DOI: 10.12809/hkmj134208
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Helicobacter pylori–negative gastric mucosa–associated lymphoid tissue lymphoma: magnifying endoscopy findings
TT Law, FRCSEd, FHKAM (Surgery);
Daniel Tong, MS, PhD;
Sam WH Wong, FRCSEd, FHKAM (Surgery);
SY Chan, FRCSEd, FHKAM (Surgery);
Simon Law, MS, FRCSEd
Division of Esophageal and Upper Gastrointestinal Surgery, Department of Surgery, The University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong
Corresponding author: Prof Simon Law (slaw@hku.hk)
Abstract
Gastric mucosa–associated lymphoid tissue
lymphoma is uncommon and most patients
have an indolent clinical course. The clinical
presentation and endoscopic findings can be
subtle and diagnosis can be missed on white light
endoscopy. Magnifying endoscopy may help identify
the abnormal microstructural and microvascular
patterns, and target biopsies can be performed.
We describe herein the case of a 64-year-old
woman with Helicobacter pylori–negative gastric
mucosa–associated lymphoid tissue lymphoma
diagnosed by screening magnification endoscopy.
Helicobacter pylori–eradication therapy was given
and she received biological therapy. She is in clinical
remission after treatment. The use of magnification
endoscopy in gastric mucosa–associated lymphoid
tissue lymphoma and its management are reviewed.
Introduction
Primary extranodal low-grade B cell lymphoma of
the mucosa-associated lymphoid tissue (MALToma)
of stomach is uncommon. The clinical presentation
is variable and there is no endoscopic hallmark.
Magnifying endoscopy (ME) may help in the
diagnosis of gastric MALToma. Herein we report
the case of a patient who had gastric MALToma
diagnosed by ME.
Case report
A 64-year-old woman had a history of cancer of
alveolus that was in remission. She was considered
at increased risk of developing squamous cell
carcinoma of the upper aero-digestive tract;
therefore, she was referred to us for endoscopic
screening in May 2012. On white light endoscopy,
a well-demarcated erythematous zone measuring
3 cm in size was identified at the distal greater
curvature of the stomach (Fig 1a). To further
delineate this suspicious lesion, ME combined with
narrow-band imaging (NBI) was used. Magnifying
endoscopy was performed with a zoom endoscope
(GIF-Q260Z; Olympus Hong Kong and China Ltd,
China) which has a magnifying power of 80 times.
The tip of the endoscope was mounted with a
transparent cap for the purpose of focusing. Under
ME in combination with NBI, a non-structural area,
ie, complete or almost-complete disappearance of
gastric crypt epithelium and abnormal mucosal
capillary pattern was identified (Fig 1a). Biopsy of
the suspicious zone revealed atypical lymphoid
cells with lymphoepithelial lesion formation.
Immunohistochemical stains confirmed that the
atypical lymphoid cells were positive for B cell
marker CD20 (pan-B-cell marker: L26, Dako, UK),
and negative for T cell markers CD3 and CD5 (Fig
2). Helicobacter pylori was not found. These findings
were consistent with gastric MALToma. Whole-body
positron-emission tomography scan did not
show abnormal uptake in the stomach and the rest
of body.
The patient was empirically treated with H
pylori–eradication therapy (esomeprazole 20 mg twice
daily, amoxicillin 1 g twice daily, and clarithromycin
500 mg twice daily for 1 week). She was then treated
with four doses of rituximab (weekly for 4 weeks);
this treatment was completed in June 2012. Repeated
endoscopy and biopsy at 4 weeks after treatment
showed a residual focus of MALToma. Radiotherapy
(30 Gy) was subsequently given for improved local
control in view of partial response to rituximab,
which was completed in December 2012. Endoscopy
performed 6 weeks after completion of radiotherapy
showed complete response, ie, recovery of gastric pit
pattern under ME (Fig 1b).
