Hong Kong Med J 2014 Oct;20(5):401–6 | Epub 20 Jun 2014
DOI: 10.12809/hkmj134140
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Initial experience with the Oncotype DX assay
in decision-making for adjuvant therapy of early
oestrogen receptor–positive breast cancer in Hong Kong
Polly SY Cheung, MB, BS, FHKAM (Surgery)1;
Adam C Tong, MB, BS, FHKAM (Radiology)2;
Roland CY Leung, MRes, BEng3;
WH Kwan, MB, BS4;
Thomas CC Yau, MD, MB, BS3
1 Breast Care Centre, Hong
Kong Sanatorium and Hospital, Happy Valley, Hong Kong
2 St George’s University of
London, St George’s Healthcare Trust,
Cranmer Terrace, London SW17 0RE, United Kingdom
3 Division of Medical
Oncology, Department of Medicine, The University of
Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong
4 Comprehensive Oncology
Centre, Hong Kong Sanatorium and Hospital, Happy Valley, Hong Kong
Corresponding author: Dr Polly SY Cheung
(pollyc@pca.hk)
Abstract
Objective: To examine
the impact of the 21-gene
Oncotype DX Breast Cancer Assay on the adjuvant
treatment decision-making process for early-stage
breast cancer in Hong Kong.
Design: Retrospective
study.
Setting: Private
hospital, Hong Kong.
Patients: Study included
cases of early-stage breast
cancer (T1-2N0-1M0, oestrogen receptor–positive,
human epidermal growth factor receptor 2–negative) that were presented at a multidisciplinary
breast meeting at a single site. Cases were selected for
Oncotype DX testing with the assistance of Adjuvant!
Online. The recommendations for adjuvant therapy
before and after obtaining the Oncotype DX
Recurrence Score results were analysed.
Results: A total of 154
cases that met the inclusion
criteria were discussed at our multidisciplinary
breast meeting. Of these, 64 cases with no clear
recommendation by the Meeting Panel were selected
for this study and reviewed. The distribution of
Recurrence Score results was similar to that reported
by others, with a somewhat higher proportion of low
Recurrence Scores. Treatment recommendation was
changed for 20 (31%) patients after the Oncotype
DX result was received. Of the changes in treatment
decisions, 16 (80%) were changes to lower-intensity
regimens (either equipoise or hormonal therapy). The number of
cases receiving an equipoise
recommendation decreased by nine (82%), based on
the additional information provided by the Oncotype
DX test.
Conclusion: The Oncotype
DX Recurrence Score
information impacts the decision-making process
for adjuvant therapy for early-stage breast cancer
in the multidisciplinary care setting in Hong Kong.
A larger-scale study is required to gain more
experience, evaluate its impact more thoroughly,
and assess its cost-effectiveness.
New knowledge added by this
study
- Application of the Oncotype DX Breast Cancer Assay reduces adjuvant chemotherapy recommendations for early-stage breast cancer in a multidisciplinary clinic environment in the Chinese population.
- Application of the Oncotype DX Breast Cancer Assay to early-stage breast cancer cases reduces the proportion of equipoise chemotherapy recommendations.
- The Oncotype DX Breast Cancer Assay can assist in making definitive treatment recommendations.
Introduction
Breast cancer is the most common cancer
among
women in Hong Kong, with an incidence of 54.8
per 100 000 population in 2010.1
Over the past two
decades, breast cancer incidence in Hong Kong
has been trending upward, from a lifetime risk of
1 in 27 women in 2000 to a lifetime risk of 1 in 19 women in 2010.
