Hong Kong Med J 2015 Aug;21(4):327–32 | Epub 19 Jun 2015
DOI: 10.12809/hkmj144329
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Efficacy and safety of hylan G-F 20 injection in treatment of knee osteoarthritis in Chinese
patients: results of a prospective, multicentre, longitudinal study
CH Yan, FHKCOS, FHKAM (Orthopaedic Surgery)1;
WL Chan, FHKCOS, FHKAM (Orthopaedic Surgery)2;
WH Yuen, FHKCOS, FHKAM (Orthopaedic Surgery)3;
Patrick SH Yung, FHKCOS, FHKAM (Orthopaedic Surgery)4;
KY Ip, FHKCOS, FHKAM (Orthopaedic Surgery)5;
Jason CH Fan, FHKCOS, FHKAM (Orthopaedic Surgery)6;
KY Chiu, FHKCOS, FHKAM (Orthopaedic Surgery)1
1 Department of Orthopaedics and Traumatology, The University of Hong
Kong, Queen Mary Hospital, Pokfulam, Hong Kong
2 Department of Orthopaedics and Traumatology, Kwong Wah Hospital,
Yaumatei, Hong Kong
3 Department of Orthopaedics and Traumatology, Queen Elizabeth
Hospital, Jordan, Hong Kong
4 Department of Orthopaedics and Traumatology, The Chinese University
of Hong Kong, Prince of Wales Hospital, Shatin, Hong Kong
5 Department of Orthopaedics and Traumatology, North District Hospital,
Sheung Shui, Hong Kong
6 Department of Orthopaedics and Traumatology, Alice Ho Miu Ling
Nethersole Hospital, Tai Po, Hong Kong
Corresponding author: Dr CH Yan (yanchunhoi@gmail.com)
Abstract
Objective: To study the efficacy and safety of
single intra-articular injection of 6-mL hylan
G-F 20 in Chinese patients with symptomatic knee
osteoarthritis.
Design: Prospective case series.
Setting: Six government hospitals in Hong Kong.
Patients: Patients with primary knee osteoarthritis
were recruited from six government hospitals from 1
October 2010 to 31 May 2012. All patients received 6-mL intra-articular injection of hylan G-F 20.
Main outcome measures: Pain visual analogue
scale, functional visual analogue scale, and 5-point
Likert scale on change of pain and function were
assessed. Adverse events were checked. Radiographs
were taken pre-injection and at 3 months and 1 year.
Results: A total of 110 knees of 95 patients
with primary knee osteoarthritis were treated.
The mean age of the patients was 62 (standard
deviation, 9.8) years. All patients completed 1 year
of follow-up. The mean pain visual analogue scale,
functional visual analogue scale, and Likert value
for pain and function showed statistically significant
improvements at 6 weeks, 3 months, 6 months, and
1 year compared with the pre-injection values. No
significant correlations were found between changes
in visual analogue scale and age, body mass index,
pre-injection radiological osteoarthritis severity,
serum erythrocyte sedimentation rate, or C-reactive
protein. Serial radiographs did not show any changes
in the radiological severity of knee osteoarthritis.
Overall, 16.4% of the patients experienced mild and
self-limiting adverse events.
Conclusion: Hylan G-F 20 is a safe and effective
therapy to relieve pain and improve function for up
to 1 year in Chinese patients with knee osteoarthritis.
New knowledge added by this
study
- This study demonstrated that hylan G-F 20 is effective and safe to treat knee osteoarthritis in Chinese patients. Past studies were only conducted in Caucasian or mixed populations.
- Viscosupplementation could be a valid option for managing patients with chronic and symptomatic knee osteoarthritis. Single injection preparation is safe and effective. Injection can be performed in an out-patient setting.