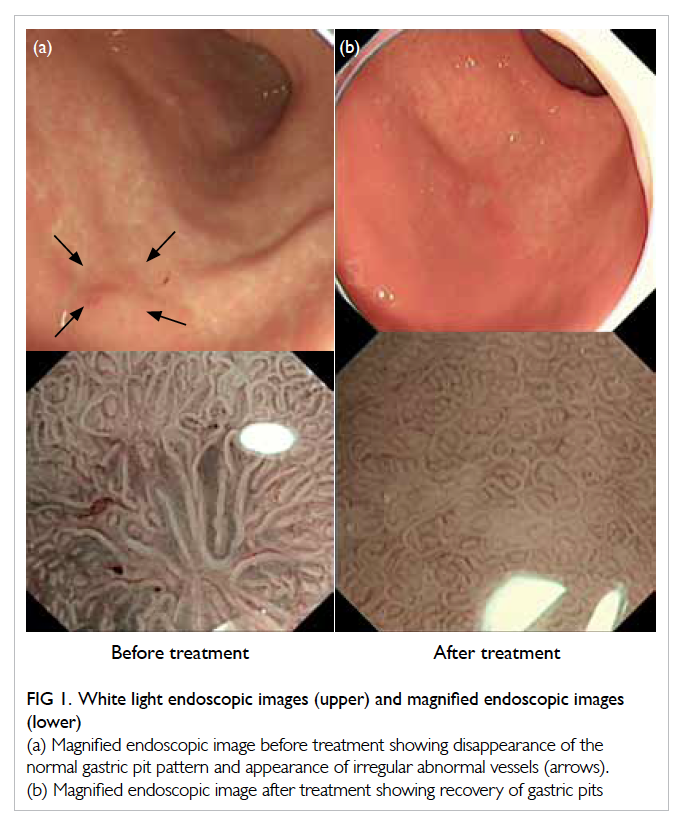
Figure 1. White light endoscopic images (upper) and magnified endoscopic images (lower)
(a) Magnified endoscopic image before treatment showing disappearance of the normal gastric pit pattern and appearance of irregular abnormal vessels (arrows). (b) Magnified endoscopic image after treatment showing recovery of gastric pits
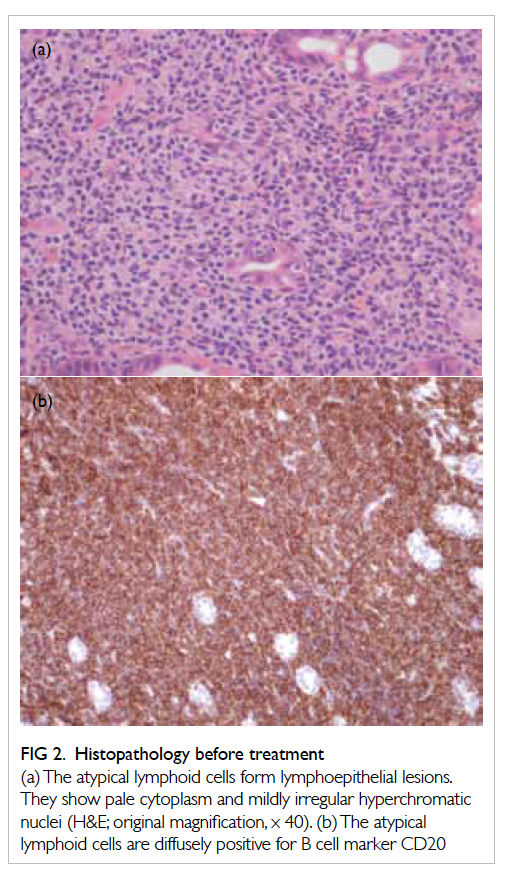
Figure 2. Histopathology before treatment
(a) The atypical lymphoid cells form lymphoepithelial lesions. They show pale cytoplasm and mildly irregular hyperchromatic nuclei (H&E; original magnification, x 40). (b) The atypical lymphoid cells are diffusely positive for B cell marker CD20
Discussion
Gastric MALToma is uncommon and accounts
for 1% to 5% of all gastric cancers. Isaacson and
Wright1 first reported a low-grade gastric lymphoma
in 1983. Lymphoid tissue is absent in the normal
gastric mucosa. Helicobacter pylori is believed to
play a causative role in the development of gastric
MALToma.2 Gastric lymphoid tissue is acquired in
response to local infection by H pylori, and there is
a strong association between H pylori infection and
gastric MALToma. Approximately 90% of gastric
MALTomas are H pylori positive. Pathogenesis of
gastric MALToma is a multistep process; H pylori
infection causes chronic gastritis, which leads to
immunological reaction with formation of lymphoid
follicles and, subsequently, to genetic abnormalities
and malignant transformation. Eradication of H
pylori may lead to remission in 50% to 90% of cases.3 4
Most patients with gastric MALToma have an
indolent clinical course; the 5-year overall survival
after H pylori eradication has been reported to be
82% to 96%.5 6
The presentation of MALToma is variable.
