DOI: 10.12809/hkmj133996
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Spontaneous intracranial hypotension: improving recognition and treatment strategies in the local setting
Gregory KY Lee, MB, BS1; Jill M Abrigo, MD1; Tom CY Cheung, FRCR, FHKAM (Radiology)1; Deyond YW Siu, FRCR, FHKAM (Radiology)1; Danny TM Chan, FRCS, FHKAM (Surgery)2
1 Department of Imaging and Interventional Radiology, The Chinese University of Hong Kong, Prince of Wales Hospital, Shatin, Hong Kong
2 Department of Neurosurgery, The Chinese University of Hong Kong, Prince of Wales Hospital, Shatin, Hong Kong
Corresponding author: Dr Gregory KY Lee (greglee011@gmail.com)
Abstract
We report a case of spontaneous intracranial
hypotension with classic symptoms of orthostatic
headache and acute presentation of subdural
haematoma on computed tomographic scan.
Conventional approach with conservative treatment
was initially adopted. The patient’s condition,
however, deteriorated after 2 weeks, requiring
surgical evacuation of the intracranial haemorrhage.
We reviewed the clinical features of this disease and
the correlated magnetic resonance imaging findings
with the pathophysiological mechanisms, and
described treatment strategies in the local setting.
Subtle findings on initial computed tomographic
scan are also reported which might improve
pathology recognition. Spontaneous intracranial
hypotension is not uncommonly encountered in
Hong Kong, and physicians must adopt a high level
of clinical suspicion to facilitate early diagnosis
and appropriate management. In addition, novel
therapeutic approaches may be required in those
with recurrent symptoms or who are refractory to
current treatment strategies.
Case report
A 54-year-old man with no history of trauma
was admitted to the Prince of Wales Hospital for
headache of progressive severity accompanied by
dizziness in August 2012. He had consulted the
emergency room 4 weeks earlier for neck pain, and
had an unremarkable computed tomographic (CT)
scan of the brain (CTB). Further enquiry revealed an
orthostatic component within the headache (worse
in upright position and relieved within minutes of
assuming supine posture), while admission CTB
revealed interval development of bilateral 5 mm–thick frontoparietal subacute subdural haematomas
(SDHs) with disproportionate tightness of the basal
cisterns.
Cerebral magnetic resonance imaging (MRI)
additionally demonstrated compression of the
midbrain, but no caudal herniation of the cerebellar
tonsils beyond the foramen magnum. Contrast
study showed diffuse pachymeningeal enhancement,
venous sinus distension, and prominent pituitary
gland. Spinal MRI was unremarkable and MRI
cisternography/myelography was negative for
cerebrospinal fluid (CSF) leakage.
The patient was advised complete bed rest
with adequate hydration. His neurological status was
intact all along. However, he reported persistent,
severe bifrontal headache which, after 2 weeks, was
accompanied with repeated projectile vomiting.
Computed tomographic scan of the brain at this
juncture revealed enlargement of the SDH with
development of acute haemorrhage. Emergency
evacuation of subdural blood was performed with
development of low intracranial pressure during the
evacuation process.
The patient’s symptoms improved markedly
thereafter, and CTB reassessment showed minor
residual blood. The patient was discharged in a
neurosurgically stable condition, and currently
remains asymptomatic.
Discussion
Intracranial hypotension is traditionally attributed
to leakage of CSF from a dural defect along the
craniospinal axis, which can occur spontaneously,
such as due to rupture of Tarlov cyst1 or dural
weakness in connective tissue disorder.2 Intracranial
hypotension can also be precipitated by direct trauma
or iatrogenic causes such as a lumbar puncture. The
commonest cause, however, is a spontaneous defect
of the dura (spontaneous intracranial hypotension
[SIH]), though a trivial traumatic event can be
elicited retrospectively in around one third of
such patients.3 4 The most common sites of leakage
identified were at the cervicothoracic junction and
thoracic region of the spinal canal.1 5 In the absence
of a dural defect, a recent alternative hypothesis
proposes increased CSF absorption from negative
pressure gradient in the inferior vena cava.6 In both
instances, CSF hypovolaemia is the main feature and
primary cause of the related clinical and imaging
findings.