Introduction
Osteoarthritis (OA) is a progressive degenerative
joint disease initiated by multiple aetiological
factors. When clinically evident, OA is characterised
by joint pain, tenderness, stiffness, crepitus, effusion,
and variable degrees of inflammation without
systemic effects. Knee OA is a leading musculoskeletal
cause of disability in elderly people around the
world, and affects both Caucasian and Chinese
populations.1 2 3 The burden of disease dramatically
impacts health care costs. A local study found that,
excluding joint replacement, the direct costs of
managing OA ranged from HK$11 690 to $40 180
per person per year and indirect costs ranged from
HK$3300 to $6640.4
There are many types of treatment for knee
OA. These therapies can be divided into two major
groups of non-surgical and surgical. Non-surgical
therapies include exercise, weight loss, physical
therapy, occupational therapy, medications (eg
paracetamol, non-steroidal anti-inflammatory
agents), and intra-articular injections (steroids,
viscosupplementation). Surgical therapies mainly
entail osteotomy and arthroplasty.5
Knee OA has been associated with a decrease
in the elasticity and viscosity of the synovial
fluid,6 7 8 which may alter the transmission of
mechanical forces to the articular cartilage,
possibly increasing its susceptibility to mechanical
damage, or wear and tear. Viscosupplementation
is an intra-articular therapeutic modality based
on the physiological importance of hyaluronan in
synovial joints. Its therapeutic goal is to restore the
viscoelasticity of synovial hyaluronan, decrease pain,
improve mobility, and restore the natural protective
functions of hyaluronan in the joint.
Hylan G-F 20 is a cross-linked sodium
hyaluronate with a high average molecular weight
of 6 million daltons. Hylan G-F 20 is used in North
America and Europe for the treatment of pain
associated with knee OA. However, there are no
data available in the literature on the clinical benefits
of the viscosupplement in Chinese populations.
Therefore, we carried out a prospective, multicentre,
longitudinal study to investigate the efficacy and
safety of hylan G-F 20 in the treatment of knee OA
in local Chinese patients over a period of 1 year.
Methods
The study protocol was approved by the Institutional
Review Boards/Ethics Committees of all six
participating centres in Hong Kong. The inclusion
criteria were: Chinese patients with primary knee OA
who fulfilled the diagnostic criteria of the American
College of Rheumatology; knee pain visual analogue
scale (VAS) score of >50 (range, 0-100) and/or functional
VAS score of >50 (0-100); and willingness to pay
for the viscosupplementation and participate in the
study. The exclusion criteria were: knee arthritis of
other aetiologies; knee surgery or previous intra-articular
injection in the knee within 1 year of
the study; known allergy to chicken extracts; or
unwillingness to pay for the viscosupplementation or
participate in the study. Weight-bearing radiographs
of the affected knee joint (standard anteroposterior
and lateral views) were taken at the screening
visit. Severity of knee OA in the tibiofemoral
and patellofemoral compartments was classified
according to the Kellgren-Lawrence (KL) system. The
overall severity was defined as the highest KL grade
in any of the compartments. We also documented
the patients’ body mass index (BMI), and checked
serum erythrocyte sedimentation rate (ESR) and C-reactive
protein (CRP) level.
Single intra-articular preparation of 6 mL of
hylan G-F was injected into the patients’ knees in
the out-patient clinic. Strict aseptic technique was
adopted with skin disinfection and draping. The
injection was administered through a direct lateral
parapatellar approach. Knee joint aspiration was
performed using a separate syringe before injection
of the viscosupplement. After injection, the patients
were allowed to resume normal activities, but were
advised against vigorous exercise for 2 to 3 days.
Ice therapy was recommended in case of transient
increase in pain and swelling. Patients could continue
with their routine analgesics on a pro re nata basis.
All patients were followed up regularly at 6
weeks, 3 months, 6 months, and 1 year. A telephone
interview was conducted at 2 weeks to record any
adverse events, if present. The severity of knee pain
and knee function using 0-100 VAS scores (where
0 represents ‘no pain’ or ‘normal function’) were
documented at each visit. Any changes in knee
pain and functional limitations were charted using
a 5-point Likert scale. Standard radiographs of the
knee were repeated at 3 months and 1 year to detect
any changes in radiological severity.
The change in pain and functional VAS scores
before and after injection during each visit was
compared using paired t test. Using correlation tests,
we tried to find out the predictive factors (including
age, sex, BMI, pre-injection KL grade, pre-injection
pain VAS, ESR, and CRP) of favourable treatment
response. All analyses were performed using the
Statistical Package for the Social Sciences (Windows
version 20.0; SPSS Inc, Chicago [IL], US). Statistical
significance was assumed if the P value was <0.05.
