Hong Kong Med J 2016 Feb;22(1):39–45 | Epub 23 Oct 2015
DOI: 10.12809/hkmj144482
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Partial nephrectomy for T1 renal cancer can
achieve an equivalent oncological outcome
to radical nephrectomy with better renal
preservation: the way to go
Terence CT Lai, MB, BS;
WK Ma, MB, ChB, FRCSEd (Urol);
MK Yiu, MB, BS, FRCSEd
Division of Urology, Department of Surgery, The University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong
Corresponding author: Dr WK Ma (kitkitma@yahoo.com)
Abstract
Introduction: Patients who undergo partial
nephrectomy have been shown to be at decreased
risk of renal impairment compared with radical
nephrectomy. We examined the oncological outcome
of patients in our centre who underwent partial or
radical nephrectomy for T1 renal cancer (7 cm or
smaller), and compared the likelihood of developing
chronic kidney disease.
Methods: This historical cohort study with internal
comparison was conducted in a tertiary hospital in
Hong Kong. A cohort of 86 patients with solitary
T1 renal cancer and a normal contralateral kidney
who underwent radical (38 patients) or partial (48
patients) nephrectomy between January 2005 and
December 2010 was included. The overall and
cancer-free survival, change in glomerular filtration
rate, and new onset of chronic kidney disease
were compared between the radical and partial
nephrectomy groups.
Results: A total of 32 (84%) radical nephrectomy
patients and 43 (90%) partial nephrectomy patients
were alive by 31 December 2012. The mean follow-up
was 43.5 (standard deviation, 22.4) months.
There was no significant difference in overall
survival (P=0.29) or cancer-free survival (P=0.29)
between the two groups. Both groups enjoyed
good oncological outcome with no recurrence in
the partial nephrectomy group. Overall, 18 (21%)
patients had pre-existing chronic kidney disease.
The partial nephrectomy group had a significantly
smaller median reduction in glomerular filtration rate (12.6%
vs 35.4%; P<0.001), and radical nephrectomy carried
a significantly higher risk of developing chronic
kidney disease (hazard ratio=5.44; 95% confidence
interval, 1.26-23.55; P=0.02).
Conclusions: Compared with radical nephrectomy,
partial nephrectomy can prevent chronic kidney
disease and still achieve an excellent oncological
outcome for T1 renal tumours, in particular T1a
tumours and tumours with a low R.E.N.A.L. score.
New knowledge added by this study
- Partial nephrectomy for T1 renal tumour is associated with excellent overall and cancer-free survival, and better renal preservation than radical nephrectomy.
- As a significant proportion of T1 renal cancers are still managed by radical nephrectomy in our locality, we recommend partial nephrectomy for T1a and selected T1b renal cancers, provided that relevant expertise is available.
Introduction
With the widespread use of advanced imaging such
as computed tomography (CT), many renal tumours
are now incidentally discovered before the patient
becomes symptomatic. These tumours are often
of small size. This has led to the emerging practice
of partial nephrectomy (PN) rather than radical
nephrectomy (RN) that has been the gold-standard
treatment for localised renal tumours for over 40
years.1 Studies have shown that cancer control can
be achieved by PN in patients with T1a tumours,2 3 4 5 and some studies also supported the extended use of PN for T1b tumours.6 7 8 9 In addition, patients who undergo PN have been shown to be at decreased risk of renal impairment.10
Nonetheless, many surgeons in Hong Kong
continue to perform RN for all renal tumours,
regardless of their size. The major concerns are the
technical difficulty of PN and the associated major
postoperative complications.
The aim of this study was to compare the
oncological outcome, survival, and changes in renal
function in patients who underwent RN or PN
for T1 renal cancer in our centre. Patients were
predominantly of Chinese ethnicity.
Methods
We retrospectively reviewed the data of patients who
underwent RN or PN at our centre between January
2005 and December 2010. Patients with a solitary
tumour of 7 cm or less in diameter and a normal
contralateral kidney were included. The decision to
perform PN was based on tumour characteristics
(size, proximity to collecting system and major
vessels) and the surgeon’s preference. Exclusion
criteria were: end-stage renal failure (glomerular
filtration rate [GFR] <15 mL/min/1.73 m2), history of
renal transplantation, known hereditary renal cancer,
known poor or non-functioning contralateral kidney,
history of nephrectomy, and preoperative
evidence of tumour metastases.
