DOI: 10.12809/hkmj154525
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE
First-trimester medical abortion service in Hong Kong
Sue ST Lo, MD, FRCOG1;
PC Ho, FRCOG, FHKAM (Obstetrics and Gynaecology)2
1 The Family Planning Association of Hong Kong, 10/F, Southorn Centre,
130 Hennessy Road, Wanchai, Hong Kong
2 Department of Obstetrics and Gynaecology, The University of Hong
Kong, Pokfulam, Hong Kong
Corresponding author: Dr Sue ST Lo (stlo@famplan.org.hk)
Abstract
Research on medical abortion has been conducted
in Hong Kong since the 1990s. It was not until 2011
that the first-trimester medical abortion service was
launched. Mifepristone was registered in Hong Kong
in April 2014 and all institutions that are listed in the
Gazette as a provider for legal abortion can purchase
mifepristone from the local provider. This article
aimed to share our 3-year experience of this service
with the local medical community. Our current
protocol is safe and effective, and advocates 200-mg
mifepristone and 400-µg sublingual misoprostol
24 to 48 hours later, followed by a second dose of
400-µg sublingual misoprostol 4 hours later if the
patient does not respond. The complete abortion
rate is 97.0% and ongoing pregnancy rate is 0.4%.
Some minor side-effects have been reported and
include diarrhoea, fever, abdominal pain, and allergy.
There have been no serious adverse events such as
heavy bleeding requiring transfusion, anaphylactic
reaction, septicaemia, or death.
Introduction
In Hong Kong, legal abortion can be performed in
registered institutions in accordance with the legal
requirements stipulated in the Offences against
the Person Ordinance Cap 212. In the past, first-trimester
pregnancies up to 12 weeks of gestation
were terminated by suction evacuation and achieved
a complete abortion rate of 99%.1 Possible risks and
complications, which could affect future fertility,
include heavy bleeding, infection, uterine perforation
with or without bowel injury, and cervical trauma.
With the introduction of mifepristone, a
progesterone antagonist, effective and safe medical
abortion became possible. Over the past three
decades, various medical abortion protocols have
been tested in different countries and Hong Kong has
participated in such research since the 1990s. Most
institutions recommended the use of mifepristone
followed by prostaglandin 24 to 48 hours later to
stimulate uterine contraction and expulsion of the
conceptus. Mifepristone was first registered for
medical abortion in France and China in 1988. By
2013, mifepristone was registered in 60 countries2
and was eventually registered in Hong Kong on 8
April 2014 for medical abortion and cervical priming
before first-trimester surgical abortion (Mifegyne;
Exelgyn, Paris, France). All institutions listed in the
Gazette as a legal abortion provider can purchase
mifepristone from a local provider. The provider
cannot sell mifepristone to an individual doctor or
pharmacy store. Although the posology of Mifegyne
indicates its use for first-trimester medical abortion
up to 63 days of gestation, the treatment is also
effective after 63 days of gestation.3
Most regulatory bodies, including Hong Kong,
approved the use of 600-mg mifepristone for medical
abortion but the International Planned Parenthood
Federation (IPPF),4 World Health Organization
(WHO),5 and Royal College of Obstetricians and
Gynaecologists (RCOG)6 recommended the use of
200 mg as it has similar efficacy and lower cost.7
A prostaglandin has to be given after
mifepristone to stimulate uterine contractions.