Results
A total of 110 knees of 95 patients (31 men and
64 women) were recruited from six government
hospitals in Hong Kong from 1 October 2010 to
31 May 2012. There were 59 left knees and 51 right
knees. All patients completed 1 year of follow-up. The
mean (± standard deviation) age of the patients was
62.0 ± 9.8 (range, 33-86) years. The mean BMI was 27.7
± 4.6 kg/m2 (range, 18.3-46.8 kg/m2). The mean ESR
was 23.35 ± 14.00 mm/h (range, 2.00-66.00 mm/h) and
the mean CRP level was 1.3 ± 1.7 mg/L (range, 0.1-7.1
mg/L). The youngest patient in the study was 33
years old. His BMI was 25.9 kg/m2. X-rays of his right
knee showed KL grade 1 OA in the patellofemoral
compartment and KL grade 2 OA in the tibiofemoral
compartment. He denied any previous injury to his
knee.
The mean pain and functional VAS scores
are shown in Figures 1 and 2, respectively. There
were statistically significant improvements in
pain and functional VAS scores after injection at
every follow-up visit when compared with the pre-injection
scores (paired t test, P<0.0001 for all).
Significant differences were also found between the
pain and functional VAS scores at 1 year and at 6
weeks (P<0.001 and P<0.01, respectively), 3 months
(P<0.003 and P<0.01, respectively), and 6 months
(P<0.01 for both). The score differences between 6 weeks,
3 months, and 6 months were not significant.
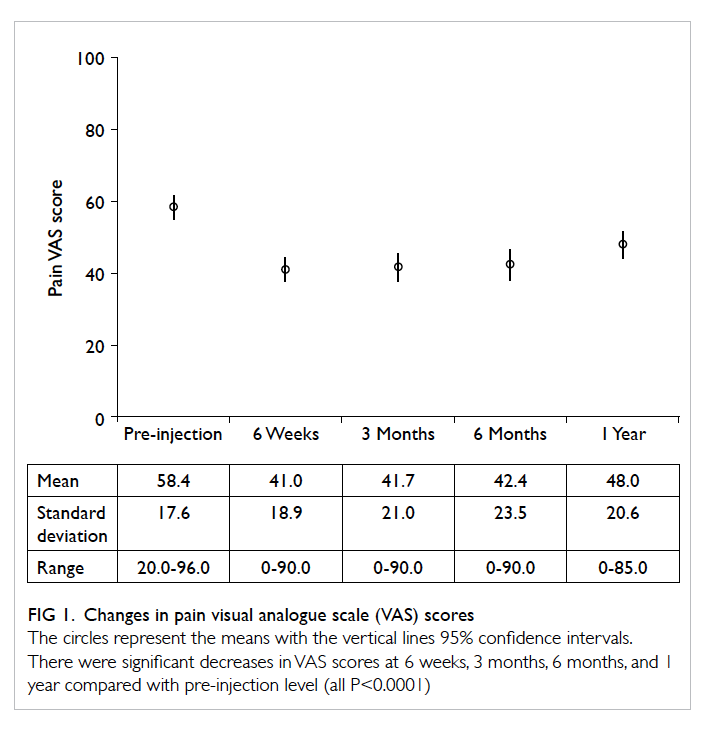
Figure 1. Changes in pain visual analogue scale (VAS) scores
The circles represent the means with the vertical lines 95% confidence intervals. There were significant decreases in VAS scores at 6 weeks, 3 months, 6 months, and 1 year compared with pre-injection level (all P<0.0001)
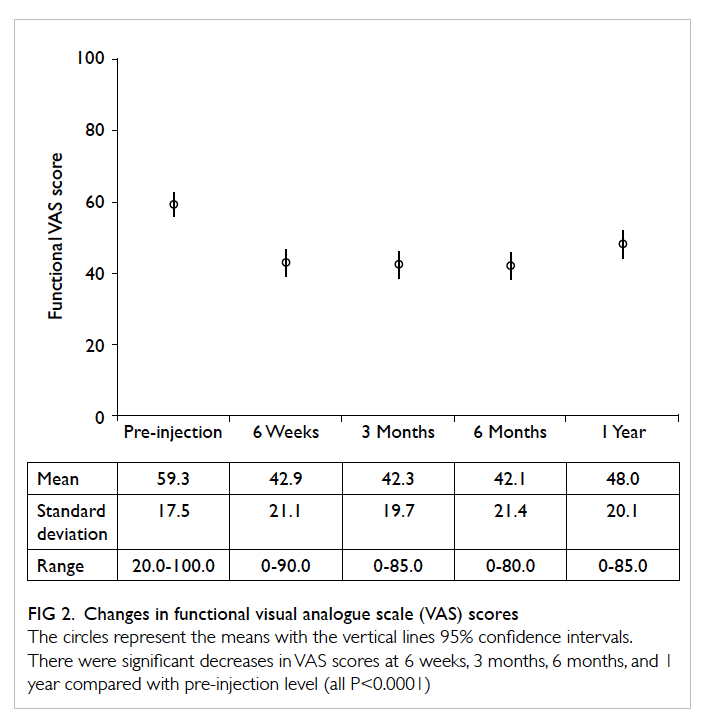
Figure 2. Changes in functional visual analogue scale (VAS) scores
The circles represent the means with the vertical lines 95% confidence intervals. There were significant decreases in VAS scores at 6 weeks, 3 months, 6 months, and 1 year compared with pre-injection level (all P<0.0001)
Likert values were coded as 1 to 5 with 3 being
no change and 1 being much reduced. A sign test
against a median of 3 and an alternate hypothesis
that the sample median was less than 3 was used.