Spontaneous intracranial hypotension is an
increasingly diagnosed cause of headache, with an
incidence of one in 50 000 individuals.7 The female-to-male incidence ratio of SIH is 2:1, with a peak
incidence occurring around the age of 40 years.3 7
The typical clinical feature of SIH is orthostatic
headache, which, according to the International
Headache Society, should occur or worsen within
15 minutes in an upright posture together with
at least one feature of meningeal irritation (neck
stiffness, tinnitus, hypacusia, photophobia, nausea)
in addition to imaging features of SIH.8 With
chronicity, the postural component may become
less prominent.3 Other clinical manifestations
include disappearance of or improvement in the
headache within 30 minutes after lying supine, and
cranial nerve palsies related to traction from caudal
displacement of the brainstem.9 Severe midbrain
compression may result in nigral dopaminergic
dysfunction and manifest as parkinsonism.9 Coma
occurs from delayed decompression of brainstem
descent.4
Subdural haematoma is a late finding and
occurs in around 10% of patients with SIH,3
commonly seen in males and those older than 35
years.10
Computed tomography is the frontline
imaging workup for headache. Typically, SIH is
not considered unless patients present with non-traumatic
SDH in the setting of a normal clotting
profile. The proposed mechanism involves rupture
of bridging veins in expanding subdural hygromas
which, in turn, results from brain sagging due to CSF
hypovolaemia.
On CT, SIH in the absence of SDH can be easily
interpreted as being unremarkable. With high level of
clinical suspicion, however, subtle imaging features
may suggest the diagnosis. For instance, the initial
CTB of our index patient showed paucity of CSF for
his age (Fig 1). On follow-up CTB, the tightness of
the basal cisterns appeared rather disproportionate
to the small amount of SDH. Thus, it may be possible
to detect CSF hypovolaemia on CT if these subtle
findings are sought and the diagnosis is borne in
mind.
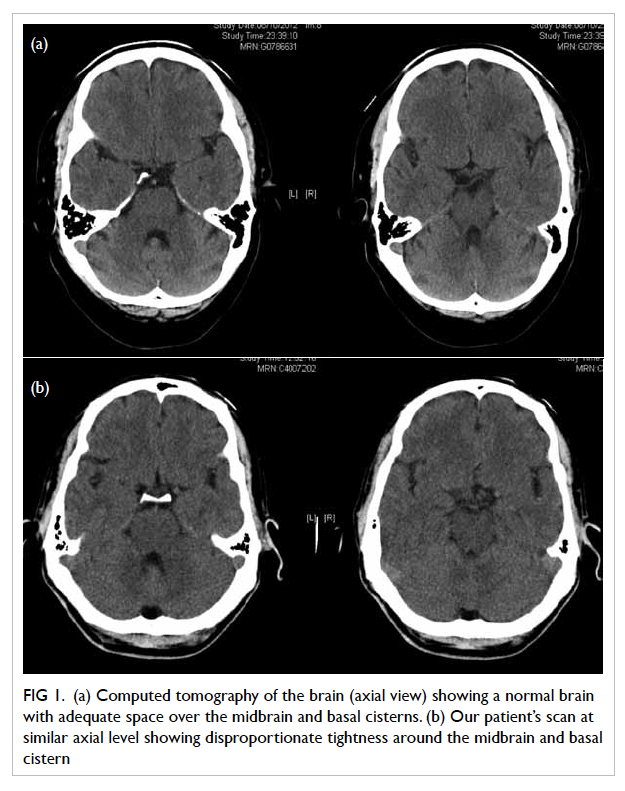
Figure 1. (a) Computed tomography of the brain (axial view) showing a normal brain with adequate space over the midbrain and basal cisterns. (b) Our patient’s scan at similar axial level showing disproportionate tightness around the midbrain and basal cistern
The MRI findings of SIH are well-described
in the literature. This preferred modality of imaging
depicts characteristic features which may obviate the
need for lumbar tap.4 Additionally, the cause or site
of dural defect can be investigated.
The MRI appearances are mainly attributed to
CSF hypovolaemia, or represent secondary reactive
changes following the Monro-Kellie doctrine.
Briefly, decrease in CSF volume prompts an increase
in dural blood flow and causes venous engorgement.
The latter, when prolonged, incites surrounding
fibro-proliferation that in turn accounts for diffuse
dural thickening and intense enhancement with
gadolinium on MRI. Such explanation is confirmed
by meningeal biopsy showing proliferation of
fibroblasts without inflammation.11 12
The primary feature of brain sagging is a very
specific MRI finding in SIH. It is a collaboration
of features, including decreased dimension of
the suprasellar cistern, bowing of optic chiasma,
flattening of the pons against the clivus, effacement
of the perimesencephalic cistern and hindbrain
herniation (Fig 2a).11 12 A quantitative measurement
of brain sagging has been described10 although the
degree of descent may be underestimated since
patients are scanned in the recumbent position.
