Hong Kong Med J 2015 Jun;21(3):201–7 | Epub 23 Apr 2015
DOI: 10.12809/hkmj144373
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Effects of a plasma heating procedure for inactivating Ebola virus on common chemical
pathology tests
YK Chong, MB, BS;
WY Ng, MB, ChB, PhD;
Sammy PL Chen, FRCPA, FHKAM (Pathology);
Chloe M Mak, FRCPA, FHKAM (Pathology)
Chemical Pathology Laboratory, Department of Pathology, Princess Margaret Hospital, Laichikok, Hong Kong
Corresponding author: Dr Chloe M Mak (makm@ha.org.hk)
Abstract
Objectives: The recent declaration of Ebola
virus disease as epidemic by the World Health
Organization indicates urgency for affected
countries and their laboratories to evaluate and
provide treatment to patients potentially infected
by the Ebola virus. A heat inactivation procedure
involving treating specimens at 60°C for 60 minutes
has been suggested for inactivation of the Ebola
virus. This study aimed at evaluating the effect of
plasma heating on common biochemical tests.
Design: Comparative experimental study.
Setting: A regional chemical pathology laboratory in Hong Kong.
Methods: Forty consecutive plasma specimens for
general chemistry analytes on Beckman Coulter
AU5822 and another 40 plasma specimens for
troponin I analysis on Access 2 Immunoassay
System were obtained, anonymised, and divided into
two aliquots. One aliquot was analysed directly and
the other was analysed after heating at 60°C for 60
minutes.
Results: A total of 20 chemical pathology tests were
evaluated. Nine tests (sodium, potassium, chloride,
urea, creatinine, total calcium, phosphate, total
protein, and glucose) were not significantly affected
by the heat inactivation procedure and remained
clinically interpretable. Results for magnesium
(15% mean increase), albumin (41% mean increase),
bilirubin (8% mean decrease), amylase (27% mean
decrease), and troponin I (76% mean decrease) were
still interpretable using regression estimation with
proportional bias. However, all enzymes studied
except amylase (alanine transaminase, aspartate
transaminase, alkaline phosphatase, gamma-glutamyltransferase,
creatine kinase, and lactate
dehydrogenase) were inactivated to a significant
degree. Their Pearson r or Spearman rho values
ranged from no significant correlation (P≥0.05) to
0.767, and most normality was rejected.
Conclusion: Heat inactivation results in no
significant change in electrolytes, glucose, and
renal function tests, but causes a significant bias
for many analytes. Recognition of the relationship
between pre- and post-heat inactivation specimens
allows clinical interpretation of affected values and
contributes to patient care. For safety and diagnostic
accuracy, we recommend use of a point-of-care
device for blood gases, electrolytes, troponin, and
liver and renal function tests within a class 2 or above
biosafety cabinet with level 3 or above biosafety
laboratory practice.
New knowledge added by this
study
- Heat inactivation results in no significant change in electrolytes, glucose, and renal function tests, but causes a significant bias for many analytes in routine biochemistry tests.
- For the analytical methodologies tested, nine tests (sodium, potassium, chloride, urea, creatinine, total calcium, phosphate, total protein, and glucose) were not significantly affected.
- Magnesium, albumin, bilirubin, amylase, and troponin I were still interpretable using regression estimation with a linear proportional bias.
- However, alanine transaminase, aspartate transaminase, alkaline phosphatase, gamma-glutamyltransferase, creatine kinase, and lactate dehydrogenase were inactivated to a significant degree with rejected normality and are not useful clinically.
- When a patient suspected of having Ebola virus disease cannot be managed in a facility with comprehensive containment facilities, a heat inactivation procedure can be applied to allow analysis of the specimens with acceptable risk, when used in concert with appropriate precautions, and still yield some clinically useful results.