Misoprostol is the prostaglandin of choice because
it is cheap, effective, and stable at room temperature
but the dose, route, and timing of administration is
not standardised. The WHO and RCOG recommend
800-µg misoprostol (vaginal, buccal, or sublingual)
24 to 48 hours later.5 6 For women at 50 to 63 days of gestation, the RCOG recommends an additional
400-µg misoprostol (vaginal or oral) 4 hours later if
abortion has not occurred.6 The IPPF recommends
800-µg misoprostol (oral, vaginal, buccal, or
sublingual) at once or in two doses 2 hours apart,
36 to 48 hours later.4 When compared with vaginal
misoprostol, sublingual misoprostol appears to be
associated with a higher rate of gastro-intestinal
side-effects, and buccal misoprostol appears to be
associated with a higher rate of diarrhoea.7
The complete abortion rate for medical
abortion is approximately 95%,1 which is slightly
lower than that for surgical abortion. The chance
of ongoing pregnancy is 1% to 2% and the baby
may suffer from congenital abnormalities due
to prostaglandin. Hence it is recommended that
suction evacuation be performed if medical
abortion fails. Bleeding and abdominal cramps are
inevitable side-effects of medical abortion although
<1% of women need emergency curettage because
of excessive bleeding1 and 0.1% require blood
transfusion.8 In a systematic review of 65 studies
with heterogeneous designs, the overall frequency of
diagnosed or treated pelvic infection after medical
abortion was 0.9%.9 The incidence of suspected or
confirmed post-abortion infection (endometritis,
pelvic inflammatory disease) quoted in the posology
of mifepristone is <5%.10 Very common and common
side-effects of mifepristone include nausea,
vomiting, diarrhoea, light or moderate abdominal
cramps, post-abortion infection, uterine cramps, and
heavy bleeding.10 The side-effects of prostaglandin
observed during medical abortion include transient
fever, chills, nausea, vomiting, diarrhoea, headache,
dizziness, and rash.4 The incidence and intensity of
prostaglandin side-effects are determined by the
dose and route of administration.
Our protocols
The Family Planning Association of Hong Kong
(FPAHK) piloted a medical abortion service for
gestation up to 63 days in November 2011. Routine
ultrasound examination was performed to ascertain
the gestation and to exclude multiple pregnancies
and ectopic pregnancy. Exclusion criteria included
hypersensitivity to mifepristone or misoprostol,
multiple pregnancies, haemoglobin level of
<100 g/L, bleeding tendency, known coagulopathy,
use of anticoagulant, current long-term systemic
corticosteroid therapy, history of adrenal tumour/steroid-dependent tumour, chronic adrenal failure,
porphyria, renal/liver impairment, cardiovascular
disease (including arrhythmia, angina, heart
failure, Raynaud’s disease, diastolic hypertension of
>95 mm Hg, arterial hypotension, history of evidence
of thromboembolism), epilepsy, on multiple
medications, inherited porphyria, severe asthma
uncontrolled by therapy, and breastfeeding.
The initial protocol was 200-mg oral mifepristone followed by
800-µg vaginal misoprostol
48 hours later (protocol 1, n=603). Both doses
were administered in the FPAHK. The woman was
permitted to leave after mifepristone administration
provided she did not feel unwell but was required
to remain for observation on the day of misoprostol
administration. Oral paracetamol, oral mefenamic
acid, and intramuscular pethidine were offered for
pain relief. Vital signs, hydration status, and blood
loss were monitored. Most women passed tissue
mass within 4 hours of misoprostol administration.
The doctor routinely performed a pelvic examination
after 4 hours to remove any tissue mass remaining in
the vagina, and to assess blood loss and vital signs.
Any tissue mass collected was sent for pathological
examination to exclude partial mole. The patient was
discharged provided bleeding was not excessive.
In January 2013, this protocol was changed
to 200-mg oral mifepristone followed by 400-µg sublingual misoprostol 48 hours later (protocol 2,
n=890). We changed from vaginal to sublingual
administration because vaginal administration
is intrusive, and requires more consultation
time and more medical consumables. Sublingual
administration requires the woman to place two
tablets of misoprostol under her tongue and then
swallow after 30 minutes. We used a half dose (400
µg) to minimise the severity of gastro-intestinal
reactions that might make patients uncomfortable
and increase the workload of nursing and auxiliary
staff. Since November 2013, women have been given
a second dose of 400-µg sublingual misoprostol
if abortion has not occurred within 4 hours of
misoprostol administration (protocol 3, n=1042).
We hope this regimen will reduce the ongoing
pregnancy rate by administration of the full dose to
selected patients.
Surgical evacuation is performed if there is
heavy bleeding after misoprostol. Manual vacuum
aspiration was introduced as an alternative to
electric vacuum aspiration in January 2014.
Women return for follow-up after 1 week
if abortion has not started on discharge. Other
women are followed up 2 to 3 weeks after the
abortion. Ultrasound examination is performed
in the presence of any abnormal symptoms or
signs. Suction evacuation is arranged for ongoing
pregnancy. Prior to the introduction of 400-µg
sublingual misoprostol after November 2013
(protocol 3), those with persistent bleeding/spotting
and/or retained products of gestation were managed
expectantly or by curettage. An additional dose of
misoprostol is not offered to women with a history
of caesarean section. Further follow-up is arranged
as required, with final follow-up 6 to 8 weeks after
abortion to confirm return of menstruation and
assess their compliance with contraception.