Preoperative parameters including age, gender,
serum creatinine, and estimated GFR were studied.
We used the modified Charlson-Romano index to
compare patient co-morbidity.11 We also compared
postoperative outcome for the RN and PN groups,
including length of hospital stay, complications,
and 90-day mortality. Severity of complications
was graded using the Clavien-Dindo classification
system.12
Our study outcome included 5-year overall
survival, cancer-free survival, change in renal
function in terms of estimated GFR (eGFR), and
new onset of chronic kidney disease (CKD)—GFR
was calculated with the four-variable Modification
of Diet in Renal Disease formula: eGFR = 32 788 x
serum creatinine (µmol/L)-1.154 x age-0.203 x [1.212
if black] x [0.742 if female]13; CKD was defined
as GFR of <60 mL/min/1.73 m2. Patients were
followed up according to international guidelines,
with slight variation in timing of imaging due to
examination waiting time issues. In general, follow-up
was scheduled every 3 months within the first
year of operation with measurement of serum
creatinine and GFR, and CT scan was performed
approximately 12 months following surgery. Patients
were subsequently followed up every 6 months, with
renal function checked at each visit, and imaging
studies (ultrasonography or CT) performed annually
for the first 5 years and thereafter once every 2 years.
For preoperative characteristics and
postoperative outcome, P value was determined by
the Chi squared test and Mann-Whitney U test for
categorical and continuous variables, respectively.
Kaplan-Meier model was used for 5-year overall
and cancer-free survival, and the postulated 5-year
probability of freedom from CKD. Risk of new onset
of CKD was calculated with Cox proportional hazards
regression, adjusted for age, Charlson Comorbidity
Index (CCI), preoperative GFR, gender, and tumour
size; with time to CKD development as dependent
variable; death, loss to follow-up, or last follow-up
date before 31 December 2012 were censored
in the analysis. Overall survival was analysed by
Cox proportional hazards regression adjusted with
Fuhrman grade of tumour, in addition to the above
factors. We did not include diabetes mellitus as it
was included in the CCI. We considered a 2-sided P
value of <0.05 as statistically significant. All statistical
analyses were performed with the Statistical Package
for the Social Sciences (Windows version 20.0; SPSS
Inc, Chicago [IL], US).
Results
A total of 86 patients were reviewed, with a mean
(± standard deviation) follow-up time of 43.5 ± 22.4
months. Four (5%) patients were lost to follow-up
with their status unknown by the end of our study.
Overall, RN was performed in 38 patients and PN in
48. Age, gender, and preoperative serum creatinine level
and GFR were similar between the two groups. The
mean age of RN and PN groups was 61 years and
63 years, respectively (P=0.48), with a respective
mean preoperative GFR of 80.8 mL/min/1.73 m2 and
75.0 mL/min/1.73 m2 (P=0.38). Six (16%) patients
in the RN group and 11 (23%) in the PN group had
a preoperative GFR of <60 mL/min/1.73 m2; one
patient in the PN group had a preoperative GFR of
<30 mL/min/1.73m2. Most patients in the RN and
PN groups had a CCI of <2 (84% vs 85%; P=0.87).