Significant reductions in pain and functional
limitations were found at 6 weeks (P<0.001 for both),
3 months (P<0.001 for both), 6 months (P<0.001 for
both), and 1 year (P<0.03 for both). The proportion of
patients feeling reduced or much reduced pain was
74% at 6 weeks, 75% at 3 months, 62% at 6 months,
and 49% at 1 year. The proportion of patients feeling
no change in pain level (when compared with pre-injection
level) was 23% at 6 weeks, 22% at 3 months,
33% at 6 months, and 43% at 1 year.
Investigation of predictive factors of good
clinical response did not demonstrate any significant
correlation with age, BMI, pre-injection VAS, ESR,
or CRP (Pearson’s tests). Sex (Welch’s t test) and pre-injection
KL grade (analysis of variance test) did not
significantly affect the treatment response.
Overall, 37 knees had KL grade IV OA, 38
had grade III OA, 30 had grade II OA, and
five had grade I OA before injection. Radiographs
showed no significant changes in KL OA grades at
3 months and 1 year. A total of 18 (16.4%) knees
experienced adverse events, including pain (14
knees), swelling (2 knees), and warmth (2 knees). All
of the adverse events were mild and self-limiting. No
patients required hospital admission or extra clinic
visits for these self-reported events.
Discussion
The evidence in the literature is still inconclusive
regarding the clinical and biological efficacy of
viscosupplementation. The 2006 Cochrane review
summarised the results of 76 randomised controlled
trials (RCTs) comparing hyaluronic acid (HA) and
various other treatment modalities.9 The authors
concluded that viscosupplementation is an effective
treatment for knee OA with beneficial effects on
pain, function, and global assessment, especially at
the 5- to 13-week post-injection period. Although
the sample size restriction may preclude any
definitive comment on the safety of the products, no
major safety issues were detected. The 2nd edition
of the American Academy of Orthopaedic Surgeons
guideline on treatment of knee OA claimed that
“we cannot recommend using HA for patients with
symptomatic knee OA”.10 This recommendation is
based on the fact that although meta-analyses of
the Western Ontario and McMaster Universities
Osteoarthritis Index (WOMAC) for pain,
function, and stiffness subscale scores all found
statistically significant treatment effects, none of the
improvements met the minimum clinically important
improvement thresholds. The latest Osteoarthritis
Research Society International guideline for nonsurgical
management of knee OA claimed that the
recommendation for intra-articular HA injection
was ‘uncertain’, because the inconsistent conclusions
among the meta-analyses and conflicting results
regarding HA’s safety influenced the panel votes.11
One of the drawbacks of the meta-analyses and
reviews is that they pooled the results of studies that
investigated different viscosupplement formulations.
These included low- and high-molecular-weight HA
preparations of avian or bacterial origin. Hylan G-F
20 is a cross-linked HA derivative of avian origin,
with relatively high molecular weight (average, 6000
kDa) and fluid rheological properties similar to those
of knee synovial fluid of healthy young individuals.
In a 26-week RCT, hylan G-F 20 single-injection
formulation resulted in significant improvements
in WOMAC pain score, observer-reported disease
status, and patient-reported health status score.12
Our study is the first in Chinese patients to
investigate the efficacy and safety of hylan G-F
20. The results show that the single 6-mL intra-articular
injection could significantly improve pain
and function in patients with primary knee OA. The beneficial effect could be sustained for
up to 6 months. The VAS scores increased again by
the 1-year follow-up visit, but the values were still
significantly lower than the pre-injection levels. The
5-point Likert scale also revealed that about 75% of
patients had reduced pain at 3 months, 62% at 6 months,
and the percentage remained decreased at 50% at the
1-year follow-up visit.