Women are encouraged to start birth control
immediately after misoprostol administration.
Hormonal injectables are given on discharge. Women
are asked to start oral pills on the evening after
misoprostol. A condom should be used consistently
and correctly thereafter. Insertion of an intrauterine
contraceptive device cannot be performed until
abortion has completed and is usually done at the
6-week follow-up.
Outcome
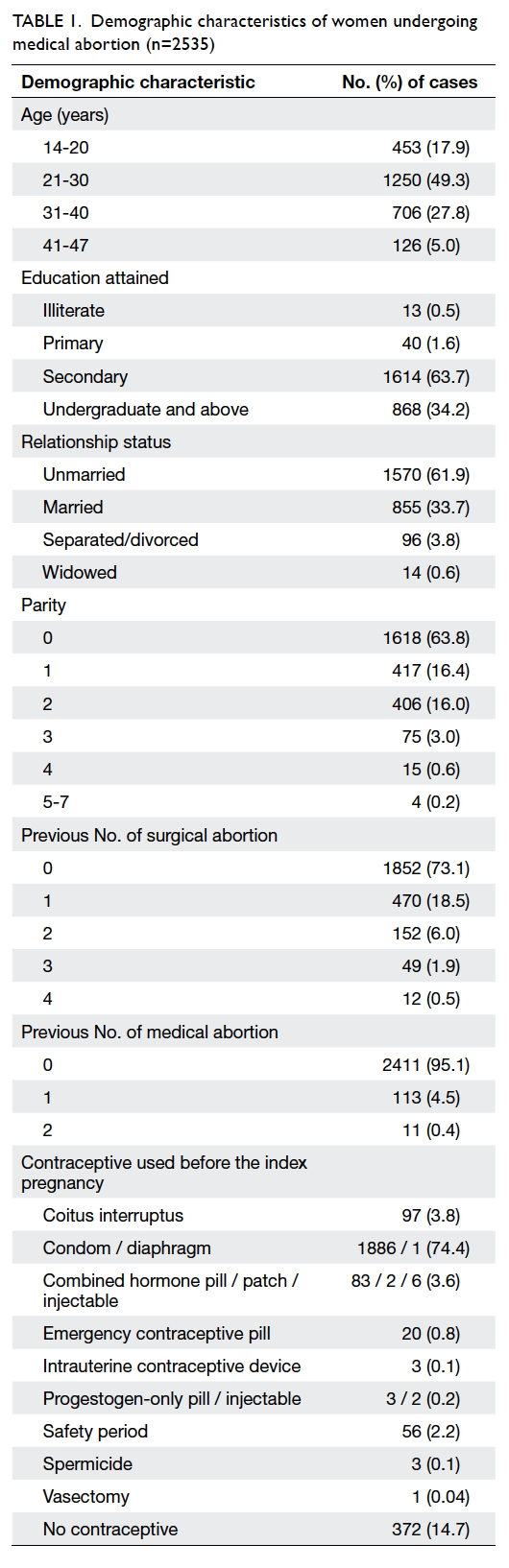
Overall, 2535 medical abortions in 2499 women
were performed in the 3 years from 2011 to 2014.
The median age was 27 years (interquartile range, 11
years). Most women were nulliparous (63.8%), and
mean parity was 0.61 (standard deviation, 0.9). Most
women had no history of surgical (73.1%)
or medical (95.1%) abortion. The demographics are
detailed in Table 1.
Five women took mifepristone only, without
misoprostol. One woman developed palmar
erythema and intense itching 30 minutes after vaginal
misoprostol administration: misoprostol tablets
were removed and douching performed. Allergic
signs and symptoms resolved 15 minutes later.
Suction evacuation was subsequently performed on that
day. This was the 84th case when doctors were still
relatively inexperienced and might have overreacted to
her symptoms. One woman was admitted to hospital
for abdominal pain after mifepristone: ultrasound
examination revealed complete abortion and she was
not given misoprostol during admission. Another
woman was admitted to hospital because of vaginal
bleeding: emergency curettage was performed.