The respective mean follow-up time was 42.1 ± 24.0
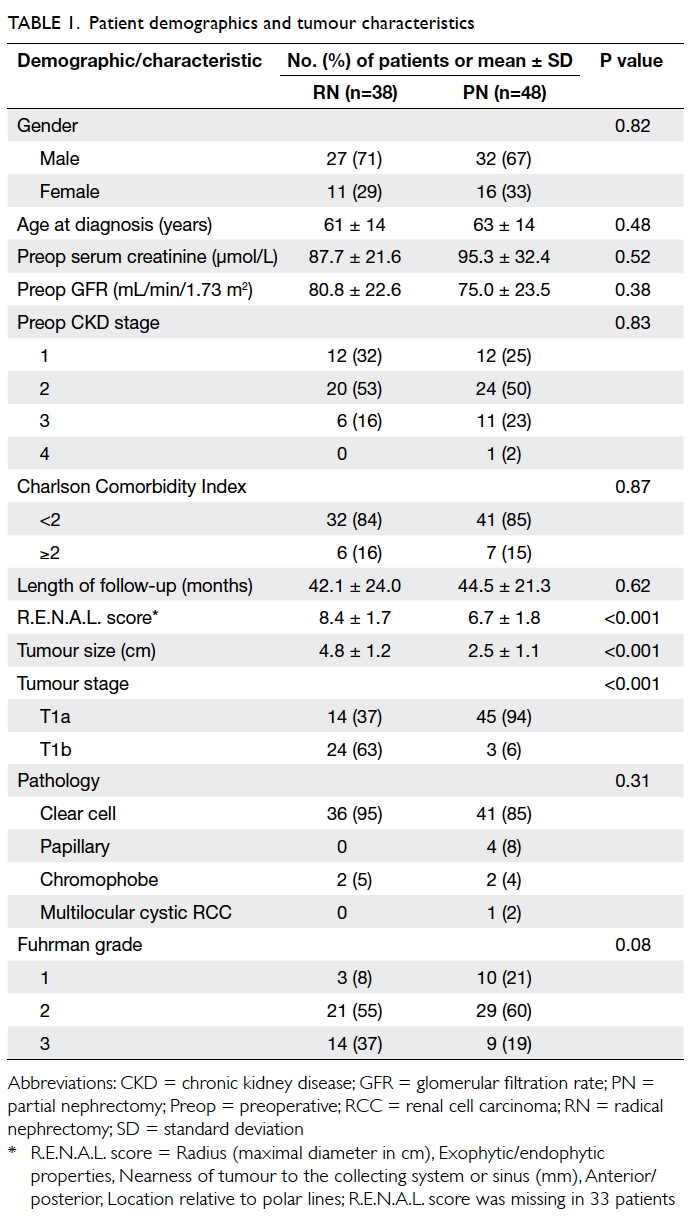
and 44.5 ± 21.3 months (P=0.62) [Table 1].
Tumours were more complex in the RN group
in terms of the R.E.N.A.L. score14 derived from
preoperative CT (8.4 vs 6.7, P<0.001). The mean
tumour size, based on final pathological examination,
was also larger in the RN group (4.8 cm vs 2.5 cm,
P<0.001). In the RN group, 24 (63%) patients had
a T1b tumour compared with three (6%) in the PN
group (P<0.001). Comparison of the first half of
our study period (2005-2008) with the second half
(2009-2010) revealed that tumour characteristics
were similar in the PN group, in terms of both size
(2.4 cm vs 2.5 cm; P=0.93) and R.E.N.A.L. score
(6.2 vs 7.0; P=0.32). Most tumours in both groups
were of the clear cell type. The RN group had more
Fuhrman grade 3 tumours but the difference was
not significant (Table 1). All resections enjoyed clear resection margins on final pathological examination.
All RNs were performed via a laparoscopic
approach. An open procedure was performed for
29 (60%) of the PNs, eight (17%) were performed via a
laparoscopic approach, and 11 (23%) with robotic
assistance. Of the latter, conversion to an open
procedure was required in two cases. Operating
time was significantly longer in the PN group (250
mins vs 345 mins; P<0.001). None of the PNs were
converted to RN.
All PNs were performed with vascular control
achieved by hilar clamping. Overall, 28 (58%) PNs
were performed with cold ischaemia using ice slush,
with a mean cold ischaemia time of 70 minutes;
among these, 23 (82%) were an open procedure
and two were conversion of robotic-assisted
laparoscopic PN to open procedure. Of the PNs, 20
(42%) were performed with warm ischaemia, and
the mean warm ischaemia time was 32 minutes;
all laparoscopic PNs were performed with warm
ischaemia. The complexity of tumour in terms of
R.E.N.A.L. score was significantly lower in the warm
ischaemia group (5.7 vs 7.5; P=0.01).
