DOI: 10.12809/hkmj134141
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Acquired factor V inhibitor in a patient receiving venous-venous extracorporeal membrane oxygenation for Legionella pneumonia
Anne KH Leung, FHKCA, FHKAM (Anaesthesiology)1; George WY Ng, FHKCP, FHKAM (Medicine)1; KC Sin, FHKCP, FHKAM (Medicine)1; SY Au, FHKCP, FHKAM (Medicine)1; KY Lai, FHKCP, FHKAM (Medicine)1; KL Lee, FHKCP, FHKAM (Medicine)2; KI Law, FHKCP, FHKAM (Medicine)2
1 Intensive Care Unit, Queen Elizabeth Hospital, Jordan, Hong Kong
2 Intensive Care Unit, United Christian Hospital, Kwun Tong, Hong Kong
Corresponding author: Dr Anne KH Leung (leungkha@ha.org.hk)
Abstract
We report a rare complication of factor V deficiency
in a patient having Legionella pneumonia. This
patient also had other complications like severe
acute respiratory distress syndrome, acute kidney
injury, and septic shock that required venous-venous
extracorporeal membrane oxygenation support.
This is the first reported case of acquired factor V
deficiency in a patient receiving extracorporeal
membrane oxygenation for Legionella pneumonia.
With the combined use of intravenous
immunoglobulin, rituximab and plasma exchange,
we achieved rapid clearance of the factor V inhibitor
within 1 week so as to allow safe decannulation of
extracorporeal membrane oxygenation.
Case report
This was the case of a 53-year-old lorry driver with
a history of pulmonary tuberculosis and chronic
smoking, who presented in December 2012 with fever,
cough, and sputum. Chest X-ray (CXR) showed left
lower zone consolidation; the patient was diagnosed
to have community-acquired pneumonia which was
treated with ceftriaxone and azithromycin. Two days
later, both the renal and liver functions worsened with
elevation of serum urea level to 23.2 mmol/L (reference
range [RR], 8-8.1 mmol/L), creatinine level to 493 µmol/L
(RR, 62-106 µmol/L), aspartate transaminase level to 300
IU/L (reference level [RL], <40 IU/L), and alanine
transaminase level up to 94 IU/L (RL, <41 IU/L). There
was severe rhabdomyolysis with increased serum
creatine kinase levels to 11 010 IU/L (RR, 39-308
IU/L). Urine tested positive for Legionella antigen.
Antibiotic was changed to piperacillin-tazobactam
and azithromycin. The patient developed respiratory
failure the next day and was admitted to the intensive
care unit (ICU) for ventilator support. His condition
gradually stabilised over the next 10 days and sputum
culture showed growth of Legionella pneumophila
serogroup 1.
By day 11 in the ICU, he developed secondary
deterioration with rapid progression of pulmonary
infiltrates on CXR, septic shock, and acute kidney
injury. Sputum culture after ICU admission
showed growth of Pseudomonas aeruginosa
and Corynebacterium species. Antibiotic was
changed to meropenem and levofloxacin. By day
12, his oxygenation could not be maintained with
conventional ventilation and the Murray score was
3.5. The patient was referred for extracorporeal
membrane oxygenation (ECMO) support.
As his condition was unstable for transfer to
the ECMO centre, venous-venous ECMO (VV-ECMO)
was initiated at the referring hospital by
percutaneous placement of two ECMO cannulas
(23F and 19F) into the femoral vein and right
internal jugular vein, respectively. The ECMO
circuitry consisted of the Quadrox-i hollow-fibre
oxygenator and Cardiohelp centrifugal pump
(Maquet Cardiopulmonary AG, Germany). The
circuit flow was started at 3.2 to 2.8 L/min during the
first ECMO day, and then subsequently increased to
4.0 to 5.0 L/min to achieve PaO2 of 8 to 10 kPa. The
ventilator setting was then decreased to peak airway
pressure of <25 cm H2O, positive end–expiratory
pressure of 10 cm H2O and FiO2 of 0.4. Heparin was
started according to protocol with bolus 70 unit/kg
after cannulation, followed by continuous infusion
at 10 unit/kg/h to achieve an activated clotting
time (ACT) of 200 to 220 seconds. The coagulation
profile, ACT, renal function, and arterial blood gas
were monitored every 4 hours.