Two women were admitted to hospital because of
persistent vomiting with dehydration. They did not
attend the appointment for misoprostol and suction
evacuation was arranged.
Among 2530 cases admitted for misoprostol,
17 (0.7%) underwent emergency suction evacuation
because of heavy bleeding after misoprostol
administration with 11 having gestation of 56 days
and above. Bleeding stopped after curettage in all
women. The outcomes of medical abortion are listed
in Table 2. After excluding 159 women who did not
return for follow-up, the overall complete abortion
rate was 97.0%. The complete abortion rate in those
with gestation of ≥56 days was significantly lower
than in those with <56 days of gestation (95.7% vs
97.7%; Fisher’s exact test, P=0.007). The complete
abortion rate for protocols 1, 2, and 3 was 96.3%,
97.5%, and 97.1% respectively, and the respective
mean gestation was 51.3 days, 51.7 days, and 51.3
days (P=0.362). The complete abortion rate did not
differ by protocol (P=0.440), parity (P=0.527), or
history of miscarriage and termination of pregnancy
(P=0.246). Among those who had curettage after
medical abortion, 64% were performed within the
first 2 weeks, 14% were between third and fourth
week, and the remaining 22% were between fifth and
12th week. Curettage within the first 4 weeks was
performed for excessive bleeding: those performed
later were for retained products of gestation and
persistent spotting.
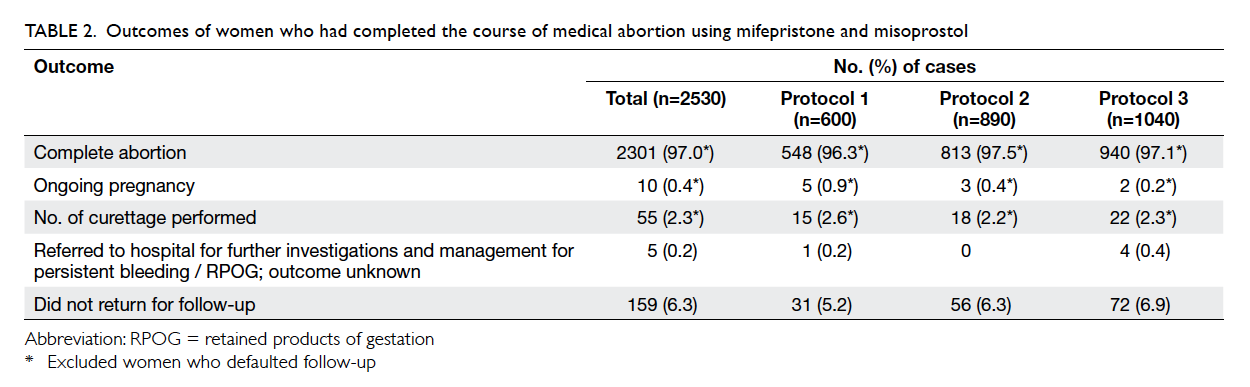
Table 2. Outcomes of women who had completed the course of medical abortion using mifepristone and misoprostol
The overall ongoing pregnancy rate was 0.4%
and gestation was between 52 and 60 days. The
ongoing pregnancy rate for protocols 1, 2, and 3 was
0.9%, 0.4%, and 0.2%, respectively (P=0.138). After
changing to protocol 3, 97 (9.3%) women received
a second dose of misoprostol. During follow-up,
49 women received additional misoprostol—seven
during first follow-up for dead fetus in situ, 37 during
first 2 weeks for moderate bleeding, one during the
fourth week for persistent bleeding, and four for
retained products between fourth and eighth week.
One required manual vacuum aspiration in the
12th week because of persistent tissue mass despite
misoprostol.