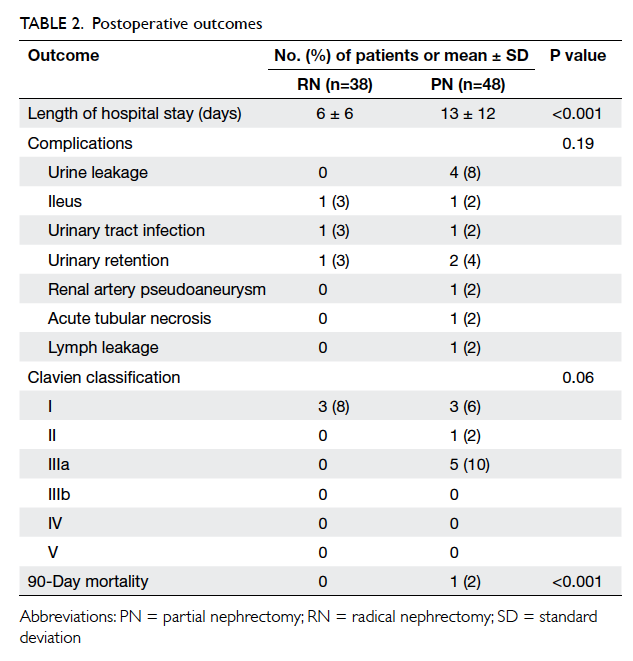
Patients in the PN group had a longer
postoperative hospital stay (6 vs 13 days; P<0.001);
one of whom died within 90 days of surgery of
cholecystitis and septic complications unrelated to
the renal cancer.
Postoperative complication rates were similar
between the two groups (P=0.19), although the
PN group had more Clavien grade III or above
complications (0% vs 10%; Table 2). Four patients
had persistent urine leakage that was successfully
treated with retrograde injection of surgical adhesive
glue; one patient developed pseudoaneurysm of the
segmental branch of the renal artery, which was
treated by angiographic embolisation. No long-term
morbidity or mortality occurred. Complication rate
was not associated with patient age, CCI, R.E.N.A.L.
score of tumour, or operative parameters (operative
approach, operating time, and ischaemia time).
An overall excellent oncological outcome was
achieved by both groups. Extensive metastases were
evident 3 months after operation in one patient in
the RN group but these were not apparent before
operation. By 31 December 2012, 32 (84%) RN and 43 (90%) PN patients were alive. The overall 5-year survival for the RN
and PN groups was 84.2% and 89.6% (P=0.29)
respectively, and the cancer-free survival was 97%
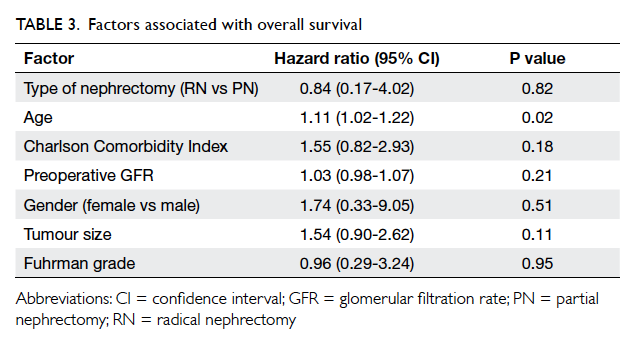
and 100% (P=0.29) respectively. Both RN and PN did
not affect overall survival (hazard ratio=0.84; 95%
confidence interval [CI], 0.17-4.02; P=0.82), after
adjustment for age, CCI, preoperative GFR, gender,
tumour size, and Fuhrman grade (Table 3).
