Hong Kong Med J 2015 Apr;21(2):155–64 | Epub 10 Mar 2015
DOI: 10.12809/hkmj144383
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE CME
Vitamin B12 deficiency in the elderly: is it worth screening?
CW Wong, FHKCP, FHKAM (Medicine)
Department of Medicine and Geriatrics, Caritas Medical Centre, Shamshuipo, Hong Kong
Corresponding author: Dr CW Wong (chitwaiwong@hotmail.com)
Abstract
Vitamin B12 deficiency is common among the elderly.
Elderly people are particularly at risk of vitamin B12
deficiency because of the high prevalence of atrophic
gastritis–associated food-cobalamin (vitamin B12)
malabsorption, and the increasing prevalence
of pernicious anaemia with advancing age. The
deficiency most often goes unrecognised because
the clinical manifestations are highly variable, often
subtle and non-specific, but if left undiagnosed the
consequences can be serious. Diagnosis of vitamin
B12 deficiency, however, is not straightforward as
laboratory tests have certain limitations. Setting a
cut-off level to define serum vitamin B12 deficiency is
difficult; though homocysteine and methylmalonic
acid are more sensitive for vitamin B12 deficiency,
it may give false result in some conditions and the
reference intervals are not standardised. At present,
there is no consensus or guideline for diagnosis of
this deficiency. It is most often based on the clinical
symptoms together with laboratory assessment
(low serum vitamin B12 level and elevated serum
homocysteine or methylmalonic acid level) and the
response to treatment to make definitive diagnosis.
Treatment and replacement with oral vitamin B12
can be as effective as parenteral administration even
in patients with pernicious anaemia. The suggested
oral vitamin B12 dose is 1 mg daily for a month, and
then maintenance dose of 125 to 250 µg for patients
with dietary insufficiency and 1 mg daily for those
with pernicious anaemia. Vitamin B12 replacement is
safe and without side-effects, but prompt treatment
is required to reverse the damage before it becomes
extensive or irreversible. At present, there is no
recommendation for mass screening for vitamin B12
in the elderly. Nevertheless, the higher prevalence with
age, increasing risk of vitamin B12 deficiency in the
elderly, symptoms being difficult to recognise, and
availability of safe treatment options make screening
a favourable option. However, the unavailability of
reliable diagnostic tool or gold standard test makes
screening difficult to carry out.
Introduction
Vitamin B12 deficiency is a common condition
affecting the elderly and tends to increase with
age. Acquirement of vitamin B12 into our body for
cell metabolism involves dietary intake of vitamin
B12–enriched foods and the absorption of vitamin
B12 into our body for utilisation. The main dietary
sources of vitamin B12 are animal products because
animals obtain vitamin B12 through microbial
symbiosis. The subsequent release of vitamin B12
from food for absorption into the body is complex
and requires intact function of stomach,
pancreas, and ileum. Pathophysiological changes,
multiple co-morbidities, coupled with multiple drug
intake, and increasing dependency associated with
ageing can lead to malnutrition due to inadequate
intake and malabsorption of vitamin B12, resulting
in deficiency. Vitamin B12 is essential for the normal
metabolism and functioning of all cells in the body.
Vitamin B12 deficiency can pose significant adverse
effects to organ systems with high cell turnover and
metabolism like the bone marrow, gastro-intestinal
tract, and brain. Fortunately, vitamin B12 deficiency
can be readily treated by vitamin B12 replacement.
Nevertheless, prompt diagnosis and treatment
are required to prevent extensive and irreversible
damage to the body.
Prevalence of vitamin B12 deficiency among the elderly
In general, vitamin B12 level declines with age and
therefore prevalence of vitamin B12 deficiency
increases with age.1 Studies have shown that
prevalence of vitamin B12 deficiency among elderly
can range between 5% and 40% depending on the
definition of vitamin B12 deficiency used.1 2 3 4 5 6 7 Many studies have used serum vitamin B12 level with or
without additional tests for its metabolites like
homocysteine and methylmalonic acid (MMA)
to estimate the prevalence of vitamin B12 in the
population. The most frequent serum vitamin B12
cut-off to diagnose vitamin B12 deficiency is 150
pmol/L (203 pg/mL). Using this serum vitamin
B12 cut-off alone, the prevalence of vitamin B12
deficiency is estimated to be in the range of 5% to
15%.3 4 5 6 However, when higher serum vitamin B12
cut-off at 258 pmol/L (350 pg/mL) or using elevated
serum homocysteine or MMA level in addition to a low or
low-to-normal serum vitamin B12 level to diagnose
vitamin B12 deficiency, the prevalence of deficiency
increases to 40.5%.1 3 Also, the prevalence of vitamin B12 deficiency appears to increase with age among
the elderly population.4 5 Furthermore, reports have indicated that institutionalised elderly with multiple
co-morbidities and with increasing dependency
are more prone to vitamin B12 deficiency than
non-institutionalised (free-living) elderly. In such
individuals, the prevalence of vitamin B12 deficiency
has been reported to reach 30% to 40%.8 9 In our unpublished study on 2096 institutionalised
elderly residents aged >65 years, the prevalence of
serum vitamin B12 level of <150 pmol/L was 34.9%,
whilst in another local study conducted on non-institutionalised
(free-living) elderly residents aged over 70 years, the prevalence of vitamin B12 level of
<140 pmol/L was only 6.6%.7
Diagnosis of vitamin B12 deficiency
There is no precise or ‘gold standard’ test to diagnose
vitamin B12 deficiency. The diagnosis is usually based
on identifying a low level of serum vitamin B12 with
clinical evidence of deficiency, which responds
to vitamin B12 replacement therapy. When there
is a clinical suspicion of vitamin B12 deficiency,
the initial laboratory assessment includes serum
vitamin B12 levels, complete blood count, and blood
film examination.10 11 12 Although the blood picture