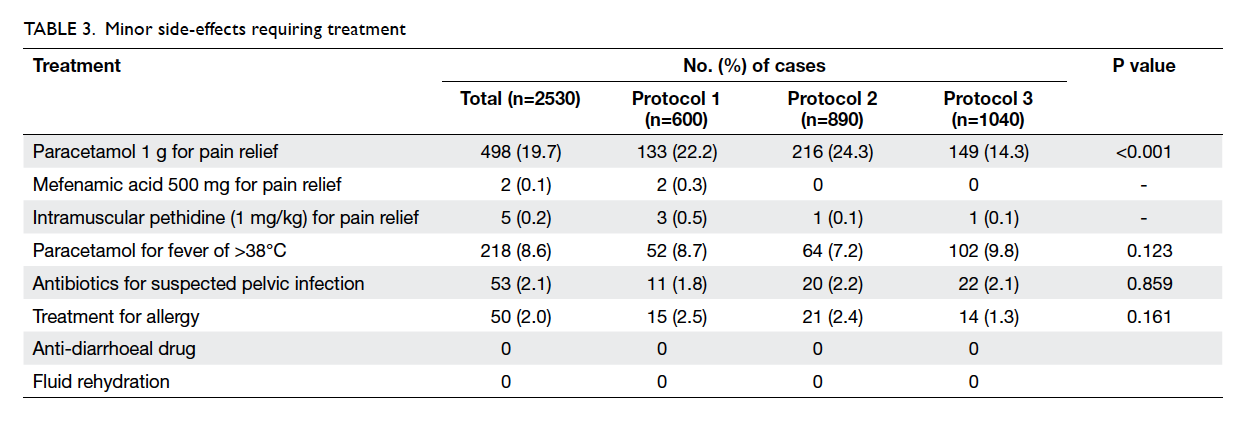
Minor side-effects that required treatment are
listed in Table 3. Treatment for allergic symptoms of
varying severity was required in 50 (2.0%) cases.
One woman developed back and neck rash after
mifepristone and was given oral chlorpheniramine
maleate 4 mg when she was admitted for
misoprostol. Another woman also reported skin
rash after mifepristone that resolved spontaneously
and no treatment was required. One or more of
the following minor allergic symptoms developed
in 94 women after misoprostol: palmar erythema,
palmar itchiness, perioral rash, and peri-orbital rash.
Symptoms resolved spontaneously in 51 women and
43 required oral chlorpheniramine maleate. Two
women received oral chlorpheniramine maleate
followed by intramuscular chlorpheniramine
maleate 10 mg because symptoms progressed: in
both cases a localised body rash progressed and
one woman became breathless. Three women
received intramuscular chlorpheniramine maleate:
one had generalised body rash with shortness of
breath, another had generalised facial rash, and
the third developed swollen lips and tongue and
palmar erythema with itchiness. One woman
developed peri-orbital rash with swelling and
blurred vision: intramuscular chlorpheniramine
maleate was administered. One hour later, her
vision normalised but peri-orbital rash with swelling
persisted. Symptoms gradually resolved following
intramuscular injection of hydrocortisone 100 mg.
During follow-up, 53 (2.1%) women
were treated with oral antibiotics for clinically
suspected pelvic infection—11 (1.8%) had received
vaginal misoprostol and 42 (2.2%) had sublingual
misoprostol (Table 3). Some were treated by their
private gynaecologists and others by doctors in
the Hospital Authority when they were admitted
for bleeding or abdominal pain. Those who were
treated by us during follow-up presented with pelvic
excitation tenderness with or without prolonged
spotting or bleeding. None of them had fever.
Empirical antibiotics were prescribed but no swabs
were taken for culture. Four (0.2%) cases of partial
mole were reported by the pathologist and these
women were referred to a specialist gynaecology
clinic of the Hospital Authority. Two women had
post-abortion anaemia due to prolonged bleeding for
over 4 weeks, with haemoglobin levels of 80 g/L and
86 g/L. In no case was bleeding sufficiently severe to
warrant transfusion, and there were no instances of
anaphylactic reaction, septicaemia, or death.
Following abortion, the three main
contraceptives of choice were male condom (38.1%),
combined oral contraceptive pill (36.2%), and
combined hormone injection (12.3%).
Discussion
When we first piloted the service, we adopted
the standard protocol of 200-mg oral mifepristone
plus 800-µg vaginal misoprostol. The side-effects,
complete abortion rate, and ongoing pregnancy
rate were as expected. After changing to sublingual
administration, the complete abortion rate was
similar and the ongoing pregnancy rate was halved
that of vaginal misoprostol. This was not a formal
study, hence women were not asked to record
the intensity of pain, diarrhoea, or discomfort.