Patients who underwent RN had a greater
median reduction in GFR than PN patients (35.4%
vs 12.6%; P<0.001), and the degree of reduction
was most distinctive in the first year after operation
(Fig 1). In the PN group, one patient with a
preoperative GFR of 30 mL/min/1.73 m2 developed
stage 5 CKD 4 years after surgery and required renal
replacement therapy. Preoperative GFR was >60 mL/min/1.73 m2 in 32 patients in the RN group and in 36
patients in the PN group. At their last follow-up, CKD
was developed in 18 (56%) patients who underwent
RN but new-onset CKD was evident in only five
(14%) of the PN group (P<0.001). The majority of the
CKD cases were stage 3 (94% in RN group and 80% in
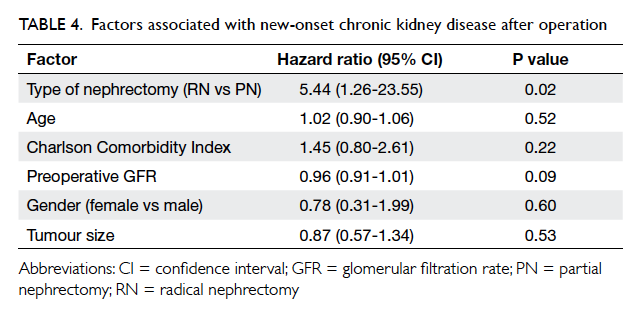
PN group); none was in stage 5. Cox proportional hazards
regression model showed that RN was the most
significant factor contributing to the development of
CKD (RN vs PN, hazard ratio=5.44; 95% CI, 1.26-23.55; P=0.02; Table 4). The postulated probability of
freedom from new-onset CKD in 5 years, by Kaplan-Meier model, was 33% and 81% in the RN and PN
groups, respectively (P<0.001; Fig 2).
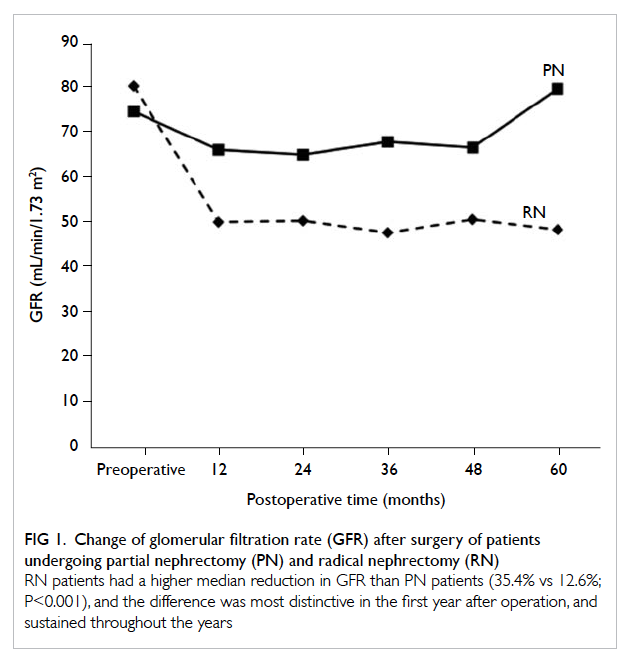
Figure 1. Change of glomerular filtration rate (GFR) after surgery of patients undergoing partial nephrectomy (PN) and radical nephrectomy (RN)
RN patients had a higher median reduction in GFR than PN patients (35.4% vs 12.6%; P<0.001), and the difference was most distinctive in the first year after operation, and sustained throughout the years
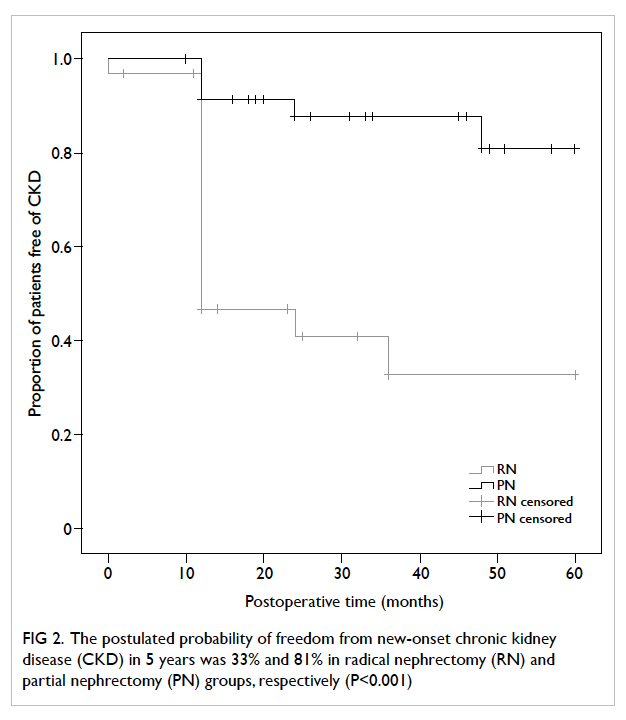
Figure 2. The postulated probability of freedom from new-onset chronic kidney disease (CKD) in 5 years was 33% and 81% in radical nephrectomy (RN) and partial nephrectomy (PN) groups, respectively (P<0.001)
Discussion
Many surgeons have underestimated the impact of
renal impairment after RN for renal cancer, on the
basis that organ donors who undergo nephrectomy
are not at increased risk of renal failure or death.15 16 17 Nonetheless, donors represent a different population
as they are often young and fit. Patients with renal
cancer are often older and have co-morbidities
such as hypertension and diabetes mellitus. In our
study, 21% of our patients had a GFR of <60 mL/min/1.73 m2 prior to surgery and 15% had a CCI of ≥2. Therefore it is logical that this cohort of patients may benefit from a surgical technique that preserves
more of their renal function.
Our study showed that PN resulted in less
renal deterioration in terms of GFR, with RN having
a hazard ratio of 5.44 for development of CKD, after
taking into consideration the patient co-morbidities,
gender, age, and tumour size. The postulated
probability of freedom from new onset of CKD in
5 years in our series was 33% following RN and 81%
following PN. This echoes the finding of Huang et
al10 who reported the 3-year probability of freedom