Introduction
Since March 2014, there has been an outbreak of
Ebola virus disease (EVD) in West Africa; the affected
countries include Guinea, Liberia, Sierra Leone and,
more recently, Nigeria. The cumulative number of
confirmed EVD cases rose exponentially from May
to June 2014.1 On 8 August 2014, the World Health
Organization (WHO) declared the EVD outbreak a
“Public Health Emergency of International Concern”,
indicating that EVD is no longer a distant and
confined issue, but a proximate and real threat.2
The Ebola virus was first discovered in 1976.3 4 It can be transmitted through direct contact with
blood, secretions, and other body fluids or tissues of
infected animals or persons.3 4 The incubation period
of EVD ranges from 2 to 21 days,5 and a case fatality
rate of 90% has been reported.5
Chemical pathology laboratory investigations
are among the most basic and common tests
requested for patients, particularly when intensive
care is required, as would be expected for patients
with EVD who are critically ill. Standard, contact, and
droplet precautions have been recommended for the
management of hospitalised patients with suspected
EVD.6 While EVD is not normally transmitted by
aerosol, there is a concern that the Ebola virus can
remain infectious in laboratory-generated aerosol.7 8 9
Hence, stringent guidelines for laboratory personnel
with respect to handling of laboratory specimens
containing Ebola virus have been published.10 11 The
WHO suggested in its interim guideline that “activities
such as micro-pipetting and centrifugation can
mechanically generate fine aerosols that might
pose a risk of transmission of infection through
inhalation as well as the risk of direct exposure”,
and recommended that gown, gloves, eye-face
protection, and particulate respirators such as the
US National Institute for Occupational Safety and
Health–certified N95 respirator should be used when
laboratory personnel are performing activities such
as aliquoting, centrifugation, and other procedures
that may generate aerosol.10
Nowadays, most general chemistry tests are
performed with analysers that aspirate specimens
from primary blood collection tubes on which
centrifugation has been performed. Flushing
the instrumental parts with Triton X-100 (Dow
Chemical Company, Midland [MI], US) or Clorox
(Clorox, Oakland [CA], US) has been suggested
for decontamination after analysis of specimens
containing Ebola virus. However, such disinfection
procedure may not achieve 100% inactivation of the
virus and is likely to affect the chemical analysis.
Therefore, general chemistry analysers are not
suitable for analysing highly infectious specimens.
Processing of these specimens without an adequate
disinfection procedure will pose an occupational
health hazard to laboratory workers. It has been
suggested that specimens that potentially contain
live Ebola virus should be processed in a class 2
biological safety cabinet following biosafety level 3
practices.6
With regard to inactivation of Ebola virus in
blood specimens of patients, the heat inactivation
procedure (incubation of serum or plasma
specimens at 60°C for 60 minutes) has been reported
to decrease viral titres in patients’ specimens.12 A
report on the effect of the same heat inactivation
procedure in the Hitachi 917 (Roche Diagnostics,
Basel, Switzerland) and Bayer ACS 180 (Bayer
Diagnostics, New York, US) biochemistry analysers
has demonstrated minimal change in concentrations
of sodium, potassium, urea, creatinine, glucose,
urate, total bilirubin, amylase, and C-reactive
protein, but significant reductions in concentrations
of troponin, bicarbonate, total protein, albumin,
total calcium, phosphate, aspartate aminotransferase
(AST), alanine aminotransferase (ALT), alkaline
phosphatase (ALP), gamma-glutamyltransferase
(GGT), and creatine kinase (CK).13 However, the
report only stated the change in percentage, with
no information provided for diagnostic utility
(eg the correlation of the post-heat inactivation
procedure result with the result obtained without
the inactivation procedure, which would indicate
retention of diagnostic information).13 14 Therefore,
this study aimed to provide a statistical delineation
of the heat inactivation procedure effects on more
common general chemistry analytes using the
AU5822 (Beckman Coulter Inc, Pasadena [CA], US)
and troponin I using the Access 2 Immunoassay
System (Beckman Coulter Inc).