and classical finding of vitamin B12 is megaloblastic
anaemia, often times this is not seen especially in mild
cases of vitamin B12 deficiency. The investigations for
vitamin B12 deficiency are traditionally recommended
for patients with macrocytosis, but macrocytosis
with or without anaemia is neither specific nor
sensitive to confirm the diagnosis.10 11 12 The reason for
this is that macrocytosis can also be found in other
conditions like folate deficiency and myelodysplastic
disorders, and up to 84% of cases would be missed if
macrocytosis is used as the only parameter to screen
for vitamin B12 deficiency.13
Tests to measure and quantify serum vitamin
B12 levels in the body are readily available and
inexpensive. However, the screening test has some
limitations and drawbacks. The main drawback is
that there is no universally accepted serum vitamin
B12 cut-off to define deficiency although the value
of <150 pmol/L (200 pg/mL) is often used, and at
this serum vitamin B12 level or below, metabolites
like serum homocysteine, serum and urine MMA,
become elevated. The World Health Organization
has suggested to use this cut-off to define vitamin
B12 deficiency since the year 2008.14 However, some
have argued that the cut-off value of 150 pmol/L is
too low and inevitably does not reflect a sufficient
level of vitamin B12 in the body, and more so
the clinical symptoms of vitamin B12 deficiency like
neurological symptoms can occur even if serum
vitamin B12 is above 150 pmol/L. Thus, a higher
cut-off value of 220 to 258 pmol/L (298-350 pg/mL)
based on more sensitive indicators of vitamin B12
status like elevated serum homocysteine and MMA levels
has been suggested.3 15 It should be noted that not all the vitamin B12 circulating in the blood is in
metabolically active form and a low serum vitamin
B12 level is not necessarily equivalent to tissue
deficiency. The falsely low vitamin B12 level can be
related to the disturbance in vitamin B12 metabolism
but may not be associated with any tissue vitamin
B12 deficiency. Such situations can occur in people
with folate deficiency, multiple myeloma, and
transcobalamin I deficiency.10 11 12 On the other hand,
falsely normal serum vitamin B12 level may occur
in the presence of liver disease, myeloproliferative
disorder, congenital transcobalamin II deficiency,
and intestinal bacterial overgrowth.10 11 12
When serum vitamin B12 results are normal
but still the clinical suspicion of deficiency exists,
additional ‘confirmatory testing’ may help to identify
vitamin B12 deficiency. There is compensatory
elevation of homocysteine and MMA levels preceding the drop in serum vitamin B12 level and
these are regarded as more sensitive indicators of
vitamin B12 deficiency than just low serum vitamin
B12 level.11 12 16 17 Elevated serum homocysteine and
MMA level (>3 standard deviations above the mean in normal subjects) has a sensitivity of 95.9% and 98.4%,
respectively to diagnose vitamin B12 deficiency.16
However, the reference intervals for serum MMA and homocysteine are variable among different
laboratories. Serum MMA of 100 to 750 nmol/L, urine MMA of 1 to 4 nmol/L, and serum homocysteine
of 6 to 29 µmol/L are the reference ranges for most methods.10 If the normalisation of elevated serum homocysteine and MMA levels in response to vitamin B12 replacement therapy is used as a diagnosis of deficiency, up to 50% of patients may be missed when
the diagnosis is based on low vitamin B12 level (150 pmol/L) alone.18 19 Rise in homocysteine level before increase in MMA is an early indicator of vitamin B12 deficiency. However, this is less specific than elevated MMA level for vitamin B12 deficiency, since such elevated homocysteine levels can occur even in vitamin B6
and folate deficiency states. Both homocysteine
and MMA levels can be elevated in renal insufficiency,
hypovolaemia, and inherited metabolic defects.12
Although elevated homocysteine and MMA levels can
aid in the diagnosis of vitamin B12 deficiency in
people with ‘normal’ serum vitamin B12 levels, there
are concerns about these metabolite assays. Some
have reported that serum MMA and homocysteine
levels increase with age and the prevalence of
elevated MMA and homocysteine levels is higher than
the prevalence of low vitamin B12 or clinically
evident vitamin B12 deficiency in the elderly.19 20 21 22 In this regard, using the assay for metabolites alone
may result in overdiagnosis and overtreatment. The
rationale for these findings is uncertain and some
have suggested that it may be related to the increased
prevalence of subclinical vitamin B12 deficiency in
the elderly. Moreover, these add to the controversies
about whether to use metabolite estimation as the
initial test to diagnose vitamin B12 deficiency. Besides,
other important considerations are that they are
more expensive, not readily available, and reference
intervals are not standardised. Currently, the initial
test for vitamin B12 deficiency is to assess serum
vitamin B12 levels, and only when there is low normal
vitamin B12 level, metabolite assay is most often
suggested.11 12 However, the consensus for vitamin B12
threshold levels for ordering the additional tests has
not yet been reached.
In addition to elevation in homocysteine and
MMA levels, a decrease in serum holotranscobalamin
level is also considered an early marker for vitamin
B12 deficiency. Holotranscobalamin is composed
of vitamin B12 attached to a transport protein,
transcobalamin II. It is a biologically active fraction
of vitamin B12 that can be readily taken up by all
cells and represents only 6% to 20% of total serum vitamin B12.23 In vitamin B12 deficiency, serum level of holotranscobalamin decreases even before elevation in homocysteine and MMA levels occurs.24 It has been shown that holotranscobalamin is the most sensitive marker for vitamin B12 deficiency, followed by MMA.23 25 Like homocysteine and MMA, holotranscobalamin cannot be tested in renal patients as its level increases in renal impairment.23
Furthermore, higher cost and lesser availability than homocysteine and MMA testing make it difficult to
acquire wide clinical acceptance.