from new onset of CKD as 35% after RN and 80%
after PN; RN remained an independent risk factor
for development of new-onset CKD with a hazard
ratio of 3.82.
A community-based study showed that CKD
was an independent risk factor for the development
of cardiovascular events, hospitalisation, and death.18
In a population-based cohort of 7769 patients, RN
was associated with a 1.23-fold increase in overall
mortality compared with PN (P=0.001), and a higher
rate of non–cancer-related mortality.19 Huang et al10
also demonstrated that RN was associated with a
1.46-fold increased risk of overall mortality, although
the risk of a cardiovascular event was not increased
in the RN group. Evidence that PN decreases overall
mortality remains contradictory. A randomised
controlled trial showed that RN had comparable
overall survival with PN after a median follow-up of
9.3 years.20 In our study we did not show a significant
difference in overall survival between PN and RN
groups.
Another concern of PN is its cancer control,
since the prevention of local recurrence is of
paramount importance. The extent of resection is
affected by tumour size, proximity to the collecting
system, and location and degree of exophytic growth.
It is generally accepted that PN can achieve excellent
oncological outcome for tumours smaller than 4
cm, with a long-term 5-year and 10-year cancer-free
survival rates of 92% to 100%.2 21 22 23 Studies have
demonstrated the feasibility of PN for tumours larger
than 4 cm without compromising the oncological
outcome, although there was a higher risk of peri-operative
bleeding and other complications.23 24
In our cohort, we achieved a 100% clear surgical
resection margin with PN, and no local recurrence
was found after 5 years.
Increased peri-operative morbidity is
traditionally a concern in PN. There were more
Clavien grade III complications and a longer hospital
stay for patients who underwent PN in our cohort.
The most common complication in an open PN
series was urine leakage, with a mean incidence of
6.5% (range, 2.1%-17%).25 26 In a multicentre review
of 51 laparoscopic PNs, postoperative urine leakage
was observed in three (6%) patients.27 The results
of a previous series by Gill et al,28 supported by
our results, suggested that both tumour location
and diameter were not related to the occurrence of
urine leakage. In contrast to the logical thinking that
calyceal entry was not observed during renorrhaphy,
it has been suggested that central coagulation
necrosis with electrocautery is responsible for
fistula formation.27 The use of a ureteral catheter and
retrograde dye injection after haemostasis has been
advocated to help identify any calyceal opening,
but this was not supported in a retrospective series
by Bove et al29 that involved 54 patients with and
49 patients without ureteral catheter placement.