Methods
Forty consecutive plasma specimens were obtained,
anonymised, and divided into two aliquots. One
aliquot of the specimens was analysed immediately,
whereas the other aliquot was analysed after heat
inactivation at 60°C for 60 minutes. In addition, as
a pilot study, 20 specimens with elevated cardiac
troponin I (>0.04 ng/mL, 99th percentile of the
reference interval), together with 20 specimens with
cardiac troponin I that were not elevated (below the
99th percentile) were analysed in the same manner.
Common general chemistry tests—including
sodium, potassium, chloride, urea, creatinine,
glucose, total protein, albumin, total bilirubin, ALT,
AST, ALP, GGT, amylase, total calcium, phosphate,
magnesium, CK, and lactate dehydrogenase
(LDH)—were performed on the AU5822 analyser
and the troponin I test was done on the Access 2
Immunoassay System. The methodologies for the
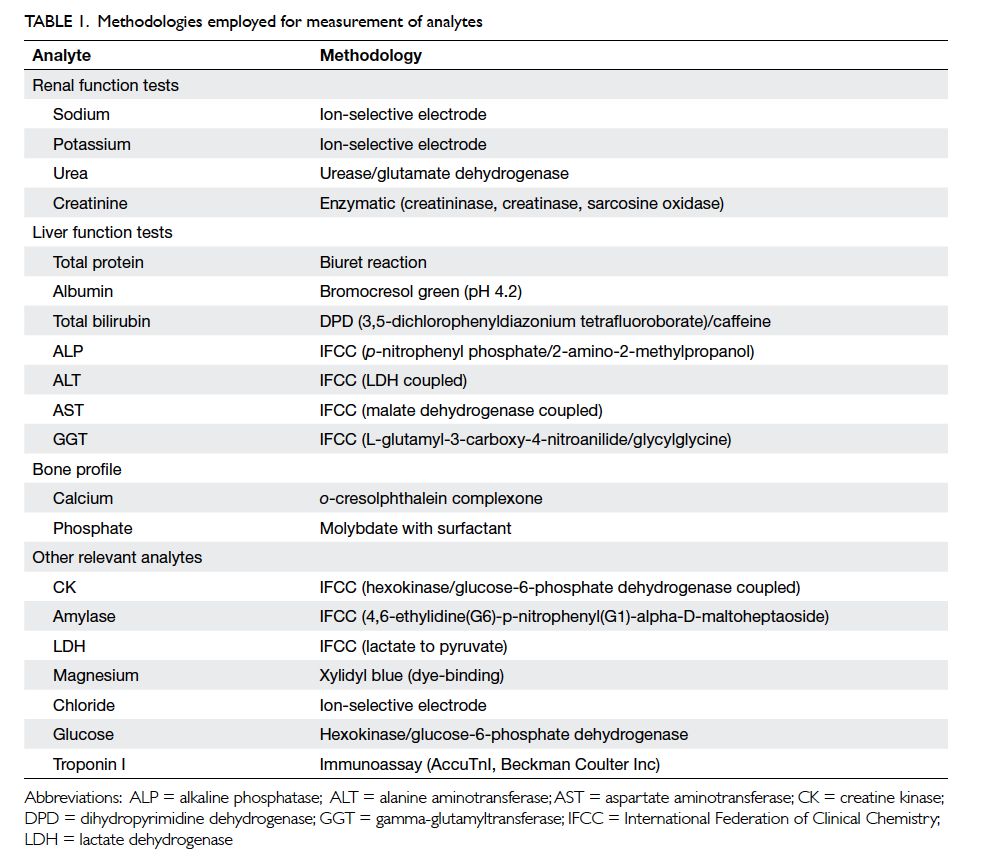
analytes are listed in Table 1 to allow laboratory
staff using different analysers, but with similar
methodologies, to adopt the results from the present
study.