Before initiation of VV-ECMO, the baseline
international normalised ratio (INR) was 1.1 and
activated partial thromboplastin time (APTT)
was 33.7 seconds (RR, 28-34.6 seconds). A full
blood count showed a haemoglobin concentration
of 68 g/L, white cell count of 16 x 109 /L, and a
platelet count of 169 x 109 /L. Continuous veno-venous
haemofiltration (CVVH) was started for
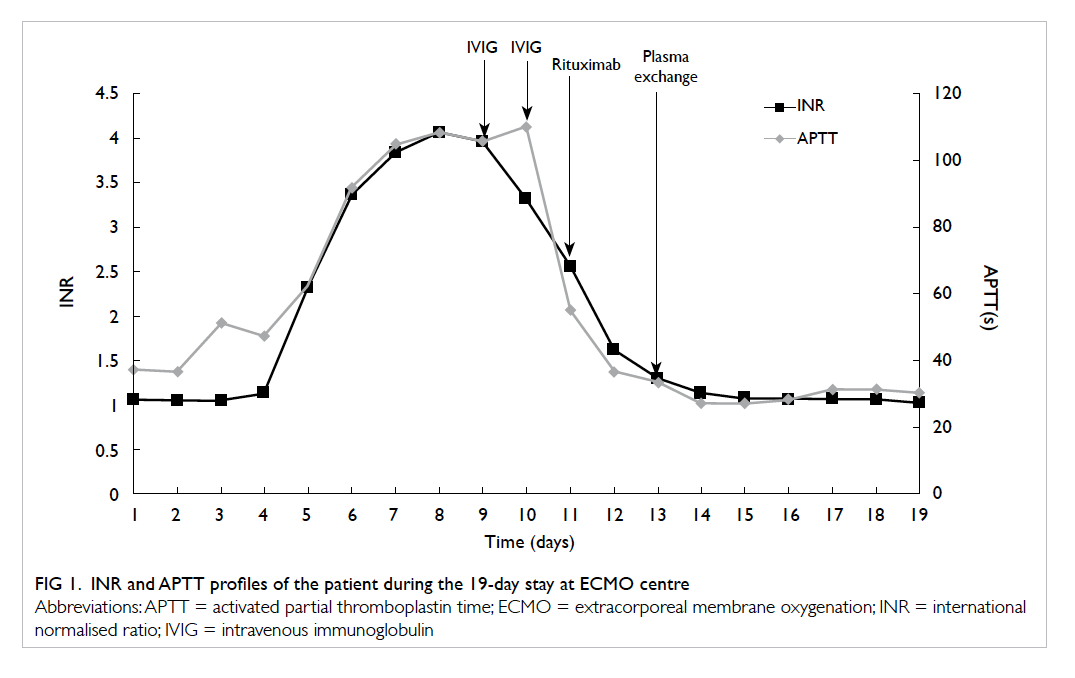
renal support. By day 4 of ECMO, INR started to
prolong (1.61) and gradually increased to 2.32 and
3.36 over the next 2 days. Heparin was stopped,
and vitamin K 10 mg and repeated fresh frozen
plasma (FFP) transfusions ranging from 8 to 14
units per day were given. Throughout this period,
the fibrinogen level remained normal at 4.44 g/L (RR,
2-4.5 g/L) and platelet count was greater than 100
x 109 /L. Liver function and ammonia level were
normal. The coagulopathy could not be corrected
by FFP. A haematologist was consulted and further
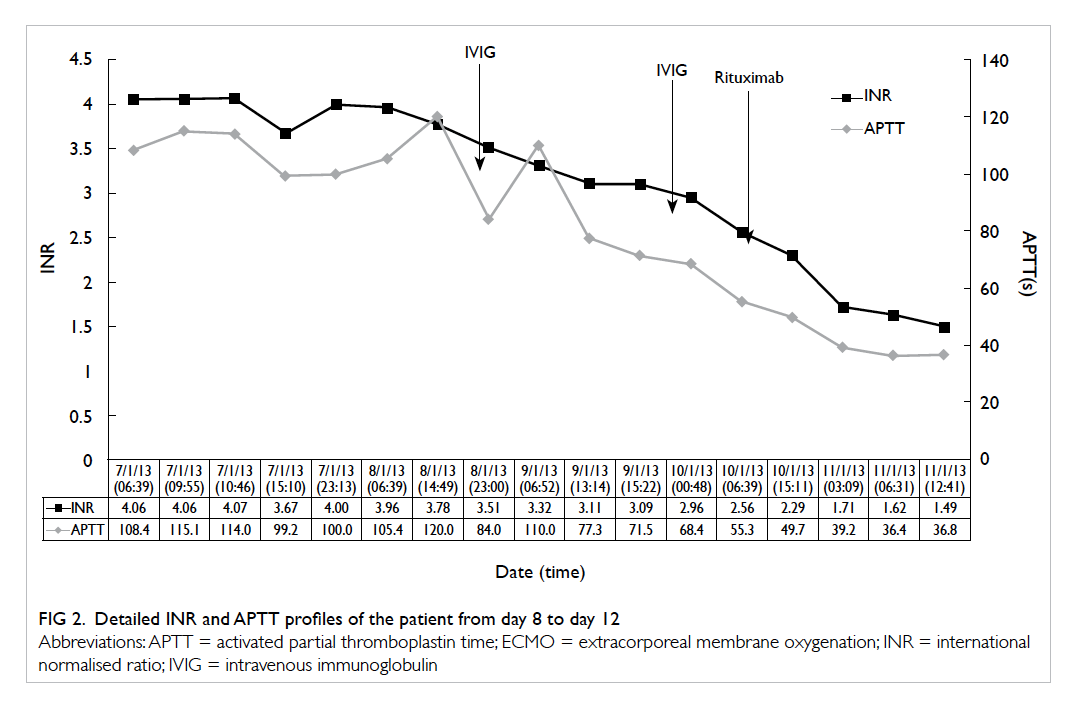
tests were arranged. By day 8, the INR peaked at
4.06, and APTT increased to 115 seconds with
slight prolongation of thrombin time (TT) to 15.3
seconds (TT control = 14.4 seconds). Coagulation
factor assay showed factor V of 1% (RR, 50-200%)
while factor VII, VIII, IX, X, XI and XII levels were
within reference intervals. Factor V inhibitor assay
showed levels increased up to 6 Bethesda units. The
diagnosis of acquired factor V inhibitors was made.
In the presence of significantly high levels of