Patients may present with non-specific symptoms
such as epigastric pain, vomiting, and weight loss.
Endoscopy with biopsy is diagnostic but there is no
endoscopic hallmark. The endoscopic appearance is
also non-specific. It is difficult to differentiate gastric
MALToma from gastric erosion or gastritis on
conventional white light endoscopy. Diagnosis can
be easily missed if multiple biopsies are not taken.
Narrow-band imaging allows enhanced visualisation
of microvascular and microsurface structures in
the superficial part of the mucosa. The use of ME
with NBI is useful for the diagnosis of early gastric
cancer.7 Recently, retrospective studies have shown
the characteristics of gastric MALToma on ME. Chiu
et al8 reported abnormal spider-like vasculature and
disappearance of gastric pits in nine patients with
histological diagnoses of gastric MALToma, while
Ono et al9 reported that non-structural areas with
abnormal vessels were observed in 11 patients before
treatment. The use of ME may help the endoscopist
to take targeted biopsies from the abnormal areas.10
The histological criteria of gastric MALToma
were established according to the World Health
Organization criteria in 2001, which included
(i) invasion of epithelial structures resulting in
‘lymphoepithelial lesions’; (ii) small lymphocytes,
marginal zone cells, and/or monocytoid B cells; (iii)
infiltration of diffuse, perifollicular, interfollicular, or
even follicular type due to colonisation of reactive
follicles.11
Surgery has been replaced by medical therapy
in the management of gastric MALToma.12 It is
generally recommended that H pylori–eradication
therapy be the first-line therapy for H pylori–positive patients with localised gastric MALToma;
eradication therapy should also be given to H pylori–negative patients, as there may be false-negative results.13 About 60% to 90% of patients with gastric
MALToma achieve complete response after H pylori
eradication.13 The presence of atrophic-like mucosa
is a characteristic finding after treatment of gastric
MALToma. Reported post-treatment ME findings
include recovery of gastric pits and resolution of
abnormal vascular pattern.8 It was demonstrated
that the disappearance of non-structural areas
with abnormal vessels was related to pathological
remission.10
There is no consensus on treatment for those
who fail to respond to eradication therapy. It is
evident that patients with progressive disease or
clinically evident relapse should undergo further
treatment. Different treatment modalities include
chemotherapy and radiotherapy. In a recently
published multicentre cohort, high response rate
to radiotherapy (94%) and chemotherapy (88%)
was reported when used as second-line treatment
in 82 non-responders and eight responders with
relapse.14 Nine patients (out of 420 patients) showed
transformation into diffuse large B cell lymphoma
at a median follow-up period of 6 years.14 The
management of patients with clinically partial
remission is not well defined. A ‘watch and wait’
strategy has been advocated, and patients are
followed up with interval endoscopies for evidence of
progressive disease before considering oncological
treatment.14 Rituximab, a chimeric antibody directed
against CD20, is another therapeutic option. CD20 is
a B cell–specific antigen expressed abundantly by the
neoplastic cells of MALToma. The response rate has
been reported to be around 70% in small series.15
In summary, patients with gastric MALToma
present with non-specific symptoms, and there is no
endoscopic hallmark. Endoscopy and biopsy are the
gold standard for diagnosis. Magnifying endoscopy
findings in MALToma include abnormal vessel
patterns and disappearance of gastric pits; however,
these findings have not been confirmed by large-scale
prospective studies. Helicobacter pylori eradication
is the first-line treatment. While the majority of
patients respond to eradication therapy, those who
fail to respond and develop progressive disease
require oncological treatment. The management
of partial responders is uncertain. Surveillance
endoscopy is recommended in the follow-up of
patients with MALToma in order to detect relapse.