The heat inactivation procedure was
performed according to a WHO guideline.12 Briefly,
separated plasma aliquots were heated at 60°C for 60
minutes in a water bath while sealed in Eppendorf
Tubes (Hamburg, Germany). The post-treatment
specimens were then mixed inside the sealed tubes,
allowed to settle, and analysed in the same manner
as the untreated specimens.
Statistical analysis
Statistical tests were performed by MedCalc version
12.5 (MedCalc Software bvba, Ostend, Belgium).
The pre- and post-treatment results were analysed
with regard to normality by the Kolmogorov-Smirnov test, proportional change by Passing-Bablok regression, constant bias by paired t test,
and maintenance of diagnostic value by Pearson
correlation coefficient. When normality was not
accepted by the Kolmogorov-Smirnov test, Wilcoxon
test was used in place of paired t test, and Spearman
rho in place of Pearson correlation coefficient. The P
value of correlation was calculated for each analyte,
with the linear regression accepted if the P value
was <0.05, and linearity model accepted with the
CUSUM (cumulative sum) test.
The effect of the heat inactivation procedure
on an analyte was considered insignificant if the
slope of the regression line was between 0.95
and 1.05, the linearity was preserved, and the
correlation coefficient/rank correlation was ≥0.9.
The constant bias was evaluated as to its statistical
significance by paired t test or Wilcoxon test, and
by consideration of clinical interpretation by two
chemical pathologists. The effect of heat inactivation