factor V inhibitor and risk of spontaneous intracranial
bleed, intravenous immunoglobulin (IVIG) at 60
g/day was given for 2 days. The patient’s INR
decreased from 3.96 to 2.56 and APTT decreased
from 105.4 to 55.3 seconds. The workup for immune
markers including C3, C4, rheumatoid factor,
antinuclear antibody, antineutrophil cytoplasmic and perinuclear neutrophil antibodies, anti-extractable
nuclear antigen, and anti-cardiolipin antibodies
was negative. The tumour markers were negative
as well. The patient received no surgical procedure.
He had been put on four antibiotics after ECMO
including azithromycin, meropenem, fluconazole,
and linezolid. By day 4 of ECMO, fluconazole was
replaced with anidulafungin for fungal cover.
Despite IVIG, the patient developed significant
clinical bleeding with full-stream haematuria and
bronchoscopy showed extensive blood clots in the
left lower lobe. At the same time, his pulmonary
mechanics and CXR started to improve after 10
days of ECMO support and he appeared ready to
be weaned off from ECMO. It was decided to give
him one dose of rituximab 700 mg on day 11 of
ECMO. His INR decreased to 1.29 and APTT to
33.6 seconds over the next 2 days (Figs 1 and 2).
The patient was successfully decannulated on day
13 of ECMO. The haematuria remained severe and
required continuous bladder irrigation. Citrate
CVVH was started for renal support. One session
of plasma exchange was given after decannulation.