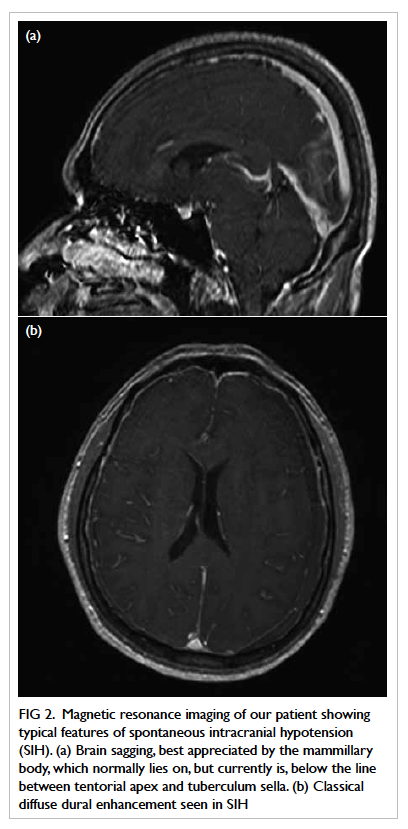
Figure 2. Magnetic resonance imaging of our patient showing typical features of spontaneous intracranial hypotension (SIH). (a) Brain sagging, best appreciated by the mammillary body, which normally lies on, but currently is, below the line between tentorial apex and tuberculum sella. (b) Classical diffuse dural enhancement seen in SIH
Secondary and less-specific signs of SIH
are more readily appreciated but represent a later
stage of the disease. Findings of MRI in the brain
classically show diffuse pachymeningeal thickening
which has a sensitivity of up to 94% (Fig 2b).9 The
venous distention sign may also be seen and is best
appreciated in the mid-portion of the dominant
transverse sinus12; on sagittal sections, the sinus,
which normally adopts a concave or straight inferior
border, bulges with a convex contour. Venous
engorgement at the dura mater across the sella
turcica could produce reactive hyperaemia and
possible increase in size of the pituitary gland. In the
spine, MRI findings mirror those of the brain with
diffuse dural enhancement, engorgement of venous
plexus and extrathecal CSF collection.3
Magnetic resonance imaging cisternography
or myelography can be easily added to routine
MRI examination. Using a heavy T2-weighted
sequence with fat signal suppression, spinal fluid
outside the craniospinal axis may be detected with
equal or improved accuracy than CT myelography.5
Radiological improvement lags behind clinical
recovery. Meningeal enhancement resolves
considerably earlier than brain sagging.12 13
Computed tomography myelography and
radionuclide cisternography are available locally
but seldom performed. These are more invasive
and time-consuming to perform, and entail
intrathecal injection of contrast/radioisotope label,
with fluoroscopic screening or serial imaging with
gamma cameras to visualise extrathecal contrast/tracer activity.
Conservative management of SIH includes
Trendelenburg positioning, aggressive hydration,
caffeine intake and abdominal binder, and has
been reported to be successful in most cases. In
those patients with increased persistent headache
or neurological deterioration, CTB should be
immediately performed to rule out expanding SDH.
The timing of active surgical drainage is
controversial. In a particular series, surgical drainage
was advised in those with focal neurological deficits,
decreased level of consciousness, or subdural
collection of >1 cm.4 Most surgically managed patients
show transient improvement but have high likelihood
of re-accumulation of subdural fluid.13
A more active strategy that is gaining
international acceptance is epidural blood patch
(EBP), which aims at sealing off the spinal leak3
but appears to be useful in those without a definite
dural defect.6 The treatment involves placing 10 to
20 mL of autologous blood in the epidural space
at the thoracolumbar level. As the patient is put in
Trendelenburg position, the blood patch distributes
along the epidural space and clots at the site of
leakage.
The overall success rate for headache
improvement is 30% to 70% after the first EBP and
30% to 50% for the remainder with repeated EBP.10
Epidural blood patch has a success rate of 85% in
reverting patients who were comatose due to SIH.13
Under fluoroscopic guidance, EBP may yield a high
rate of pain relief and is preferred for those with
altered anatomy or failure in the initial attempt.14
A novel technique of multisite EBP via continuous
infusion has been described.15
The newest treatment approach developed at
the Stanford University recommends emergency
subdural clot evacuation in the absence of
improvement in Trendelenburg positioning,
presence of dilated pupils, and large SDH with mass
effect. Otherwise, EBP is the treatment of choice.13
In those with initial improvement with EBP,
studies have shown concomitant spontaneous
resolution of significant SDH.13 Epidural blood patch
may even be performed after surgical evacuation, and
has been proven to further reduce the recurrence of
headache and SDH.13 16 As the brain has a tendency to
sag downwards in SIH, pneumocephalus during clot
evacuation may cause further downward herniation
of the brain. Hence, surgical evacuation after EBP
may not be advisable. Most of these patients, like
our index case, have shown low-pressure subdural
collection during craniotomy.