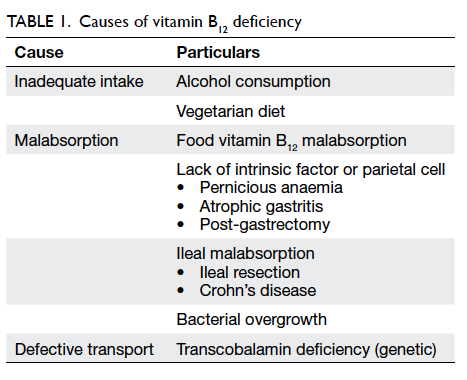
Causes of vitamin B12 deficiency in the elderly
As we know elderly people are particularly at risk
of vitamin B12 deficiency. The main aetiologies can
be divided under two main categories: inadequate
dietary intake and impaired absorption of vitamin
B12 (Table 1).
It is believed that in developed countries, the
most common cause for vitamin B12 deficiency in the
elderly is inadequate dietary intake.1 15 However, studies have shown that this is far from real. A French study
showed that among 172 elderly patients with vitamin
B12 deficiency, only 2% accounted for inadequate
intake,26 while in a hospital-based Chinese study
on 52 patients, only 3.8% (median age, 73.5 years)
with megaloblastic anaemia (98% had vitamin B12
deficiency) had inadequate dietary intake.27 However,
this can be a problem in strict vegans because animal
products are the only dietary source of vitamin B12.
Usually, 2 to 3 mg of vitamin B12 reserves are stored
in the body primarily in the liver, and our daily
requirement of vitamin B12 is only about 2 to 3 µg.
Thus, even with vegan diets, deficiency generally
takes several years to develop. According to a local
study on 119 older Chinese vegetarian women, the
prevalence of deficiency was 42%.28 Besides, factors
like poor health conditions, especially in those living
in institutions, lead to inadequate nutritional intake
and vitamin B12 deficiency.
Often, vitamin B12 deficiency can be seen even
among the elderly consuming meat and animal
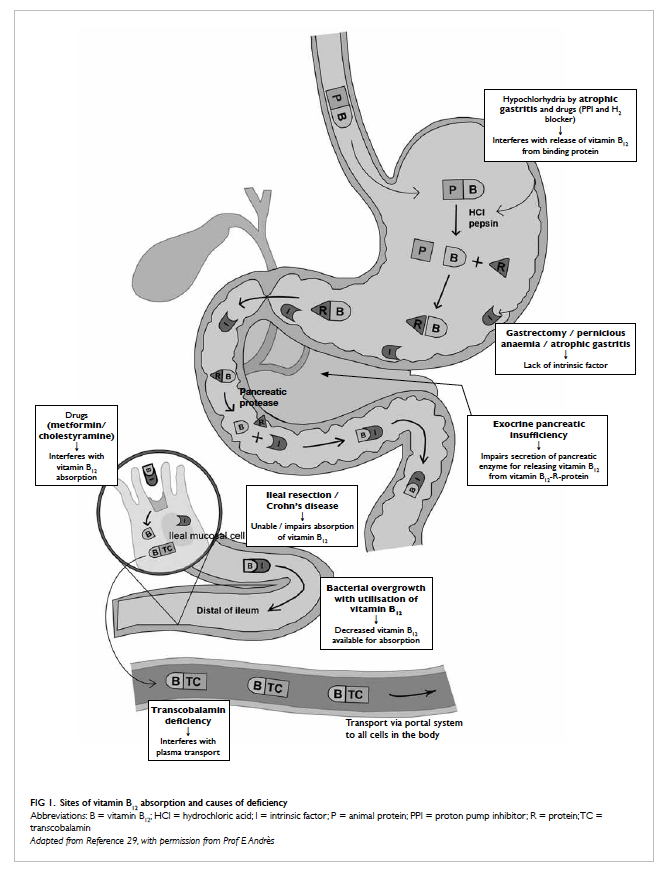
proteins and this is because of malabsorption.
