DOI: 10.12809/hkmj134084
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PICTORIAL MEDICINE
Trachyonychia in a patient with chronic myeloid leukaemia after imatinib mesylate
YM Lau, FHKCP, FHKAM (Medicine); YK Lam, FHKCP, FHKAM (Medicine); KH Leung, MRCP;
SY Lin, FHKCP, FHKAM (Medicine)
Department of Medicine and Geriatrics, United Christian Hospital, Kwun Tong, Hong Kong
Corresponding author: Dr YM Lau (lym570@ha.org.hk)
An 86-year-old man presented with leukocytosis
in December 2009. Bone marrow biopsy showed
chronic myeloid leukaemia in chronic phase and
cytogenetic studies showed t(9;22)(q34;q11.2)
translocation. He was initially put on imatinib 300 mg
daily; subsequently, this was increased to 400 mg
daily. He developed pruritic skin rash within 3
months of initiating imatinib. Initially, the skin
condition improved with topical steroid. However,
there was progressive development of white streaks
and scaling of skin over the face, scalp, trunk, limbs,
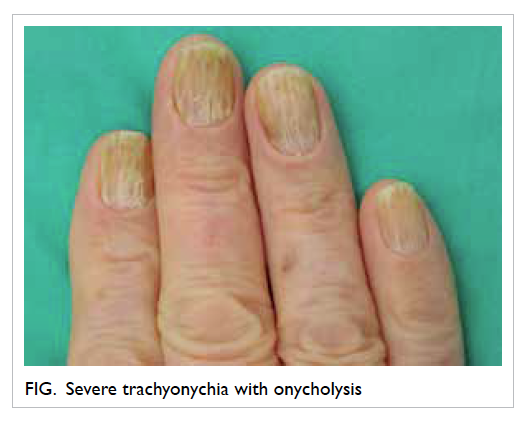
and trachyonychia with onycholysis of fingers and
toes. There were no mucosal lesions. Skin biopsy
findings were consistent with lichenoid drug reaction.
Imatinib was stopped and changed to nilotinib. The
skin and nail conditions progressively improved
while the patient was on nilotinib.
Imatinib mesylate has been the standard treatment for chronic myeloid leukaemia for 10 years.1
Imatinib mesylate inhibits tyrosine kinases of bcr/abl, c-kit, and platelet-derived growth factor receptors,
and cutaneous reactions are the commonest side-effects in patients receiving this drug.2 3 Trachyonychia results from disruption of the nail matrix cells,
and can be induced by chemotherapeutic agents.4 Although paronychial inflammation is commonly induced by kinase inhibitors, trachyonychia is rarely reported. Cross-reactivity between different tyrosine kinases has rarely been reported.5 The absence of cross-reactivity between imatinib and nilotinib in this patient suggests that the mechanism of drug reaction is not related to the inhibition of tyrosine kinase.
References
1. Peggs K, Mackinnon S. Imatinib mesylate—the new gold
standard for treatment of chronic myeloid leukemia. N
Engl J Med 2003;348:1048-50. CrossRef
2. Gardembas M, Rousselot P, Tulliez M, et al. Results of a
prospective phase 2 study combining imatinib mesylate
and cytarabine for the treatment of Philadelphia-positive
patients with chronic myelogenous leukemia in chronic
phase. Blood 2003;102:4298-305. CrossRef
3. Wahiduzzaman M, Pubalan M. Oral and cutaneous
lichenoid reaction with nail changes secondary to
imatinib: report of a case and literature review. Dermatol
Online J 2008;14:14.
4. Chen W, Yu YS, Liu YH, Sheen JM, Hsiao CC. Nail
changes associated with chemotherapy in children. J Eur
Acad Dermatol Venereol 2007;21:186-90. CrossRef
5. Novitzky-Basso I, Craddock C. Cross-intolerance to
imatinib, dasatinib and nilotinib therapy in a patient with
chronic myeloid leukaemia. Eur J Haematol 2011;86:548-9. CrossRef