarek RA, Gluck M, et al. Preference
for colonoscopy versus computerized tomographic
colonography: a systematic review and meta-analysis of
observational studies. J Gen Intern Med 2012;27:1349-60. Crossref
53. Ou G, Rosenfeld G, Fu YT, et al. Patient satisfaction and
preferences: colonoscopy or computed tomography
colonography for colorectal cancer screening. Gastrointest
Endosc 2012;75 Suppl:AB140. Crossref
54. Hale MF, Sidhu R, McAlindon ME. Capsule endoscopy:
current practice and future directions. World J
Gastroenterol 2014;20:7752-9. Crossref
55. Eliakim R, Yassin K, Niv Y, et al. Prospective multicenter
performance evaluation of the second-generation
colon capsule compared with colonoscopy. Endoscopy
2009;41:1026-31. Crossref
56. Spada C, Hassan C, Munoz-Navas M, et al. Second-generation
colon capsule endoscopy compared with
colonoscopy. Gastrointest Endosc 2011;74:581-9.e1. Crossref
57. Rex DK, Adler SN, Aisenberg J, et al. Accuracy of PillCam
COLON 2 for detecting subjects with adenomas ≥6 mm.
Gastrointest Endosc 2013;77 Suppl 1:AB29.
58. Spada C, Hassan C, Galmiche JP, et al. Colon capsule
endoscopy: European Society of Gastrointestinal
Endoscopy (ESGE) Guideline. Endoscopy 2012;44:527-36. Crossref
59. Pioche M, de Leusse A, Filoche B, et al. Prospective
multicenter evaluation of colon capsule examination
indicated by colonoscopy failure or anesthesia
contraindication. Endoscopy 2012;44:911-6. Crossref
60. Negreanu L, Babiuc R, Bengus A, Sadagurschi R. PillCam
Colon 2 capsule in patients unable or unwilling to undergo
colonoscopy. World J Gastrointest Endosc 2013;5:559-67. Crossref
61. Sieg A, Friedrich K, Sieg U. Is PillCam COLON capsule
endoscopy ready for colorectal cancer screening?
A prospective feasibility study in a community
gastroenterology practice. Am J Gastroenterol
2009;104:848-54. Crossref
62. Bouchard S, Ibrahim M, van Gossum A. Video capsule
endoscopy: perspectives of a revolutionary technique.
World J Gastroenterol 2014;20:17330-44. Crossref
63. Spada C, Barbaro F, Andrisani G, et al. Colon capsule
endoscopy: What we know and what we would like to
know. World J Gastroenterol 2014;20:16948-55. Crossref
64. Liao Z, Gao R, Xu C, Li ZS. Indications and detection,
completion, and retention rates of small-bowel capsule
endoscopy: a systematic review. Gastrointest Endosc
2010;71:280-6. Crossref
65. Triantafyllou K, Beintaris I, Dimitriadis GD. Is there a role
for colon capsule endoscopy beyond colorectal cancer
screening? A literature review. World J Gastroenterol
2014;20:13006-14. Crossref
66. Regge D, Iussich G, Senore C, et al. Population screening
for colorectal cancer by flexible sigmoidoscopy or CT
colonography: study protocol for a multicenter randomized
trial. Trials 2014;15:97. Crossref
67. Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible
sigmoidoscopy screening in prevention of colorectal
cancer: a multicentre randomised controlled trial. Lancet
2010;375:1624-33. Crossref
68. Hoff G, Grotmol T, Skovlund E, Bretthauer M;
Norwegian Colorectal Cancer Prevention Study Group.
Risk of colorectal cancer seven years after flexible
sigmoidoscopy screening: randomised controlled trial.
BMJ 2009;338:b1846.
69. Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer
incidence and mortality with screening flexible
sigmoidoscopy. N Engl J Med 2012;366:2345-57. Crossref
70. Denis B, Gendre I, Aman F, Ribstein F, Maurin P, Perrin
P. Colorectal cancer screening with the addition of flexible
sigmoidoscopy to guaiac-based faecal occult blood testing:
A French population-based controlled study (Wintzenheim
trial). Eur J Cancer 2009;45:3282-90. Crossref
71. Holme Ø, Løberg M, Kalager M, et al. Effect of flexible
sigmoidoscopy screening on colorectal cancer incidence
and mortality: a randomized clinical trial. JAMA
2014;312:606-15. Crossref
72. Mansouri D, McMillan DC, Roxburgh CS, Moug SJ,
Crighton EM, Horgan PG. Flexible sigmoidoscopy
following a positive faecal occult blood test within a
bowel screening programme may reduce the detection of
neoplasia. Colorectal Dis 2013;15:1375-81. Crossref
73. Laiyemo AO, Doubeni C, Pinsky PF, et al. Factors associated
with inadequate colorectal cancer screening with flexible
sigmoidoscopy. Cancer Epidemiol 2012;36:395-9. Crossref
74. Shum NF, Lui YL, Choi HK, Lau SC, Ho JW. A
comprehensive training programme for nurse endoscopist
performing flexible sigmoidoscopy in Hong Kong. J Clin
Nurs 2010;19:1891-6. Crossref
75. Blakeborough A, Sheridan MB, Chapman AH.
Complications of barium enema examinations: A survey
of UK consultant radiologists 1992 to 1994. Clin Radiol
1997;52:142-8. Crossref
76. Winawer SJ, Stewart ET, Zauber AG, et al. A comparison
of colonoscopy and double-contrast barium enema for
surveillance after polypectomy. National Polyp Study
Work Group. N Engl J Med 2000;342:1766-72. Crossref
77. Canon CL. Is there still a role for double-contrast
barium enema examination? Clin Gastroenterol Hepatol
2008;6:389-92. Crossref
78. Robinson MH, Hardcastle JD, Moss SM, et al. The risks
of screening: data from the Nottingham randomised
controlled trial of faecal occult blood screening for
colorectal cancer. Gut 1999;45:588-92. Crossref
79. Rex DK, Weddle RA, Lehman GA, et al. Flexible
sigmoidoscopy plus air contrast barium enema versus
colonoscopy for suspected lower gastrointestinal bleeding.
Gastroenterology 1990;98:855-61. Crossref
80. Rex DK, Mark D, Clarke B, Lappas JC, Lehman GA.
Flexible sigmoidoscopy plus air-contrast barium enema
versus colonoscopy for evaluation of symptomatic patients
without evidence of bleeding. Gastrointest Endosc
1995;42:132-8. Crossref
81. Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke
CA, Inadomi JM; American College of Gastroenterology.
American College of Gastroenterology guidelines for
colorectal cancer screening 2009 [corrected]. Am J
Gastroenterol 2009;104:739-50. Crossref
82. Bowel cancer—screening. Available from: http://www.nhs.uk/Conditions/Cancer-of-the-colon-rectum-or-bowel/Pages/Screeningforbowelcancer.aspx. Accessed Dec 2015.
83. von Karsa L, Patnick J, Segnan N. European guidelines
for quality assurance in colorectal cancer screening and
diagnosis. First Edition—Executive summary. Endoscopy
2012;44 Suppl 3:SE1-8.
84. Thomas R, Michael S, Finlay M, et al. Australian Clinical
Practice Guidelines for the Prevention, Early Detection
and Management of Colorectal Cancer. Natl Heal Med Res
Counc Aust Gov; 2005.
85. Early detection and prevention. Available from: http://www.cancer-fund.org/colorectal/html/eng/detection.html. Accessed Dec 2015.
86. Francisco D, Rankin L, Kim SC. Adherence to colorectal
cancer and polyps screening recommendations among
filipino-americans. Gastroenterol Nurs 2014;37:384-90. Crossref