As a result, Hong Kong now has
an intermediate-to-high breast cancer incidence
compared with other Asian countries. The median
age of diagnosis of breast cancer is 53 years. Early-stage
breast cancer (ESBC; defined as stages 0 to II)
is most common at diagnosis, accounting for 81.3%
of cases.2 The most common
stage at diagnosis is stage II (39.7%).2
Among those diagnosed with ESBC in Hong
Kong, 98% undergo surgery, 62% receive adjuvant
chemotherapy, and 66% receive hormonal therapy
(HT). Adjuvant therapy has been shown to increase
survival,3 which includes
HT, chemotherapy, or
both. The decision to administer adjuvant therapy
depends on clinical, pathological, and histochemical
features of the tumour, which influence the risk
of recurrence.4 5 At our institution, it has been the
practice since 2003 to discuss all breast cancer cases
at the multidisciplinary breast meeting (MDM) prior
to making adjuvant treatment recommendations. In
this model, cases are submitted for weekly review
by a group of health care professionals including
surgeons, oncologists, pathologists, and experts from
other disciplines who can add value to optimising
the treatment plan for each patient.
The Oncotype DX Breast Cancer Assay
(Oncotype DX; Genomic Health, Inc, Redwood City
[CA], US) has been validated to measure the risk
of recurrence in patients with oestrogen receptor–positive (ER+), human epidermal growth factor
receptor 2–negative (HER2-), and lymph node
negative tumours. The Oncotype DX test analysed
21 genes and generated a Recurrence Score which
is used to quantify the likelihood of distant disease recurrence
at 10 years post-treatment. For prognostic
use, the Recurrence Score value is categorised into
low- (<18), intermediate- (18-30), and high-risk (>30)
groups. Typically, patients receiving a low Recurrence
Score result will receive HT in the absence of other
factors that increase the risk of recurrence. Patients
receiving high scores have a higher risk of recurrence
and are more likely to respond to chemotherapy;
therefore, these patients often receive a combination
of chemotherapy followed by HT. The appropriate
therapy for patients with an intermediate score
is the subject of ongoing clinical trials. Several
prospective studies have validated its prognostic and
predictive significance using data from the NSABP-B14,
NSABP-20, and SWOG 8814 trials.6
7 Oncotype
DX has now been incorporated into the National
Comprehensive Cancer Network and the St Gallen
guidelines for use.4 5
Significant toxicity and cost can accrue to
patients undergoing adjuvant chemotherapy, but
only a small proportion experience survival benefits.
The Recurrence Score result can be used to assess the
10-year risk of recurrence and the potential benefit
from adjuvant chemotherapy and, thereby, assist in
development of a treatment plan that makes optimal
use of resources for the patient’s benefit.
The aim of this study was to examine the
impact of the additional information provided by
the Oncotype DX test on the clinical treatment
decisions for patients diagnosed with ESBC. The
study compared treatment regimens proposed by
a multidisciplinary breast cancer team before and
after receipt of the Oncotype DX results.
Methods
Study design
This single-centre study was conducted at
the Hong
Kong Sanatorium and Hospital, a private institution
in Hong Kong. This study was a retrospective
review of patients with breast cancer who had
surgery between 2008 and 2011, whose cases had
been reviewed by the MDM, and who had received
Oncotype DX assay testing to obtain additional
information on recurrence risk. Recurrence risk
was assessed by the MDM using clinical factors
(including age, tumour size, number of positive
lymph nodes, and grade) and Adjuvant! Online,8 and
a provisional treatment recommendation was made.
The Oncotype DX test was ordered after the MDM to
obtain additional recurrence risk information when
there was a difference of opinion on interpretation
of available information. The test was not ordered
when a consensus of opinion on treatment
recommendation was reached. Cost of testing was
borne by insurance or the patient. For each case,
the MDM made final treatment recommendations
after consideration of the Recurrence Score results; the actual
treatment received took into account
patient preference, and might have differed from
that recommended by the MDM. Eligible patients
had ESBC (T1-2N0-1M0 tumours) that was
determined to be ER+, HER2-, and with at most one
positive lymph node. In addition, the patient profile
was consistent with that prescribed by international
guidelines for application of this assessment.4 5
The Recurrence Score result was discussed for all
patients and a recommendation was made by simple
majority of opinion. Therapy recommendations
before Recurrence Score result were categorised
as chemohormonal therapy (CHT), equipoise where
a clear recommendation for either CHT or HT was
not possible, or HT. Changes in intensity of therapy
were categorised as increased intensity from HT to
equipoise or CHT and equipoise to CHT; changes were
categorised as decreased intensity for changes from
CHT to equipoise or HT and equipoise to HT.