Vitamin B12 in animal food is bound to a protein, and
after ingestion, it is broken down in the stomach by
pepsin and hydrochloric acid to release free vitamin
B12 (Fig 129). The free vitamin B12 is then bound to
R-protein (transcobalamin I) found in saliva and
gastric juice. The vitamin B12-R-protein complex
is also secreted in bile from the enterohepatic
circulation. These complexes are then degraded by
pancreatic enzyme to release free vitamin B12 in
the duodenum. The free vitamin B12 is then bound
to intrinsic factor secreted by the gastric parietal
cells, and then they travel undisturbed until the
distal 80 cm of ileum where they bind to mucosal
cell receptors. Subsequently, vitamin B12 is carried by
transport protein, transcobalamin, via the portal
system to all cells in the body for utilisation. About
60% of vitamin B12 from food is absorbed through
this pathway, and any pathophysiological changes in
stomach, pancreas, and intestine result in disturbance
of vitamin B12 absorption. Food-cobalamin (vitamin
B12) malabsorption, first described by Carmel in
1995,30 is the most common cause of vitamin B12
deficiency in the elderly and accounts for about
40% to 70% of cases.26 29 31 It is characterised by the
inability to release vitamin B12 from food or from
its binding protein and thus, preventing vitamin
B12 from being taken up by intrinsic factor for
absorption. It is defined by vitamin B12 deficiency in
the presence of sufficient dietary vitamin B12 intake,
negative Schilling test, and lack of anti-intrinsic
factor antibodies.30 Clinically, it is diagnosed by
exclusion of other disorders or factors causing
vitamin B12 deficiency. It can be corrected simply
with oral vitamin B12 supplement since free vitamin
B12 absorption is not affected.31 Any process that
interferes with the release of free vitamin B12, such
as decreased production of gastric acid and pepsin
for releasing vitamin B12 from food, and impaired
secretion of pancreatic enzyme for releasing vitamin
B12 from vitamin B12-R-protein complex, can lead to
malabsorption. Atrophic gastritis is the main cause of
food-cobalamin malabsorption in the elderly. In the
stomach, hypochlorhydria associated with atrophic
gastritis interferes with vitamin B12 release from
the food and causes intestinal bacterial overgrowth
to compete for vitamin B12 uptake, resulting in a
decline in vitamin B12 in the body. The prevalence of
atrophic gastritis in the elderly ranges from 20% to
50% and generally increases with age.26 32 According to Framingham Heart Study, the prevalence in age-group
of 60 to 69 years was 24% and increased to 37%
in people aged >80 years.33 Chronic Helicobacter
pylori infection is strongly associated with atrophic
gastritis,34 35 and a study reported that H pylori was found in 56% of people with vitamin B12 deficiency.35 Other causes of food-cobalamin malabsorption include long-term consumption of proton pump
inhibitors,36 histamine H2 blockers,36 chronic alcohol consumption, gastric bypass surgery, and pancreatic
insufficiency in patients with alcohol abuse and
cystic fibrosis. Food-cobalamin malabsorption often
produces a slow, progressive depletion of vitamin
B12. Clinical manifestations tend to be subtle and
mild,2 although progression to more severe form,
like pernicious anaemia (PA), can still occur in a
minority of patients.26
Pernicious anaemia, a result of autoimmune
atrophic gastritis (type A atrophic gastritis), is
most often diagnosed in the elderly. Earlier studies
suggested that PA was restricted to Northern
Europeans, but subsequent studies indicate that
PA affects virtually all ethnic groups.37 Pernicious
anaemia was considered a classical cause of vitamin
B12 deficiency before food-cobalamin malabsorption
was described, and accounted for 15% to 25% of
vitamin B12 deficiency in the elderly in studies.9 In a
local study on 296 Chinese patients, definite PA was
diagnosed in 61% of patients having megaloblastic
anaemia with vitamin B12 or folate deficiency.38
Pernicious anaemia is characterised by destruction
of gastric mucosa, especially fundal mucosa,
primarily by a cell-mediated mechanism.39 There is
progressive destruction and eventual loss of intrinsic
factor producing gastric parietal cells. Moreover,
auto-antibodies in gastric juices bind and block
the vitamin B12–binding site of intrinsic factor and
prevents the uptake of vitamin B12. The end result
is gastric atrophy and depletion of intrinsic factor
leading to poor absorption of food-bound, free, and
biliary vitamin B12.2 Malabsorption is more complete
and severe in PA compared to food-cobalamin
malabsorption which is more partial in nature,2 and
so the manifestations are more overt and severe in
PA. Two antibodies, anti-parietal cell antibody and
anti-intrinsic factor antibody, have been described
in PA. Anti-parietal cell antibody is more sensitive
(90%) but less specific (50%) for diagnosis of PA as it
can also be found in other autoimmune diseases.29 39 On the other hand, anti-intrinsic factor antibody
is less sensitive (50%) but more specific (98%), and
its presence is almost diagnostic of PA.29 39 Schilling test, traditionally used to diagnose intrinsic factor–related malabsorption, is now rarely performed. Although PA is associated with excess risk of gastric
carcinoma and gastric carcinoid tumour,40 the
benefit of endoscopic surveillance has still not been established. Once the patient is diagnosed with PA,
single endoscopic screening for gastric cancer or carcinoid tumours is recommended, but subsequent
routine endoscopic surveillance recommendation is inconclusive.41
In the elderly, long-term use of medications for
co-morbidities can interfere or reduce vitamin B12 absorption. These include proton pump inhibitors
and histamine H2 blockers, which suppress gastric acid secretion and prevent release of vitamin B12 from food.42 Other drugs like metformin reduces intestinal availability of free calcium ions for vitamin B12–intrinsic factor complex uptake by ileal cell
membrane receptors,43 and cholestyramine interferes
with vitamin B12 absorption from intestine.44
Clinical manifestations of vitamin B12 deficiency
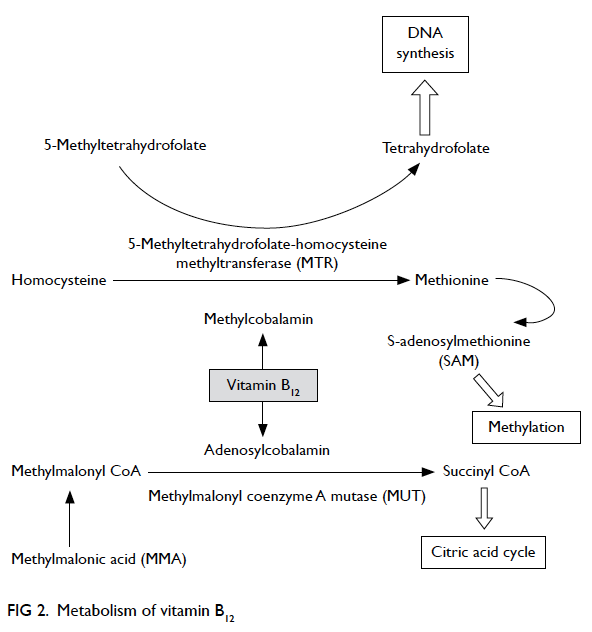
Vitamin B12 is essential for metabolism of all
cells in our body. In humans, two enzymatic
reactions are dependent on vitamin B12—methylmalonyl coenzyme A mutase (MUT) reaction
and 5-methyltetrahydrofolate-homocysteine
methyltransferase (MTR) reaction (Fig 2). The MUT
reaction is an important step in the extraction of
energy from protein and fat in the mitochondrial
citric acid cycle. In the MTR reaction, vitamin B12
and folic acid are required for the conversion of
homocysteine to methionine that is important
for maintaining the integrity of nervous system.