was considered interpretable if, despite a proportion
bias, the linearity was preserved, and correlation
coefficient/rank correlation was ≥0.9. If an analyte
did not fulfil the above two criteria, the post-treatment
measurement result was considered to
have lost the diagnostic values.
Results
A total of 20 chemical pathology tests were
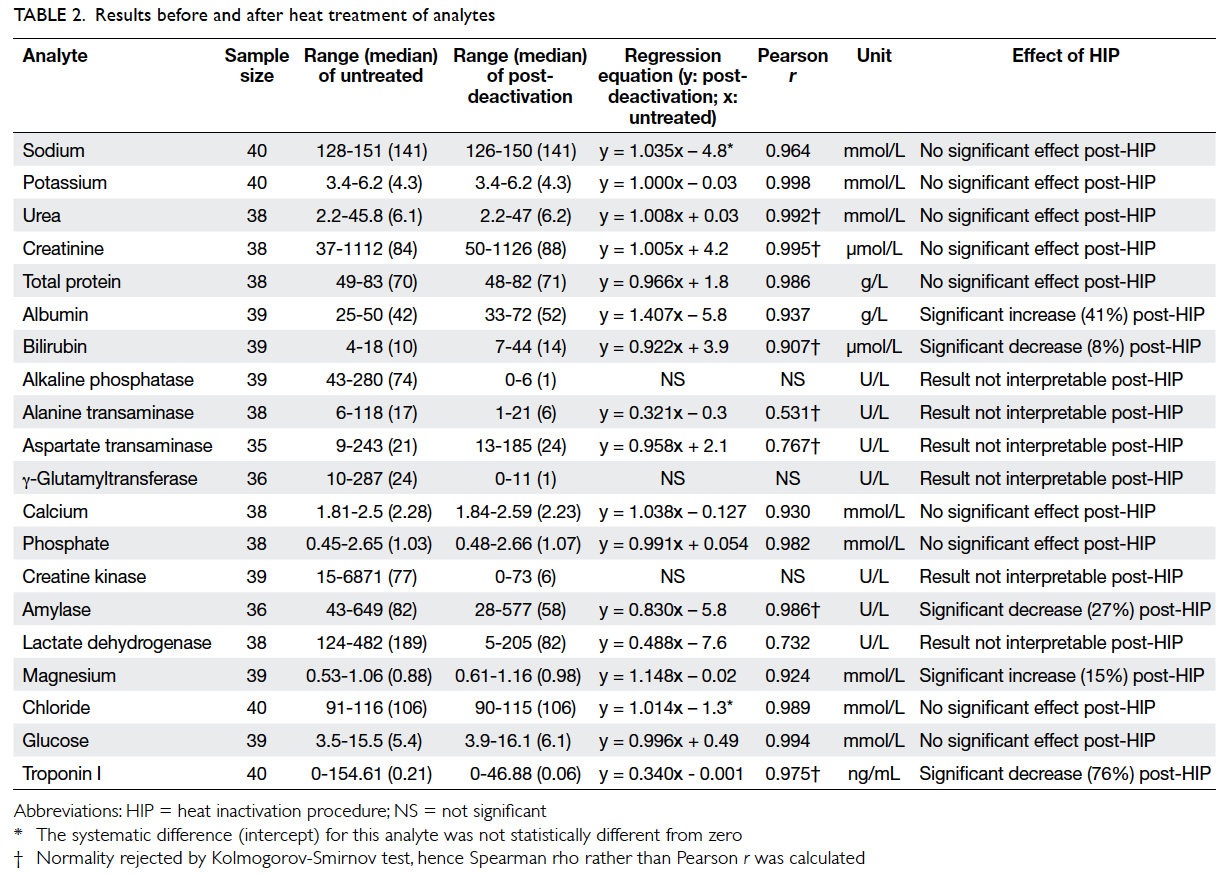
evaluated. Figure 1 shows the percentage change in concentrations of each analyte, and Table 2 shows the range of concentration, regression equation, and
Pearson correlation coefficient of each analyte before
and after heating. Among the analytes, nine tests
(sodium, potassium, chloride, urea, creatinine, total
calcium, phosphate, total protein, and glucose) were
not significantly affected by the heat inactivation
procedure and remained clinically interpretable.
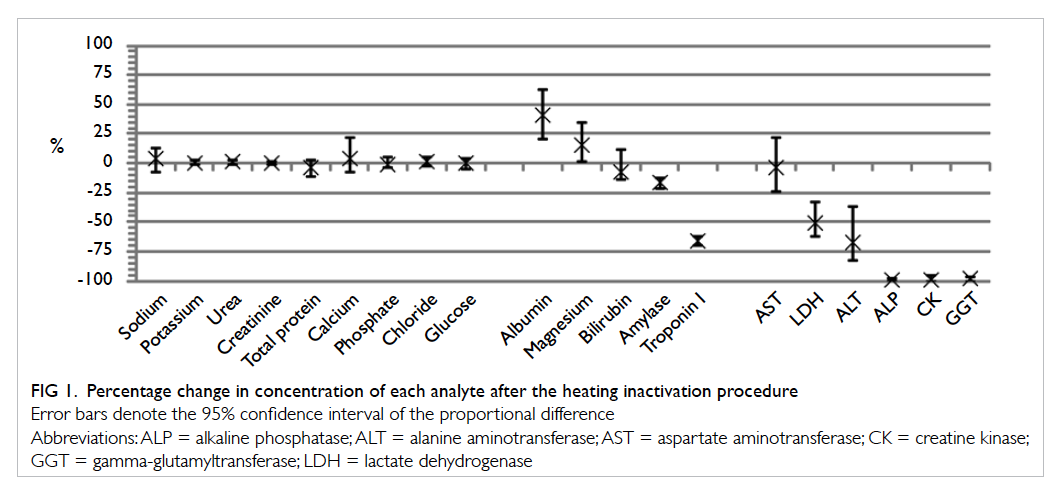
Figure 1. Percentage change in concentration of each analyte after the heating inactivation procedure
Despite the proportional differences, analytical
results for magnesium (15% mean increase), albumin
(41% mean increase), bilirubin (8% mean decrease),
amylase (27% mean decrease), and troponin I (76% mean decrease) were still interpretable, as the pre- and
post-heat inactivation procedure results for these
analytes were found to have significant correlation
(P<0.0001 for the aforementioned analytes, with
Pearson r or Spearman rho >0.9). These results can
be interpreted with estimation from the significant
proportional bias using the regression equation.