We believe that the adoption of cold cutting and
elevation of the tumour from the tumour bed by the
suction cannula, which also simultaneously aspirates
the blood, can avoid coagulation necrosis and a
clear operative field can be maintained so that any
breaching of calyceal integrity can be identified. The
use of a ureteral catheter can be an adjunct measure
in equivocal cases when the tumour is abutting the
calyceal lining on preoperative imaging.
Our study have some limitations. Since it was
a retrospective study, patients were not randomised
and there was a selection of smaller and less complex
tumours in the PN group. There were also other
confounding factors such as patient’s smoking status
that were not included, hence the two groups were
not totally comparable, although other patient
demographics were similar. As a proportion of
preoperative imaging could not be retrieved, the
R.E.N.A.L. score could not be calculated for every
patient included, and this contributed another
confounding factor. Only three patients had T1b
renal cancer in the PN group, thus the oncological
outcome may be more certain in T1a renal cancer.
Our stratified analysis in T1a renal cancer had
a similar result with PN having an equivalent
oncological outcome and superior renal function
preservation, although the result is not shown here.
In addition, the risk of tumour recurrence, negative
effect of CKD and their effect on survival might not
be truly reflected in our relatively short follow-up
time. Nonetheless with experience, renal tumours of
4 cm or more in diameter may be amenable to safe
PN with equivalent oncological outcome and a lower
chance of progression to CKD. This may translate
into improved overall survival.30
Conclusions
Partial nephrectomy can preserve more renal
function and reduce the risk of development of CKD
compared with RN. Excellent cancer control and a
low local recurrence rate can still be achieved with
PN for T1 tumours, in particular T1a tumours and
tumours with a low R.E.N.A.L. score. Although
RN continues to constitute a significant proportion
of surgical procedures for T1 renal cancer in our
locality, we recommend that, if technically feasible,
PN should be performed for all T1a and selected T1b
renal cancers.
References
1. Robson CJ, Churchill BM, Anderson W. The results of
radical nephrectomy for renal cell carcinoma. J Urol
1969;101:297-301.
2. Fergany AF, Hafez KS, Novick AC. Long-term results of
nephron sparing surgery for localized renal cell carcinoma:
10-year followup. J Urol 2000;163:442-5. Crossref
3. Lee CT, Katz J, Shi W, Thaler HT, Reuter VE, Russo P.
Surgical management of renal tumors 4 cm or less in a
contemporary cohort. J Urol 2000;163:730-6. Crossref
4. Touijer K, Jacqmin D, Kavoussi LR, et al. The expanding role
of partial nephrectomy: a critical analysis of indications,
results, and complications. Eur Urol 2010;57:214-22. Crossref
5. Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective
randomized EORTC intergroup phase 3 study comparing
the complications of elective nephron-sparing surgery and
radical nephrectomy for low-stage renal cell carcinoma.
Eur Urol 2007;51:1606-15. Crossref
6. Patard JJ, Shvarts O, Lam JS, et al. Safety and efficacy
of partial nephrectomy for all T1 tumors based on an
international multicenter experience. J Urol 2004;171:2181-5. Crossref
7. Leibovich BC, Blute M, Cheville JC, Lohse CM, Weaver
AL, Zincke H. Nephron sparing surgery for appropriately
selected renal cell carcinoma between 4 and 7 cm results
in outcome similar to radical nephrectomy. J Urol
2004;171:1066-70. Crossref
8. Dash A, Vickers AJ, Schachter LR, Bach AM, Snyder ME,
Russo P. Comparison of outcomes in elective partial vs
radical nephrectomy for clear cell renal cell carcinoma of
4-7 cm. BJU Int 2006;97:939-45. Crossref
9. Antonelli A, Cozzoli A, Nicolai M, et al. Nephron-sparing
surgery versus radical nephrectomy in the treatment of
intracapsular renal cell carcinoma up to 7 cm. Eur Urol
2008;53:803-9. Crossref
10. Huang WC, Levey AS, Serio AM, et al. Chronic kidney
disease after nephrectomy in patients with renal cortical
tumours: a retrospective cohort study. Lancet Oncol
2006;7:735-40. Crossref
11. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A
new method of classifying prognostic comorbidity in
longitudinal studies: development and validation. J Chronic
Dis 1987;40:373-83. Crossref
12. Dindo D, Demartines N, Clavien PA. Classification of
surgical complications: a new proposal with evaluation in
a cohort of 6336 patients and results of a survey. Ann Surg
2004;240:205-13. Crossref
13. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D.