The haematuria eventually stopped by day 16. Two
days later, urinary output returned to normal and
the patient was successfully extubated. By day 19, the
patient was transferred back to the parent hospital
with INR of 1.02, APTT of 30.4 seconds, and factor
V assay of 173%. The patient was discharged 3 weeks
later and his coagulation profile remained normal
without further eradication therapy. At the time of
discharge, the patient was able to walk with the help
of a walking stick, could perform activities of daily
living independently, and was dialysis-independent.
Discussion
Factor V deficiency: causes, clinical course, laboratory finding, treatment, and outcome
Factor V is a plasma cofactor that activates
prothrombin to thrombin, thus, affecting the
common final pathway of the coagulation cascade.
About 20% of the circulating factor V is found
within platelet α granules.1 The first reported case
of congenital factor V deficiency was from Germany
in 1955,2 and to date, about 200 reported cases have
been reported.1 Congenital factor V deficiency is a
rare autosomal recessive disease with a prevalence
of 1 in 1 000 000.1 In acquired cases, it is related
to the presence of factor V inhibitor.3 In one case
series of 78 patients, the commonest cause was
the use of antibiotics (42%), including β-lactam
antibiotics, aminoglycosides, cephalosporins,
tetracyclines, and quinolones. The next common
cause was surgical procedure (31%) with exposure
to bovine thrombin, which is a topical haemostatic
agent widely used in cardiovascular or neurosurgical
procedures.4 Infection, cancer, and autoimmune
disease were present in 23%, 22%, and 13% of the
cases, respectively. About 16 (21%) cases had no
identifiable causes.3
The median age of presentation was 69 years,
with a tendency for male predominance.3 Overall, 81% of cases had bleeding, and the mucous membranes of most frequently reported sites including gastro-intestinal tract, genito-urinary tract, and the airway were noted in up to 62% of cases.3 Cerebral haemorrhage
occurred in only 8% of cases, but was associated with
50% mortality.3 Some cases were associated with
thrombotic complications rather than haemorrhage.5
Laboratory findings included a prolonged
prothrombin time and APTT that failed to be
corrected by mixing studies. Thrombin time was
usually normal unless there is presence of thrombin
inhibitor. Bethesda assay is used to detect and
quantify the presence of inhibitors. One Bethesda
unit is defined as the amount that decreases factor
V concentration by 50%.4 5 Bleeding correlated with
factor V activity with median factor V activity being
1% in bleeders and 3% in non-bleeders.3
Treatment mainly consists of controlling
bleeding and eradication of the autoantibody.
Daily infusion of 15 to 20 mL/kg of FFP is usually
sufficient.1 In refractory cases, recombinant factor
VIIa, activated prothrombin complex concentrate,
and platelet transfusion are therapeutic options.1 3 6 Plasmapheresis and immunoadsorption can rapidly
reduce antibody titres. For immunosuppression,
corticosteroids and cyclophosphamide have shown
a success rate of 63%.3 Use of high-dose IVIG and
anti-20 monoclonal antibody rituximab were
associated with rapidly increasing factor V activity,
although results were conflicting.3 6
The alloantibody against factor V was
polyclonal immunoglobulin G7 and it disappeared
in the majority of cases (69%) either after eradiation
therapy (43/78 patients) or spontaneously (12/78
patients).3 7 For those patients who survived, factor V
inhibitor persisted for a mean period of 5.1 months8
(range, <1 month to several years).6 7 For those related
to bovine thrombin, the inhibitor emerged after
a mean of 8.3 days of exposure and persisted for a
shorter time of 2.3 months.8 Overall, 72% of patients
with acquired factor V inhibitors suffered bleeding
complications, with 17% of those being fatal.8 For
those with acquired factor V deficiency with a
known cause like bovine thrombin–induced factor V
inhibitor, bleeding was less common (33%) and was
associated with better prognosis and lower fatality
(6%).8 The highest mortality was found in patients
with autoimmune disorder (30%) or cancer (24%).3
Use of extracorporeal membrane