Statistical analysis
Data were summarised by using descriptive
statistics.
For cases considered in this study, the distribution
of parameter values in the sample was described
by calculating the mean, median, and range where
appropriate.
Results
During the study period from 1 August 2008
to
30 June 2011, a total of 620 breast cancer patients
with T1-2N0-1M0 tumours underwent surgery.
Among them, 154 were ER+ HER2- cases. A total
of 66 cases for which there was no unanimity in
the MDM were reviewed, of which 64 fulfilled the
inclusion criteria for this study; two cases were
excluded because HER2 status was determined to
be overexpressed by immunohistochemistry. The
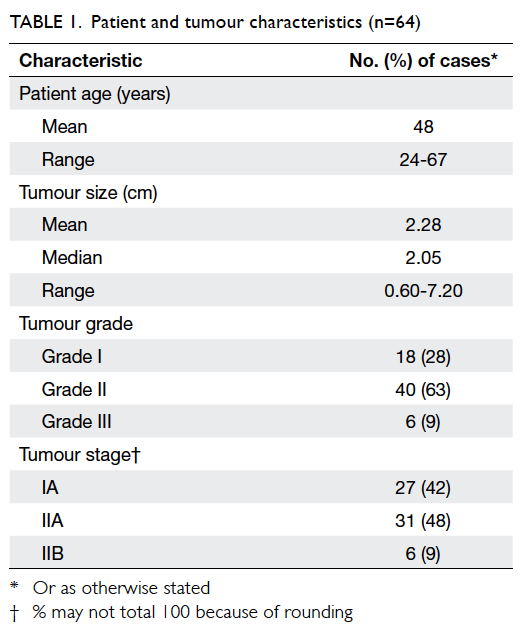
tumours were predominantly grade II (63%) and
similar proportions were stage IA (42%) and stage
IIA (48%), with small number of stage IIB cases (9%)
[Table 1]. Nine patients with positive lymph
nodes
(N1 or N1a) were included in the study based on
clinical and pathological assessments suggesting
less-aggressive disease.
The Recurrence Score values were
categorised
as low- (<18), intermediate- (18-30), and high-risk
(>30) according to the Oncotype DX assay
recommendation which gave an estimated distant
recurrence rate after the use of HT alone. The
panel discussed the possible benefit of adding
chemotherapy to the treatment regimen for each
patient to reach a consensus recommendation
specific for the patient. In this study, the majority
of patients had a low-risk Recurrence Score (64%)
whilst patients with intermediate- and high-risk
Recurrence Score values were less frequent (30% and
6%, respectively). The distribution of Recurrence
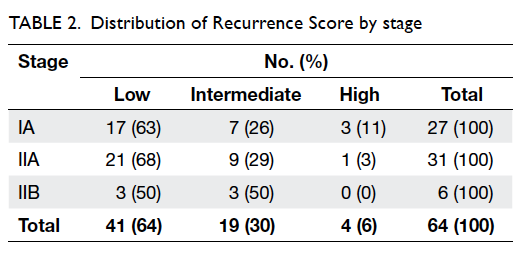
Score results by tumour stage is shown in Table 2. In this cohort, the distribution of
Recurrence Score
results by stage was similar to the overall distribution
of the Recurrence Score results. Stage IA tumours
were comprised of 63% low and 26% intermediate
Recurrence Score results, while stage IIA tumours
were comprised of 68% low and 29% intermediate
Recurrence Score results. Stage IIB tumours were
evenly split between low and intermediate scores.