A more accurate method to estimate glomerular filtration
rate from serum creatinine: a new prediction equation.
Modification of Diet in Renal Disease Study Group. Ann
Intern Med 1999;130:461-70. Crossref
14. Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a
comprehensive standardized system for quantitating renal
tumor size, location and depth. J Urol 2009;182:844-53. Crossref
15. Najarian JS, Chavers BM, McHugh LE, Matas AJ. 20 Years
or more of follow-up of living kidney donors. Lancet
1992;340:807-10. Crossref
16. Fehrman-Ekholm I, Dunér F, Brink B, Tydén G, Elinder
CG. No evidence of accelerated loss of kidney function in
living kidney donors: results from a cross-sectional follow-up.
Transplantation 2001;72:444-9. Crossref
17. Fehrman-Ekholm I, Elinder CG, Stenbeck M, Tydén G,
Groth CG. Kidney donors live longer. Transplantation
1997;64:976-8. Crossref
18. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu
CY. Chronic kidney disease and the risks of death,
cardiovascular events, and hospitalization. N Engl J Med
2004;351:1296-305. Crossref
19. Zini L, Perrotte P, Capitanio U, et al. Radical versus partial
nephrectomy: effect on overall and noncancer mortality.
Cancer 2009;115:1465-71. Crossref
20. Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective,
randomised EORTC intergroup phase 3 study comparing
the oncologic outcome of elective nephron-sparing
surgery and radical nephrectomy for low-stage renal cell
carcinoma. Eur Urol 2011;59:543-52. Crossref
21. Uzzo RG, Novick AC. Nephron sparing surgery for renal
tumors: indications, techniques and outcomes. J Urol
2001;166:6-18. Crossref
22. Herr HW. Partial nephrectomy for unilateral renal
carcinoma and a normal contralateral kidney: 10-year
followup. J Urol 1999;161:33-4; discussion 34-5. Crossref
23. Becker F, Siemer S, Humke U, Hack M, Ziegler M,
Stöckle M. Elective nephron sparing surgery should
become standard treatment for small unilateral renal cell
carcinoma: long-term survival data of 216 patients. Eur
Urol 2006;49:308-13. Crossref
24. Patard JJ, Pantuck AJ, Crepel M, et al. Morbidity and
clinical outcome of nephron-sparing surgery in relation to
tumour size and indication. Eur Urol 2007;52:148-54. Crossref
25. Campbell SC, Novick AC, Streem SB, Klein E, Licht
M. Complications of nephron sparing surgery for renal
tumors. J Urol 1994;151:1177-80.
26. Kim FJ, Rha KH, Hernandez F, Jarrett TW, Pinto
PA, Kavoussi LR. Laparoscopic radical versus partial
nephrectomy: assessment of complications. J Urol
2003;170:408-11. Crossref
27. Jeschke K, Peschel R, Wakonig J, Schellander L, Bartsch
G, Henning K. Laparoscopic nephron-sparing surgery for
renal tumors. Urology 2001;58:688-92. Crossref
28. Gill IS, Desai MM, Kaouk JH, et al. Laparoscopic partial
nephrectomy for renal tumor: duplicating open surgical
techniques. J Urol 2002;167:469-76. Crossref
29. Bove P, Bhayani SB, Rha KH, Allaf ME, Jarrett TW, Kavoussi
LR. Necessity of ureteral catheter during laparoscopic
partial nephrectomy. J Urol 2004;172:458-60. Crossref
30. Weight CJ, Larson BT, Gao T, et al. Elective partial
nephrectomy in patients with clinical T1b renal tumors
is associated with improved overall survival. Urology
2010;76:631-7. Crossref