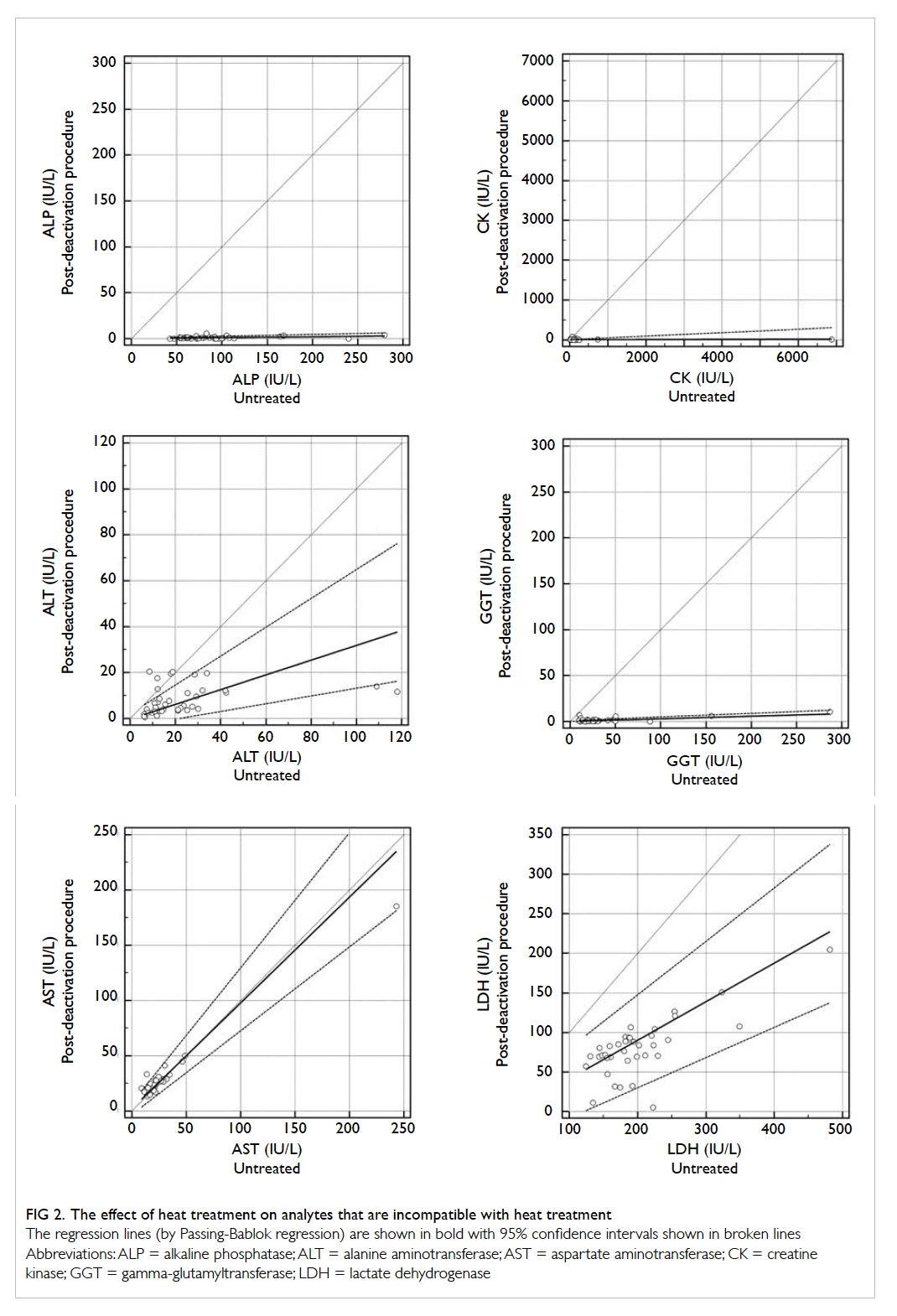
All the enzymes except for amylase (ALP,
ALT, AST, CK, GGT, and LDH) were inactivated
to a significant degree by the heat inactivation
procedure, such that the results were considered
uninterpretable. The Pearson r or Spearman rho
values ranged from no significant correlation
(P≥0.05) to 0.767, and most normality was rejected.