A total of 16.4% of patients experienced mild
and self-limiting local adverse reactions. No pseudoseptic
reaction or severe acute inflammatory reaction
was reported.13 The interview was conducted by
telephone at 2 weeks after injection, and was partly
carried out by research assistants or nurses. Some
patients might have confused ‘additional/new
pain over the injection site’ with ‘pre-existing OA
pain’, which led to the higher self-reported adverse
event incidence. There are reports on HA causing
adverse reactions; the most common of which is an
inflammatory reaction or flare at the injection site
characterised by injection site pain and swelling.14 15 16
Hypersensitivity reactions to HA or avian proteins
are listed as contra-indications for use of many of the
HA products. Many of the inflammatory responses
appear to be due to the molecular structure of the
HA, as naturally derived hyaluronan sources appear
to be better tolerated than highly cross-linked
hyaluronan.13 17 18 Leopold et al19 demonstrated
increased frequency of acute local reaction to
hylan G-F 20 in patients receiving more than one
course of treatment. Recently a murine model study
showed a single injection of hylan G-F 20 led to less
inflammation and lower antibody reaction when
compared with a three-shot series of injections.
In our study, the 6-mL single injection preparation
was used. This approach offers another advantage
over a multiple-injection regimen as it reduces the
number of consultations, therefore saves money and
manpower in government hospitals with limited
health care resources.
The serial knee radiographs in our study did not
show any changes (either progression or regression)
of the radiological severity of OA after hylan G-F
20 injection. To date, there is no concrete evidence
in the literature to support the disease-modifying
effect of HA injection. The radiographic KL grading
system may not be sensitive enough to detect minor
changes in the articular cartilage. We also did not
have a control group for comparison. In a magnetic
resonance imaging–based RCT on articular cartilage
volume change after four courses of hylan G-F 20
injection at 6-month intervals, the authors claimed
that there was less cartilage loss in the treatment
group at 24 months (2.7% over the medial tibial
plateau and 2.6% over the lateral tibial plateau).20
Whether these differences are clinically significant
is doubtful, however. We could not find any specific
factors predicting good clinical response in our
patients. This could be due to the relatively small
sample size and the heterogeneity of our patients.
The pre-injection parameters we investigated may
not be sufficiently sensitive to survive the analysis.
There are a few limitations to this study. The
pain and functional VAS scores were used because we
believe they are patient-reported outcome measures,
which would better reflect the clinical efficacy from
the patients’ perspectives. The VAS scores are also easy
to use, especially in the setting of a multicentre study.
We did not include parameters such as knee range of
motion, walking tolerance, or other knee scores as
they were not the primary objectives of our study. It
is well known that the placebo effect may account for
30% of the perceived benefits of medical treatment.21
We could not ascertain how much of the pain relief
and functional improvement were attributable to
the true therapeutic effects of hylan G-F 20 in view
of the nature of our study design. A prospective,
blinded, RCT may be able to eliminate the potential
confounding factors and information bias. Changes
in other treatment modalities during the follow-up
period were not compared because of the potential
complexity. It is difficult to standardise conservative
treatment in terms of oral medication and exercise
therapy, simply because patients with advanced knee OA
may need stronger analgesics. Patients
would also have a high non-compliance rate if we
forced them to follow a single regimen. We decided to
let all patients carry on with their usual conservative
management and asked them if there were any
changes in the pain and functional VAS scores during
each follow-up after the viscosupplement injection.
Conclusion
This prospective, multicentre study showed that
single intra-articular injection of 6-mL hylan G-F
20 was effective in providing statistically significant
pain relief and functional improvement up to 1 year
in Chinese patients with primary knee OA. Although
adverse events were not uncommon, all of them were
mild and self-limiting. Viscosupplementation with
hylan G-F 20 could be a safe and beneficial option in
managing patients with knee OA.
Declaration
The authors had not received any forms of financial or
non-financial support from commercial companies.
All patients purchased their own injection.
Acknowledgements
The authors would like to thank Drs Fu-yuen Ng
(Queen Mary Hospital), Kan-yip Law (Prince of
Wales Hospital), and Paul SC Yip (Queen Elizabeth
Hospital) for their contribution of in-patient
recruitment and data collection for the study.