oxygenation for Legionella pneumonia
Use of ECMO has been reported locally for treating
influenza H1N1 with good outcome.9 Use of ECMO
in Legionella pneumonia with acute respiratory
distress syndrome has been reported,10 11 12 with survival
rate ranging from 67% to 84% in the UK series.10 11
Acute renal failure was a common complication of
legionellosis with 53.7% requiring renal replacement
therapy. The prognosis for this subgroup of patients
was poor with only 33% (vs 70% in those without
acute renal failure) surviving to decannulation
and mortality increasing from 15% to 53%.10 Major
bleeding complications reported in these series
included intra-abdominal bleeding, cardiac
tamponade, chest drain–related haemorrhage, and
gastro-intestinal and intracranial bleeding.9 10 11
This is the first reported case of acquired
factor V inhibition in a patient put on VV-ECMO
for Legionella pneumonia. Although our patient had
acute renal failure and ECMO was instituted late
in his course of illness (13 days after intubation),
he responded favourably. The cause of the acquired
factor V inhibition was uncertain. It may be related
to the underlying infection, use of antibiotics, or
be idiopathic in nature. The coagulopathy was
not corrected by FFP transfusion and the patient
had symptomatic bleeding with haematuria
and pulmonary haemorrhage despite IVIG
therapy. Although we could wait for the natural
disappearance of the factor V inhibitor, it might
prolong weaning from ECMO and increase the risk
of fatal complications like intracranial bleeding. Yet,
too early prescription of rituximab as in this patient
might mask the effect of IVIG. Lastly, there was a
remote possibility that the observed decrease in
INR and APTT could be due to natural progression
of the underlying disease rather than a treatment
effect as only 15% of patients have spontaneous
resolution of disease and the factor V inhibitors
can persist in the body for months.3 6 7 In one case report, INR remained elevated for 10 days despite
immunosuppressive therapy and returned to
normal over the next 2 weeks.4 The need for ECMO
decannulation and presence of active symptoms
made correction of coagulopathy more imminent.
The use of multimodal therapy including IVIG,
rituximab, and plasma exchange in this patient
successfully halted the progress of the factor V
inhibitor and allowed safe decannulation within a
period of 1 week.
References
1. Huang JN, Koerper MA. Factor V deficiency: a concise
review. Haemophilia 2008;14:1164-9. Crossref
2. Horder MH. Isolated factor V deficiency caused by a specific inhibitor [in German]. Acta Haematol 1955;13:235-41.
3. Franchini M, Lippi G. Acquired factor V inhibitors: a
systematic review. J Thromb Thrombolysis 2011;31:449-57. Crossref
4. Morris CJ, Curry N. Acquired factor V inhibitor in a
critically ill patient. Anaesthesia 2009;64:1014-7. Crossref
5. Crookston K, Rosenbaum L, Gober-Wilcox J. Coagulation.
Acquired bleeding disorders. Factor V inhibitor.
Available from: http://www.pathologyoutlines.com/topic/coagulationfactorVinhibitor.html. Accessed Nov 2013.
6. Lu L, Liu Y, Wei J, Zhang L, Zhang L, Yang R. Acquired
inhibitor of factor V: first report in China and literature
review. Haemophilia 2004;10:661-4. Crossref
7. van Spronsen DJ, Oosting JD, Hoffmann JJ, Breed WP.
Factor V inhibitor associated with cold agglutinin disease.
Ann Hematol 1998;76:49-50. Crossref
8. Streiff MB, Ness PM. Acquired FV inhibitors: a needless
iatrogenic complication of bovine thrombin exposure.
Transfusion 2002;42:18-26. Crossref
9. Chan KK, Lee KL, Lam PK, Law KI, Joynt GM, Yan WW.
Hong Kong’s experience on the use of extracorporeal
membrane oxygenation for the treatment of influenza A
(H1N1). Hong Kong Med J 2010;16:447-54.
10. Bryner B, Miskulin J, Smith C, et al. Extracorporeal life
support for acute respiratory distress syndrome due to
severe Legionella pneumonia. Perfusion 2014;29:39-43. Crossref
11. Noah MA, Ramachandra G, Hickey MM, et al.
Extracorporeal membrane oxygenation and severe acute
respiratory distress secondary to Legionella: 10 year
experience. ASAIO J 2013;59:328-30.
12. Harris DJ, Duke GJ, McMillan J. Extracorporeal membrane
oxygenation for Legionnaires disease: a case report. Crit Care Resusc 2002;4:28-30.