The effect of the heat inactivation procedure on the
analytes that were considered uninterpretable are
shown in Figure 2.
Discussion
The need for more stringent statistical considerations
in the evaluation of disinfection procedures such
as the heat inactivation procedure in the present
study is evident. For example, the effect of the heat
inactivation procedure on AST was quoted to have
a mean reduction of 26% in one of the reports.13 An
even lower average reduction of enzymatic activity
(4.2%) was found in the present study although,
despite the low reduction, diagnostic information
was significantly destroyed (Spearman rho rank-order
coefficient was only 0.767) after the heat
inactivation procedure. In addition, consideration
should be taken in interpretation of the clinical
usefulness of post-heating results as some effects may
be statistically significant but clinically insignificant
in certain pathological conditions.
Our assays of total protein, total calcium, and
phosphate showed no significant difference despite
more adverse effects reported by Bhagat et al.13
This may be due to differences in analytical assays.
Therefore, local laboratories should evaluate their
assays.
It must be noted that the heat inactivation
procedure is only one of the many facets of safe
laboratory practice involving highly infectious
materials. As centrifugation and micro-pipetting
leads to aerosolisation,10 11 the use of gel separator
tubes allows the heat inactivation procedure
to be performed without micro-pipetting after
centrifugation. As gel separator tubes have previously
been reported to cause analytical interference in the
analysis of biochemical analytes such as therapeutic
drugs and steroid hormones,15 16 for hospitals that
do not use gel separator tubes, verification of assay
performance with gel separator tubes is necessary,
although in the experience of the authors, the effect
of gel separator tubes is usually minimal among
general chemistry analytes.
Apart from general chemistry analytes,
another panel of tests most commonly employed in
the management of patients is blood gas analysis.
It is, however, impossible to inactivate blood gas
specimens using heat treatment as such treatment
would invariably affect the acid-base, and the partial
pressure of oxygen and carbon dioxide in blood,
rendering the specimen unsuitable for analysis.
Disinfection of routine blood gas analysers after the
processing of infected specimens is also problematic;
the glass electrode and membranes used for blood
gas analysis are often not compatible with the high
active chlorine content of bleach or the presence of
surfactants in buffers used for disinfection purposes.
For blood gas analysis, the use of point-of-care
analysers such as the i-STAT (Abbott Laboratories,
Chicago [IL], US), whereby the test cartridge on
which a blood specimen is applied is only connected
to the analyser via electrical contacts, is seen as a
viable alternative by the authors, as the test cartridge
is single-use, can be disposed of safely, and the
analyser can be cleaned and disinfected because of
the vulnerable glass electrode, and the membranes
on Clark- or Severinghaus-type electrodes (used
for measurement of oxygen and carbon dioxide
tensions) are not situated on the analyser properly.
Lastly, rather than performing the inactivation
procedure in the laboratory, another option for
analysis is the use of point-of-care analysers in an
appropriate enclosure. Blood gas, electrolytes, and
renal and liver function tests can all be performed
in modern point-of-care analysers. In concordance
with guidelines, it is recommended that the point-of-care analyser should be housed within a class 2 or
above biosafety cabinet in a level 3 or above biosafety
laboratory operating with appropriate precautions.11
Conclusion
We have presented the effect of the heat inactivation
procedure on common biochemistry analytes,
with statistical procedures applied to determine
the diagnostic utility of the analyte concentrations.