References
1. Felson DT, Naimark A, Anderson J, Kazis L, Castelli W,
Meenan RF. The prevalence of knee osteoarthritis in the
elderly. The Framingham Osteoarthritis Study. Arthritis
Rheum 1987;30:914-8. Crossref
2. Zhang Y, Xu L, Nevitt MC, et al. Comparison of the
prevalence of knee osteoarthritis between the elderly
Chinese population in Beijing and whites in the United
States: The Beijing Osteoarthritis Study. Arthritis Rheum
2001;44:2065-71. Crossref
3. Woo J, Leung J, Lau E. Prevalence and correlates of
musculoskeletal pain in Chinese elderly and the impact on
4-year physical function and quality of life. Public Health
2009;123:549-56. Crossref
4. Woo J, Lau E, Lau CS, et al. Socioeconomic impact of
osteoarthritis in Hong Kong: utilization of health and
social services, and direct and indirect costs. Arthritis
Rheum 2003;49:526-34. Crossref
5. Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM.
A systematic review of recommendations and guidelines
for the management of osteoarthritis: The chronic
osteoarthritis management initiative of the U.S. bone and
joint initiative. Semin Arthritis Rheum 2013;43:701-12. Crossref
6. Mazzucco D, McKinley G, Scott RD, Spector M. Rheology
of joint fluid in total knee arthroplasty patients. J Orthop
Res 2002;20:1157-63. Crossref
7. Fam H, Bryant JT, Kontopoulou M. Rheological properties
of synovial fluids. Biorheology 2007;44:59-74.
8. Schurz J, Ribitsch V. Rheology of synovial fluid. Biorheology
1987;24:385-99.
9. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R,
Wells G. Viscosupplementation for the treatment of
osteoarthritis of the knee. Cochrane Database Syst Rev
2005;(2):CD005321. Crossref
10. Jevsevar DS, Brown GA, Jones DL, et al. The American
Academy of Orthopaedic Surgeons evidence-based
guideline on: treatment of osteoarthritis of the knee, 2nd
edition. J Bone Joint Surg Am 2013;95:1885-6.
11. McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI
guidelines for the non-surgical management of knee
osteoarthritis. Osteoarthritis Cartilage 2014;22:363-88. Crossref
12. Frampton JE. Hylan G-F 20 single-injection formulation.
Drugs Aging 2010;27:77-85. Crossref
13. Goldberg VM, Coutts RD. Pseudoseptic reactions to hylan
viscosupplementation: diagnosis and treatment. Clin
Orthop Relat Res 2004;(419):130-7. Crossref
14. Lussier A, Cividino AA, McFarlane CA, Olszynski WP,
Potashner WJ, De Médicis R. Viscosupplementation with
hylan for the treatment of osteoarthritis: findings from
clinical practice in Canada. J Rheumatol 1996;23:1579-85.
15. Hyalgan: prescribing information. Available from: http://products.sanofi.us/hyalgan/hyalgan.html. Accessed Jan 2009.
16. Synvisc: information for prescribers. Available from: http://synviscone.com/~/media/SynviscOneUS/Files/Synvisc-OnePI-70240104.pdf. Accessed Jan 2010.
17. Reichenbach S, Blank S, Rutjes AW, et al. Hylan versus
hyaluronic acid for osteoarthritis of the knee: a systematic
review and meta-analysis. Arthritis Rheum 2007;57:1410-8. Crossref
18. Jüni P, Reichenbach S, Trelle S, et al. Efficacy and safety of
intraarticular hylan or hyaluronic acids for osteoarthritis of
the knee: a randomized controlled trial. Arthritis Rheum
2007;56:3610-9. Crossref
19. Leopold SS, Warme WJ, Pettis PD, Shott S. Increased
frequency of acute local reaction to intra-articular hylan
GF-20 (synvisc) in patients receiving more than one course
of treatment. J Bone Joint Surg Am 2002;84-A:1619-23.
20. Wang Y, Hall S, Hanna F, et al. Effects of Hylan G-F 20
supplementation on cartilage preservation detected by
magnetic resonance imaging in osteoarthritis of the knee:
a two-year single-blind clinical trial. BMC Musculoskelet
Disord 2011;12:195. Crossref
21. Shapiro A, Moris L. The placebo effect in medical and
psychological therapies. In: Bergin A, Garfield S, editors.
Handbook of psychotherapy and behavior change: an
empirical analysis. 2nd ed. New York: Wiley; 1978.