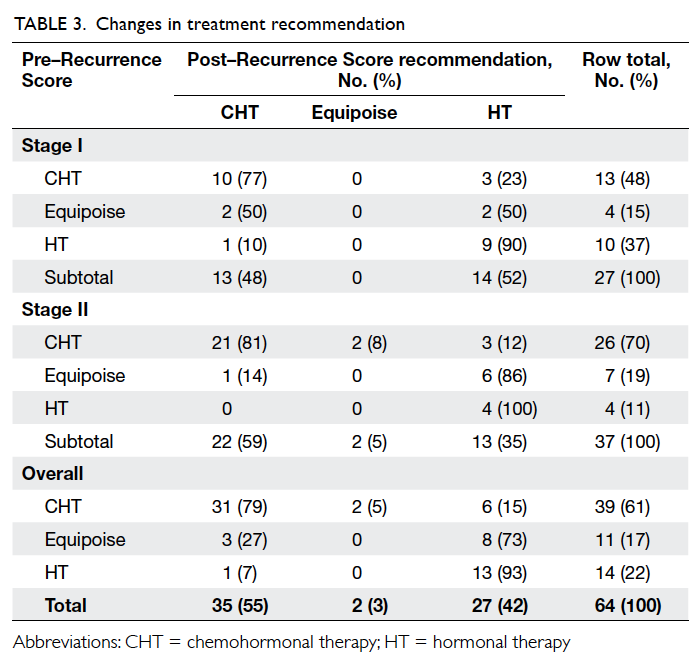
The specific changes in treatment
recommendations for all patients in the study
are shown in Table 3. Overall, the treatment
recommendations for 20 (31%) of the 64 patients
changed intensity when the Recurrence Score result
was considered. The changes in treatment decisions
were predominantly to HT (14/20; 70% of changed
treatment recommendations) for the entire cohort.
Other changes included two recommendations (10%
of changed recommendations) that were changed
from CHT to equipoise and four (20%) which resulted
in a higher-intensity CHT recommendation over HT
or equipoise. Interestingly, five of six stage IIB cases received
CHT recommendations both pre– and post–Recurrence Score result. In the sixth stage IIB case,
a patient with lobular carcinoma staged as T3N0M0
with a Recurrence Score result of 8, the treatment
recommendation was changed from CHT to HT upon
receipt of the score.
The distribution of treatment
recommendations
by stage before and after Oncotype DX
testing is shown in Table 3. For stage I patients,
recommendations were changed in eight (30%) of 27
patients, while for stage II patients recommendations
were changed in 12 (32%) of 37 patients. As for the
entire cohort, the changes in treatment decisions
were predominantly to HT for both stage I (5/8,
63%) and stage II (9/12, 75%) patients. In the stage I
tumours, all four equipoise recommendations (15%
of recommendations prior to Oncotype DX testing)
were changed after testing. The proportion of CHT
recommendations remained the same at 13 (48%)
and HT recommendations increased from 10 (37%)
to 14 (52%) after receipt of the Recurrence Score
result. In stage II tumours, the proportion receiving
recommendations for equipoise decreased from 7
(19%) to 2 (5%). The CHT recommendations decreased
somewhat from 26 (70%) to 22 (59%), while the
proportion receiving a HT recommendation
increased from 4 (11%) to 13 (35%).
The distribution of Recurrence Score
categories
by therapy recommendation before and after receipt
of Oncotype DX results is shown in the Figure. The
number of low Recurrence Score cases in the CHT
group decreased from 20 before Recurrence Score
information to 13 after the Recurrence Score result
was obtained, while the number of low Recurrence
Score cases in the group that did not require
chemotherapy increased from 21 to 28 once the
Recurrence Score information was available. While
two patients in the high Recurrence Score group did
not receive a recommendation for CHT pre–Oncotype
DX, all the cases with high Recurrence Scores
received a recommendation for CHT post–Oncotype
DX.