Nonetheless, the incidence of side-effects for each
of the three protocols appears to be similar. Fewer
women treated according to protocol 3 required
pain relief but the underlying reasons are unclear.
The change from protocol 1 to 2 to 3 reduced the
ongoing pregnancy rate but the difference was not
statistically significant, probably because of the low
incidence of ongoing pregnancy. We are continuing
to use protocol 3 because it may achieve the best
clinical result without increasing patient discomfort.
Our nursing staff did not perceive any requirement
for increased patient care after changing to
sublingual misoprostol. Although one more dose of
misoprostol was given in protocol 3, this applied to
<10% of patients and did not adversely affect nursing
workload. In addition, auxiliary staff did not report
any increased need for cleaning after changing to
sublingual misoprostol. All patients, including those
treated with protocol 3, can be discharged before the
ward closes at 5 pm.
Unlike a research protocol, there is no time
limit for performing surgical curettage during follow-up
for incomplete medical abortion. Curettage is
recommended if a moderate amount of blood is
found in the vagina during speculum examination at
follow-up, when prolonged bleeding causes anaemia
or if tissue mass is present after 6 weeks. Patients
can also request curettage if persistent bleeding is
inconvenient.
Those who do not pass tissue mass after
misoprostol are followed up after 1 week with
routine pelvic ultrasound. Those with a live fetus, ie
ongoing pregnancy, will undergo suction evacuation.
Occasionally, a dead fetus is detected. During the
initial phase of service delivery, doctors arranged
curettage to remove the dead fetus. Our doctors
are accustomed to surgical abortion and hence
are anxious to clear any retained products. At our
case discussions, they are reassured that retained
products of gestation, even a dead fetus, is part of the
natural process of medical abortion and they can try
expectant management first. In most cases, watchful
waiting and subsequent follow-up 2 weeks later will
reveal that the fetus has gone. With the adoption
of protocol 3, the option of an additional dose of
misoprostol can be offered alongside expectant
management.
Collecting tissue mass passed and sending
it for pathological examination is important as a
partial mole cannot be diagnosed by ultrasound. The
incidence of partial mole is similar to that reported in
women undergoing surgical abortion in the FPAHK.
Follow-up is important because the failure rate for
medical abortion is higher than that for surgical
abortion, and misoprostol is potentially teratogenic.
The follow-up visits also provide an opportunity
to evaluate contraceptive use. To prevent further
unplanned pregnancies, contraceptive counselling
is provided at each visit. The consistent use of
contraceptive is emphasised and women are
encouraged to discuss any problems they experience
with contraception to maximise compliance.
Conclusion
A medical abortion service can be safely provided in
a non-hospital setting that also provides emergency
suction evacuation.
References
1. Medical management of first-trimester abortion.
Contraception 2014;89:148-61. Crossref
2. Medical abortion overview. Available from: http://gynuity.org/programs/more/overview/. Accessed 10 Dec 2014.
3. Pazol K, Creanga AA, Zane SB, Burley KD, Jamieson
DJ; Centers for Disease Control and Prevention (CDC).
Abortion surveillance—United States, 2009. MMWR
Surveill Summ 2012;61:1-44.
4. First trimester abortion guidelines and protocols.
International Planned Parenthood Federation; 2008.
5. Safe abortion: technical and policy guidance for health
systems. 2nd ed. Geneva: World Health Organization; 2012.
6. The care of women requesting induced abortion: evidence-based
clinical guideline No. 7. London: Royal College of
Obstetricians and Gynaecologists Press; 2011.
7. Kulier R, Kapp N, Gülmezoglu AM, Hofmeyr GJ, Cheng L,
Campana A. Medical methods for first trimester abortion.
Cochrane Database Syst Rev 2011;(11):CD002855. Crossref
8. Raymond EG, Shannon C, Weaver MA, Winikoff B.
First-trimester medical abortion with mifepristone 200
mg and misoprostol: a systematic review. Contraception
2013;87:26-37. Crossref
9. Shannon C, Brothers LP, Philip NM, Winikoff B. Infection
after medical abortion: a review of the literature.
Contraception 2004;70:183-90. Crossref
10. Mifegyne. Physician leaflet. Exelgyn, France. HK-62757. 8
Apr 2014.