Acknowledgement
We thank Dr Florence Loong (Department of
Pathology, The University of Hong Kong Li Ka
Shing Faculty of Medicine, Queen Mary Hospital) of
providing the histopathology slides.
References
1. Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated
lymphoid tissue. A distinctive type of B-cell
lymphoma. Cancer 1983;52:1410-6. Crossref
2. Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson
PG. Helicobacter pylori–associated gastritis and primary
B-cell gastric lymphoma. Lancet 1991;338:1175-6. Crossref
3. Wotherspoon AC, Doglioni C, Diss TC, et al. Regression
of primary low-grade B-cell gastric lymphoma of mucosa-associated
lymphoid tissue type after eradication of
Helicobacter pylori. Lancet 1993;342:575-7. Crossref
4. Zullo A, Hassan C, Cristofari F, et al. Effects of Helicobacter
pylori eradication on early stage gastric mucosa-associated
lymphoid tissue lymphoma. Clin Gastroenterol Hepatol
2010;8:105-10. Crossref
5. Stathis A, Chini C, Bertoni F, et al. Long-term outcome
following Helicobacter pylori eradication in a retrospective
study of 105 patients with localized gastric marginal zone
B-cell lymphoma of MALT type. Ann Oncol 2009;20:1086-93. Crossref
6. Zullo A, Hassan C, Andriani A, et al. Eradication therapy
for Helicobacter pylori in patients with gastric MALT
lymphoma: a pooled data analysis. Am J Gastroenterol
2009;104:1932-7. Crossref
7. Yao K, Oishi T, Matsui T, Yao T, Iwashita A. Novel
magnified endoscopic findings of microvascular
architecture in intramucosal gastric cancer. Gastrointest
Endosc 2002;56:279-84. Crossref
8. Chiu PW, Wong TC, Teoh AY, et al. Recognition of
changes in microvascular and microstructural patterns
upon magnifying endoscopy predicted the presence of
extranodal gastric MALToma. J Interv Gastroenterol
2012;2:3-7. Crossref
9. Ono S, Kato M, Ono Y, et al. Characteristics of magnified
endoscopic images of gastric extranodal marginal zone
B-cell lymphoma of the mucosa-associated lymphoid
tissue, including changes after treatment. Gastrointest
Endosc 2008;68:624-31. Crossref
10. Ono S, Kato M, Ono Y, et al. Target biopsy using magnifying
endoscopy in clinical management of gastric mucosa-associated
lymphoid tissue lymphoma. J Gastroenterol
Hepatol 2011;26:1133-8. Crossref
11. Isaacson PG, Muller-Hermelink HK, Paris MA, et al.
Extranodal marginal zone B-cell lymphoma of mucosa-associated
lymphoid tissue (MALT lymphoma). In: Jaffe
ES, Harris NL, Stein H, Vardiman JW, editors. World
Health Organization classification of tumors. Pathology
and genetics of tumors of haematopoietic and lymphoid
tissues. Lyon: IARC Press; 2001: 157-60.
12. Avilés A, Nambo MJ, Neri N, et al. The role of surgery in
primary gastric lymphoma: results of a controlled clinical
trial. Ann Surg 2004;240:44-50. Crossref
13. Fischbach W, Goebeler-Kolve ME, Dragosics B, Greiner
A, Stolte M. Long term outcome of patients with gastric
marginal zone B cell lymphoma of mucosa associated
lymphoid tissue (MALT) following exclusive Helicobacter
pylori eradication therapy: experience from a large
prospective series. Gut 2004;53:34-7. Crossref
14. Nakamura S, Sugiyama T, Matsumoto T, et al. Long-term
clinical outcome of gastric MALT lymphoma after
eradication of Helicobacter pylori: a multicentre cohort
follow-up study of 420 patients in Japan. Gut 2012;61:507-13. Crossref
15. Martinelli G, Laszlo D, Ferreri AJ, et al. Clinical activity
of rituximab in gastric marginal zone non-Hodgkin’s
lymphoma resistant to or not eligible for anti–Helicobacter
pylori therapy. J Clin Oncol 2005;23:1979-83. Crossref