This serves to aid clinicians and laboratory staff in
managing suspected and confirmed patients with
EVD.
Acknowledgements
The authors would like to acknowledge Mr Kelvin
Yu, Ms Kitty Soo, and Mr Philip Chiu for their kind
assistance in performing the tests, and Ms Alision
Lam for her kind assistance in the preparation of this
manuscript.
References
1. World Health Organization. Disease Outbreak News
(DONs) 2014 [12/8/2014]. Available from: http://www.who.int/csr/don/2014_08_11_ebola/en/. Accessed 12 Aug
2014.
2. IHR Emergency Committee Members and Advisers.
WHO Statement on the Meeting of the International
Health Regulations Emergency Committee Regarding the
2014 Ebola Outbreak in West Africa 2014 [12/8/2014].
Available from: http://www.who.int/mediacentre/news/statements/2014/ebola-20140808/en/. Accessed 12 Aug
2014.
3. Leroy EM, Kumulungui B, Pourrut X, et al. Fruit bats as
reservoirs of Ebola virus. Nature 2005;438:575-6. Crossref
4. Wilson JA, Hevey M, Bakken R, et al. Epitopes involved
in antibody-mediated protection from Ebola virus. Science
2000;287:1664-6. Crossref
5. Feldmann H, Geisbert TW. Ebola haemorrhagic fever.
Lancet 2011;377:849-62. Crossref
6. Kortepeter MG, Martin JW, Rusnak JM, et al. Managing
potential laboratory exposure to Ebola virus by using
a patient biocontainment care unit. Emerg Infect Dis
2008;14:881-7. Crossref
7. Jaax N, Jahrling P, Geisbert T, et al. Transmission of Ebola
virus (Zaire strain) to uninfected control monkeys in a
biocontainment laboratory. Lancet 1995;346:1669-71. Crossref
8. Leffel EK, Reed DS. Marburg and Ebola viruses as aerosol
threats. Biosecur Bioterror 2004;2:186-91. Crossref
9. Sewell DL. Laboratory-associated infections and biosafety.
Clin Microbiol Rev 1995;8:389-405.
10. Interim infection prevention and control guidance for
care of patients with suspected or confirmed Filovirus
haemorrhagic fever in health-care settings, with focus
on Ebola 2014. Available from: http://www.who.int/csr/resources/who-ipc-guidance-ebolafinal-09082014.pdf.
Accessed 12 Aug 2014.
11. Interim guidance for specimen collection, transport, testing,
and submission for patients with suspected infection with
Ebola virus disease 2014 [12/8/2014]. Available from: http://www.cdc.gov/vhf/ebola/hcp/interim-guidance-specimencollection-submission-patients-suspected-infection-ebola.html. Accessed 12 Aug 2014.
12. WHO recommended guidelines for epidemic preparedness
and response: Ebola haemorrhagic fever (EHF). Geneva:
World Health Organization; 1997.
13. Bhagat CI, Lewer M, Prins A, Beilby J. Effects of heating
plasma at 56 degrees C for 30 min and at 60 degrees C
for 60 min on routine biochemistry analytes. Ann Clin
Biochem 2000;37:802-4. Crossref
14. Hersberger M, Nusbaumer C, Scholer A, Knöpfli V, von
Eckardstein A. Influence of practicable virus inactivation
procedures on tests for frequently measured analytes in
plasma. Clin Chem 2004;50:944-6. Crossref
15. Ferry JD, Collins S, Sykes E. Effect of serum volume
and time of exposure to gel barrier tubes on results for
progesterone by Roche Diagnostics Elecsys 2010. Clin
Chem 1999;45:1574-5.
16. Bush V, Blennerhasset J, Wells A, Dasgupta A. Stability of
therapeutic drugs in serum collected in vacutainer serum
separator tubes containing a new gel (SST II). Ther Drug
Monit 2001;23:259-62. Crossref