Tetrahydrofolate is also regenerated via the MTR
reaction for DNA synthesis. Hence, in vitamin B12
deficiency, multi-organ systems can be affected and
hence associated with wide spectrum of clinical
manifestations. However, clinically overt vitamin
B12 deficiency with classical feature of macrocytic
anaemia and neuropathy is infrequently seen in
the elderly.2 Very often they have mild, subclinical
deficiency, which are usually asymptomatic.2
Clinical manifestations of vitamin B12
deficiency are usually non-specific and are highly
variable according to severity or organ systems
involved.9 There is no one clinical feature unique to
all patients with vitamin B12 deficiency. Non-specific symptoms and signs are loss of appetite, diarrhoea, fatigue and weakness, shortness of breath, low blood pressure, confusion, and change in mental states.9 29 Classical manifestations include Hunter’s glossitis, megaloblastic anaemia, and subacute combined degeneration of spinal cord (Table 29).
Vitamin B12 deficiency and atherosclerotic vascular disease
Hyperhomocysteinaemia, as an independent risk
factor for cardiovascular disease, has been receiving
increased attention. Elevated homocysteine level is
associated with an increased risk for atherosclerotic
and thrombotic events.45 Meta-analysis of 30 studies
involving 5073 ischaemic heart disease (IHD) events suggested that elevated homocysteine level was at most
a modest independent predictor of IHD and stroke risk in healthy populations, and a 25% reduction in
homocysteine levels was associated with 11% and 19% reduction in IHD and stroke, respectively.46 Another meta-analysis also provided a strong evidence of
the causal association between homocysteine and cardiovascular disease, and showed that lowering
homocysteine level by 3 µmol/L could reduce the risk of IHD by 16% and stroke by 24%.47
Vitamin B12, folic acid, and vitamin B6 are
required for homocysteine metabolism, and often nutritional deficiency of these vitamins can cause
hyperhomocysteinaemia. In contrast to severe hyperhomocysteinaemia associated with genetic
disorders, hyperhomocysteinaemia resulted from nutritional deficiency is mild but is still associated
with increased risk of atherothrombosis. The proposed mechanism for hyperhomocysteinaemia
on inducing endothelial dysfunction and thus atherosclerosis includes homocysteine-induced
endoplasmic reticulum stress, oxidative stress, and proinflammatory response.48 Animal models of hyperhomocysteinaemia have confirmed the causal relationship between hyperhomocysteinaemia and the development of endothelial dysfunction and accelerated atherosclerosis.48
Although meta-analyses have shown reduction
of cardiovascular risk with reduction of homocysteine
levels,46 47 vitamin supplementation (with vitamin B6, vitamin B12, and folic acid) to lower homocysteine in the body may not be transformed into clinically beneficial vascular outcomes. In a double-blind, randomised controlled trial of 3680 adults with non-disabling cerebral infarction, subjects who received a combination of vitamin B6, vitamin B12, and folic acid showed moderate reduction in total homocysteine levels, but there was no effect on vascular outcomes (recurrent ischaemic stroke and coronary heart disease) during 2 years of follow-up.49 Probably a
longer duration of treatment may be necessary or
there may be other factors governing the clinical
response. Therefore, we need more controlled
trials to explore the vascular benefits of vitamin
supplementation.
Vitamin B12 deficiency and neuropsychiatric illness
Neuropsychiatric manifestations in the absence of
haematological abnormalities are commonly seen in
the elderly.2 50 These include paraesthesia, weakness,
gait abnormalities, and cognitive or behavioural
changes. Although the exact mechanism of how
vitamin B12 deficiency causes neuropsychiatric
disorder is unclear, the disruption of both MUT
and MTR vitamin B12–dependent reactions seem to
play a role. Vitamin B12 deficiency disrupts MUT
reaction with accumulation of MMA; MMA is a
myelin destabiliser and can affect normal myelin
formation. Besides, disruption of MTR reaction
leads to insufficient supply of methionine and S-adenosylmethionine (SAM), which is essential for
the myelination of myelin sheath, phospholipids
and neurotransmitter synthesis, for maintaining
brain and nervous system function.51 Furthermore,
high levels of homocysteine due to vitamin
B12 deficiency are associated with an increased risk
of atherosclerotic vascular disease, and this in turn
may increase the risk of cognitive impairment or
dementia. It has been shown that low serum vitamin
B12 is associated with a 2- to 4-fold higher risk of
cognitive impairment.50 The prevalence of low serum
vitamin B12 has been reported to be significantly
higher in the people with Alzheimer’s disease
(AD).52 However, the causal relationship between
vitamin B12 deficiency and the development of AD
remains controversial. Amyloid deposition and
hyperphosphorylation of tau protein are believed
to be involved in the mechanism of AD. The SAM-dependent
methylation is involved in the regulation
of mechanism of presenilin I expression, γ-secretase
activity, and thus amyloid levels; SAM is also
involved in the regulation of tau phosphorylation.51
Moreover, hyperhomocysteinaemia has been
shown to be associated with a significant increase
in amyloid level and amyloid deposition on cortex
and hippocampus in mouse models of AD.53 Overall,
vitamin B12 deficiency may have implications in the
neuropathological process of AD.