Conclusion
The diagnosis of SIH should be considered for
any patient presenting with headache and neck
pain. A high level of clinical suspicion could assist
identification of subtle signs on initial CT which
could facilitate early recognition and prompt
treatment and, consequently, improve outcomes.
However, MRI remains an important, highly
sensitive, and specific method for depicting imaging
markers that allow confident clinical diagnosis.
Further, MRI cisternography/myelography for CSF
leak localisation can be added with ease. Currently,
the medical practice in Hong Kong for SIH
predominantly comprises conservative treatment
and symptomatic clot evacuation. More novel
interventions that have been successfully employed
overseas may need to be reconsidered to improve
current therapeutic strategies.
References
1. Cheng MF, Pan MH, Wu YE, Tsai WC, Yen RF, Tzen KY.
Radionuclide cisternography in diagnosing spontaneous
intracranial hypotension. Ann Nucl Med Sci 2004;17:167-72.
2. Schievink WI, Gordon OK, Tourje J. Connective tissue
disorders with spontaneous spinal cerebrospinal fluid
leaks and intracranial hypotension: a prospective study.
Neurosurgery 2004;54:65-70; discussion 70-1. CrossRef
3. Schievink WI. Spontaneous spinal cerebrospinal fluid
leaks: a review. Neurosurg Focus 2000;9:e8. CrossRef
4. Chen HH, Huang CI, Hseu SS, Lirng JF. Bilateral
subdural hematomas caused by spontaneous intracranial
hypotension. J Chin Med Assoc 2008;71:147-51. CrossRef
5. Wang YF, Lirng JF, Fuh JL, Hseu SS, Wang SJ. Heavily
T2-weighted MR myelography vs CT myelography
in spontaneous intracranial hypotension. Neurology
2009;73:1892-8. CrossRef
6. Franzini A, Messina G, Nazzi V, et al. Spontaneous
intracranial hypotension syndrome: a novel speculative
physiopathological hypothesis and a novel patch method
in a series of 28 consecutive patients. J Neurosurg
2010;112:300-6. CrossRef
7. Diaz JH. Epidemiology and outcome of postural headache
management in spontaneous intracranial hypotension. Reg
Anesth Pain Med 2001;26:582-7. CrossRef
8. International Headache Society, Headache Classification
Subcommittee. The International classification of headache
disorders, 2nd ed. Cephalalgia 2004; ICHD–II code: 7.2.3.
9. Pakiam AS, Lee C, Lang AE. Intracranial hypotension with
parkinsonism, ataxia, and bulbar weakness. Arch Neurol
1999;56:869-72. CrossRef
10. Rahman M, Bidari SS, Quisling RG, Friedman WA.
Spontaneous intracranial hypotension: dilemmas in
diagnosis. Neurosurgery 2011;69:4-14. CrossRef
11. Metafratzi Z, Argyropoulou MI, Mokou-Kanta C,
Konitsiotis S, Zikou A, Efremidis SC. Spontaneous
intracranial hypotension: morphological findings and CSF
flow dynamics studied by MRI. Eur Radiol 2004;14:1013-6. CrossRef
12. Farb RI, Forghani R, Lee SK, Mikulis DJ, Agid R. The
venous distension sign: a diagnostic sign of intracranial
hypotension at MR imaging of the brain. AJNR Am J
Neuroradiol 2007;28:1489-93. CrossRef
13. Loya JJ, Mindea SA, Yu H, Venkatasubramanian C,
Chang SD, Burns TC. Intracranial hypotension producing
reversible coma: a systematic review, including three new
cases. J Neurosurg 2012;117:615-28. CrossRef
14. Watanabe K, Hashizume K, Kawaguchi M, Fujiwara A,
Sasaoka N, Furuya H. Fluoroscopically guided epidural
blood patch with subsequent spinal CT scans in the
treatment of spontaneous cerebrospinal fluid hypovolemia.
J Neurosurg 2011;114:1731-5. CrossRef
15. Ohtonari T, Ota S, Nishihara N, et al. A novel technique of
multiple-site epidural blood patch administration for the
treatment of cerebrospinal fluid hypovolemia. J Neurosurg
2012;116:1049-53. CrossRef
16. Mendes FF, Gonçalces AN, Novelo B, Mariano da
Rocha CR, Marques NF. Spontaneous intracranial
hypotension treated with epidural blood patch. Revista Dor
2012;13:1806-13.