Depression is a common psychiatric manifestation of vitamin B12 deficiency. Involved in the synthesis of neurotransmitters, SAM may be
implicated in mood disorders. In a population-based
study of 3884 elderly people, deficiency of vitamin
B12 was associated with almost 70% more likelihood
of having a depressive disorder.54 In another cross-sectional
study of 700 community-dwelling, physically disabled women aged ≥65 years, vitamin
B12–deficient women were twice more likely to have
severe depressive symptoms.55 Although controlled
studies to show response to vitamin B12 replacement
therapy in depression are lacking, it is recommended
that all patients with vitamin B12 deficiency should be
managed as part of depression treatment. Psychosis,
including delusion and hallucination, has also been
reported in vitamin B12–deficient patients. Although
the exact mechanism is unknown, vitamin B12
replacement even after a prolonged period (at least
up to 2 years) has shown good outcomes in patients
with psychosis.56
Therapeutic management
In general, vitamin B12 replacement therapy helps
to reverse the haematological abnormalities and
psychiatric disorders. However, even after correcting
serum vitamin B12 and its metabolite levels, or
haematological abnormalities, the ability to reverse
cognitive impairment (dementia) and neurological
disorders is not promising.50 51 52 The longer the time
the neurological disorder or cognitive impairment
presents before treatment, the less likely it can be
reversed. It is suggested that prompt correction of
deficiency should be done within 6 to 12 months of
cognitive impairment in order to obtain maximum
response.57 Nevertheless, continuous replacement
therapy may still help to prevent symptoms from
deteriorating. Treatment for subtle or subclinical
deficiency is still debatable although prompt
diagnosis and treatment might prevent the progress
to clinically overt deficiency.
Classical treatment for vitamin B12 deficiency
is parenteral administration, usually intramuscular
injection, to correct the deficiency and build up
tissue storage. There are two forms of vitamin B12
for parenteral administration: cyanocobalamin
and hydroxocobalamin. It is believed that
hydroxocobalamin is converted to active enzyme
more easily and retained in the body for a longer
period of time than cyanocobalamin, and therefore be
administered in intervals of 3 months. The regimen
for vitamin B12 therapy varies across countries and
between individual practices. Generally, the schedule
for vitamin B12 replacement is 1 mg daily for a week
or 1 mg 3 times a week for 2 weeks, followed by 1 mg
per week for 1 month, and then 1 mg per month as
maintenance dose.9
Around 1% to 5% of free vitamin B12
can be absorbed along the entire intestine by
passive diffusion. Oral vitamin B12 replacement
is theoretically as effective as parenteral
administration even in patients with PA or ileal
disease, provided that the dosage is high. However,
the unpredictable absorption by passive diffusion
makes recommendation of a standard dose difficult.
A Cochrane review supports the use of high-dose
vitamin B12 (1 mg and 2 mg daily) in elevating serum
vitamin B12 level and achieving haematological and
neurological responses, even in patients with PA or
with ileal resection.58 The recommendation for oral
replacement is 1 mg daily for a month, and then 125
to 250 µg daily as maintenance dose for patients
with dietary insufficiency and food-cobalamin
malabsorption, while for PA the maintenance dose is
1 mg daily.29
Vitamin B12 does not have side-effects
even when prescribed in large doses.59 However,
hypokalaemia, resulting from uptake of circulating
potassium by newly growing and dividing
haematopoietic cells, can be severe or sometimes
life-threatening. Transient potassium replacement at
the initial stage of vitamin B12 replacement, especially
in those with low-normal serum potassium, can
prevent subsequent hypokalaemia.
Correction of risk factors associated with
vitamin B12 deficiency, like antibiotics for H pylori
infection and intestinal bacterial overgrowth,
stopping or replacing offending medications are also
important in the management and prevention of
vitamin B12 deficiency. Some institutions have even
recommended universal vitamin B12 supplement for
people aged ≥60 years in view of the high prevalence
of vitamin B12 deficiency among this popualation.15
Conclusion
Vitamin B12 deficiency is prevalent among the elderly.
Elderly people are particularly at risk of deficiency
because of the increasing prevalence with increasing
age of atrophic gastritis–associated food-cobalamin
malabsorption, PA, and due to drug intake for co-morbidities.
Symptoms and signs of vitamin B12
deficiency are usually vague and unrecognised.
Treatment may always be useful to correct clinical
abnormalities like vitamin B12–related haematological abnormalities, psychiatric
and depressive symptoms. For neurological disease
and dementia, prompt vitamin replacement
is necessary before it becomes irreversible or
permanent. Both oral and parenteral administration
of vitamin B12 are effective and without untoward
side-effects. Overall, we are in support of screening
for vitamin B12 deficiency in the elderly. However,
accurate diagnosis of vitamin B12 deficiency remains
controversial. To diagnose vitamin B12 deficiency,
laboratory tests have their limitations, and this
makes it difficult to choose a reliable and easily
available tool for screening. Although there is no
formal recommendation for screening for vitamin
B12 deficiency in asymptomatic elderly people, the
high prevalence, higher risk of deficiency in the
elderly, easy and safe treatment availability warrant
more liberal testing and vitamin supplementation in
the elderly.
References
1. Baik HW, Russell RM. Vitamin B12 deficiency in the
elderly. Annu Rev Nutr 1999;19:357-77. Crossref
2. Carmel R. Current concepts in cobalamin deficiency. Annu
Rev Med 2000;51:357-75. Crossref
3. Lindenbaum J, Rosenberg IH, Wilson PW, Stabler SP, Allen
RH. Prevalence of cobalamin deficiency in the Framingham
elderly population. Am J Clin Nutr 1994;60:2-11.
4. Clarke R, Refsum H, Birks J, et al. Screening for vitamin
B-12 and folate deficiency in older persons. Am J Clin Nutr
2003;77:1241-7.
5. Clarke R, Grimley Evans J, Schneede J, et al. Vitamin B12
and folate deficiency in later life. Age Ageing 2004;33:34-41. Crossref
6. Loikas S, Koskinen, Irjala K, et al. Vitamin B12 deficiency
in the aged: a population-based study. Age Ageing
2007;36:177-83. CrossRef
7. Chui CH, Lau FY, Wong R, et al. Vitamin B12 deficiency—need for a new guideline. Nutrition 2001;17:917-20. Crossref
8. Matthews JH. 12 Cobalamin and folate deficiency in the
elderly. Baillieres Clin Haematol 1995;8:679-97. Crossref
9. Dali-Youcef N, Andrè E. An update on cobalamin deficiency
in adults. QJM 2009;102:17-28. Crossref
10. Guidelines on the investigation and diagnosis of cobalamin
and folate deficiencies. A publication of the British
Committee for Standards in Haematology. BCSH General
Haematology Test Force. Clin Lab Haematol 1994;16:101-15.
11. Klee GG. Cobalamin and folate evaluation: measurement
of methylmalonic acid and homocysteine vs vitamin B12
and folate. Clin Chem 2000;46:1277-83.
12. Snow CF. Laboratory diagnosis of vitamin B12 and folate
deficiency: a guide for the primary care physician. Arch
Intern Med 1999;159:1289-98. Crossref
13. Oosterhuis WP, Niessen RW, Bossuyt PM, Sanders GT,
Sturk A. Diagnostic value of the mean corpuscular volume
in the detection of vitamin B12 deficiency. Scand J Clin Lab
Invest 2000;60:9-18. Crossref
14. Conclusions of a WHO technical consultation on folate and
vitamin B12 deficiencies. Food Nutr Bull 2008;29:S238-44.
15. Wolters M, Ströhle A, Hahn A. Cobalamin: a critical
vitamin in the elderly. Prev Med 2004;39:1256-66. Crossref
16. Savage DG, Lindenbaum J, Stabler SP, Allen RH. Sensitivity
of serum methylmalonic acid and total homocysteine
determinations for diagnosing cobalamin and folate
deficiencies. Am J Med 1994;96:239-46. Crossref
17. Lindenbaum J, Savage DG, Stabler SP, Allen RH. Diagnosis
of cobalamin deficiency: II. Relative sensitivities of serum
cobalamin, methylmalonic acid, and total homocysteine
concentrations. Am J Hematol 1990;34:99-107. Crossref
18. Stabler SP. Screening the older population for cobalamin
(vitamin B12) deficiency. J Am Geriatr Soc 1995;43:1290-7.
19. Pennypacker LC, Allen RH, Kelly JP, et al. High prevalence
of cobalamin deficiency in elderly outpatients. J Am Geriatr
Soc 1992;40:1197-204.
20. Joosten E, Lesaffre R, Riezler R. Are different reference
intervals for methylmalonic acid and total homocysteine
necessary in elderly people? Eur J Haematol 1996;57:222-6. Crossref
21. Joosten E, van den Berg A, Riezler R, et al. Metabolic
evidence that deficiencies of vitamin B-12 (cobalamin),
folate, and vitamin B-6 occur commonly in elderly people.
Am J Clin Nutr 1993;58:468-76.
22. Chanarin I, Metz J. Diagnosis of cobalamin deficiency: the
old and the new. Br J Haematol 1997;97:695-700. Crossref
23. Herrmann W, Obeid R, Schorr H, Geisel J. Functional
vitamin B12 deficiency and determination of
holotranscobalamin in populations at risk. Clin Chem Lab
Med 2003;41:1478-88. Crossref
24. Herbert V. Staging vitamin B-12 (cobalamin) status in
vegetarians. Am J Clin Nutr 1994;59(5 Suppl):1213S-22S.
25. Nexo E, Hoffmann-Lücke E. Holotranscobalamin, a marker
of vitamin B-12 status: analytical aspects and clinical
utility. Am J Clin Nutr 2011;94:359S-65S. Crossref
26. Andrès E, Affenberger S, Vinzio S, et al. Food-cobalamin
malabsorption in elderly patients: clinical manifestations
and treatment. Am J Med 2005;118:1154-9. Crossref
27. Chan CW, Liu SY, Kho CS, et al. Megaloblastic anaemia in
Chinese patients: a review of 52 cases. Hong Kong Med J
1998;4:296-74.
28. Kwok T, Cheng G, Woo J, Lai WK, Pang CP. Independent
effect of vitamin B12 deficiency on hematological status
in older Chinese vegetarian women. Am J Hematol
2002;70:186-90. Crossref
29. Andrès E, Loukili NH, Noel E, et al. Vitamin B12 (cobalamin)
deficiency in elderly patients. CMAJ 2004;171:251-9. Crossref
30. Carmel R. Malabsorption of food cobalamin. Baillieres
Clin Haematol 1995;8:639-55. Crossref
31. Carmel R. Cobalamin, the stomach, and aging. Am J Clin
Nutr 1997;66:750-9.
32. Selhub J, Bagley LC, Miller J, Rosenberg IH. B vitamins,
homocysteine, and neurocognitive function in the elderly.
Am J Clin Nutr 2000;71:614S-20S.
33. Krasinski SD, Russell RM, Samloff IM, et al. Fundi atrophic
gastritis in an elderly population. Effect on hemoglobin
and several serum nutritional indicators. J Am Geriatr Soc
1986;34:800-6.
34. Blaser MJ, Parsonnet J. Parasitism by the “slow” bacterium Helicobacter pylori leads to altered gastric homeostasis
and neoplasia. J Clin Invest 1994;94:4-8. Crossref
35. Kaptan K, Beyan C, Ural AU, et al. Helicobacter pylori—is it a novel causative agent in Vitamin B12 deficiency? Arch
Intern Med 2000;160:1349-53. Crossref
36. Valuck RJ, Ruscin JM. A case-control study on adverse
effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adults. J Clin Epidemiol 2004;57:422-8. Crossref
37. Carmel R. Ethnic and racial factors in cobalamin
metabolism and its disorders. Semin Hematol 1999;36:88-100.
38. Chan CW, Liu SY, Kho CS, et al. Pernicious
anemia in Chinese: a study of 181 patients in a Hong Kong
hospital. Medicine (Baltimore) 2006;85:129-38. Crossref
39. Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N
Engl J Med 1997;337:1441-8. Crossref
40. Hsing AW, Hansson LE, McLaughlin JK, et al. Pernicious
anemia and subsequent cancer. A population-based cohort
study. Cancer 1993;71:745-50. Crossref
41. Hirota WK, Zuckerman MJ, Adler DG, et al. ASGE
guideline: the role of endoscopy in the surveillance of
premalignant conditions of upper GI tract. Gastrointest
Endosc 2006;63:570-80. Crossref
42. Schuümann K. Interactions between drugs and vitamins at
advanced age. Int J Vitam Nutr Res 1999;69:173-8. Crossref
43. Bauman WA, Shaw S, Jayatilleke E, Spungen AM, Herbert
V. Increased intake of calcium reverses vitamin B12
malabsorption induced by metformin. Diabetes Care
2000;23:1227-31. Crossref
44. Desouza C, Keebler M, McNamara DB, Fonseca V.
Drugs affecting homocysteine metabolism: impact on
cardiovascular risk. Drugs 2002;62:605-16. Crossref
45. Welch GN, Loscalzo J. Homocysteine and
atherothrombosis. N Engl J Med 1998;338:1042-50. Crossref
46. Homocysteine Studies Collaboration. Homocysteine and
risk of ischemic heart disease and stroke: a meta-analysis.
JAMA 2002;288:2015-22. Crossref
47. Wald DS, Law M, Morris JK. Homocysteine and
cardiovascular disease: evidence on causality from a meta-analysis.
BMJ 2002;325:1202. Crossref
48. Austin RC, Lentz SR, Werstuck GH. Role of
hyperhomocysteinemia in endothelial dysfunction and
atherothrombotic disease. Cell Death Differ 2004;11 Suppl
1:S56-64. Crossref
49. Toole JF, Malinow MR, Chambless LE, et al. Lowering
homocysteine in patients with ischemic stroke to prevent
recurrent stroke, myocardial infarction, and death: the
Vitamin Intervention for Stroke Prevention (VISP)
randomized controlled trial. JAMA 2004;291:565-75. Crossref
50. Lindenbaum J, Healton EB, Savage DG, et al.
Neuropsychiatric disorders caused by cobalamin deficiency
in the absence of anemia or macrocytosis. N Engl J Med
1988;318:1720-8. Crossref
51. Vogel T, Dali-Youcef N, Kaltenbach G, Andrès E.
Homocysteine, vitamin B12, folate and cognitive functions:
a systematic and critical review of the literature. Int J Clin
Pract 2009;63:1061-7. Crossref
52. Malouf R, Areosa Sastre A. Vitamin B12 for cognition.
Cochrane Database Syst Rev 2003;(3):CD004326.
53. Zhou JM, Praticò D. Acceleration of brain amyloidosis in
an Alzheimer’s disease mouse model by a folate, vitamin
B6 and B12-deficiency diet. Exp Gerontol 2010;45:195-201. Crossref
54. Tiemeier H, van Tuijl HR, Hofman A, Meijer J, Kiliaan
AJ, Breteler MM. Vitamin B12, folate, and homocysteine
in depression: the Rotterdam study. Am J Psychiatry
2002;159:2099-101. Crossref
55. Penninx BW, Guralnik JM, Ferrucci L, Fried LP, Allen RH,
Stabler SP. Vitamin B12 deficiency and depression in
physically disabled older women: epidemiologic evidence
from Women’s Health and Aging Study. Am J Psychiatry
2000;157:715-21. Crossref
56. Sabeen S, Holroyd S. Vitamin B12 and psychiatric illness.
Ann Longterm Care 2009;17:32-6.
57. Martin DC, Francis J, Protech J, Huff FJ. Time dependency
of cognitive recovery with cobalamin replacement: report
of a pilot study. J Am Geriatr Soc 1992;40:168-72.
58. Vidal-Alaball J, Butler CC, Cannings-John R, et al.
Oral vitamin B12 versus intramuscular vitamin B12 for
vitamin B12 deficiency. Cochrane Database Syst Rev
2005;(3):CD004655.
59. Food and Nutrition Board, Institute of Medicine. Dietary
reference intakes for thiamin, riboflavin, niacin, vitamin
B6, folate, vitamin B12, pantothenic acid, biotin, and
choline. Washington, DC: National Academy Press; 1998.