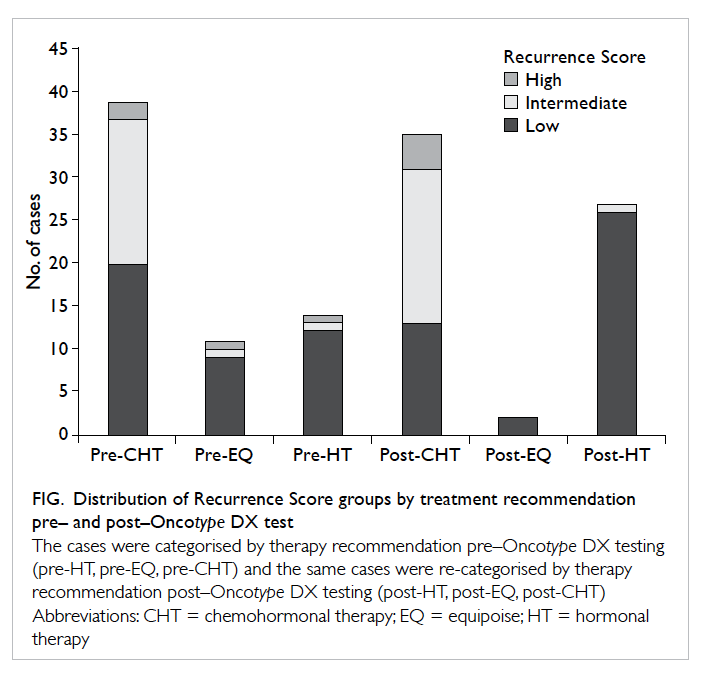
Figure. Distribution of Recurrence Score groups by treatment recommendation pre– and post–Oncotype DX test
Discussion
This first analysis of the impact of the
Oncotype DX
Breast Cancer Assay on adjuvant treatment for early-breast
cancer in Hong Kong revealed similarities
with studies in other populations worldwide with
regard to the distribution of Recurrence Score
results, proportion of treatment recommendations
that changed upon consideration of Oncotype
DX information, and shift in proportions of
chemotherapy recommendations compared with
other treatment recommendations.9
10 11 12 13
The Recurrence Score distribution observed
in
this retrospectively selected cohort of breast cancer
patients is similar to that observed in other studies
of ESBC, with predominance of lower Recurrence
Score values. These results are also comparable
to the Asia-Pacific region’s Recurrence Score
distribution reported by Genomic Health: low
risk=51%, intermediate risk=33%, and high risk=16%.14
The distribution observed in this study differed
from other studies9 12 13
in that the proportion of low Recurrence Score results was higher
and the
proportion of high Recurrence Score results was
lower than previously observed. Since these cases
were estimated to derive borderline benefit based
on their initial assessment using Adjuvant! Online
and clinical parameters, it might be expected that
Recurrence Score distribution in this cohort would
be skewed at the lower end as well. When examined
by stage, the Recurrence Score distribution did not
change substantially. In fact, all six stage IIB tumours
had low or intermediate Recurrence Scores, including
the single T3 tumour in this study. This observation
is consistent with that in other studies9
15 16 showing that
the Recurrence Score assay provides information
not inherent to traditional clinicopathological
assessments of the tumour.
Inclusion of Oncotype DX information
led to
a change in 20 (31%) of 64 treatment plans. These
results correspond with similar decision impact
studies from the US,12 13 17 European Union,9
10
and the Middle East11 that
assessed the impact of
Recurrence Score information on choice of adjuvant
therapy in ESBC. The proportion of treatment plans
that changed in these studies ranged from 25% to
40%, so the 30% observed in this study is typical.
The proportion of changes to CHT or in the
other direction to HT as a result of Oncotype
DX testing in breast cancer is also similar to
that in other studies, with the proportion of CHT
recommendations decreasing and the proportion
of HT recommendations increasing.9
10 11 12 13 Changes
were largely to lower-intensity treatments, with 80%
of the 20 changed recommendations shifting from
CHT or equipoise to a lower-intensity regimen. Most
of these transitions to lower-intensity treatment
recommendations resulted from movement of
the equipoise cases to HT (40% of changes). An
additional 30% of the changes were shifts from CHT to
HT recommendations. This effect was seen with the
only T3 tumour in the study, classified as T3N0M0, a
case which transitioned from a CHT recommendation
to HT after receiving a low-risk Recurrence Score
of 8. Recurrence Score information resulted in
increases in treatment intensity as well. The two
cases with a high score that were not originally given
a CHT recommendation were switched to CHT after
consideration of the Recurrence Score.
Adjuvant treatment for ESBC is an
important,
yet complex area faced by oncologists. To patients,
this is a life-changing decision, the outcome of
which will drastically impact their lives. The
decision whether to give chemotherapy as part
of adjuvant therapy to cancer patients can be
difficult with traditional prognostic indicators
as, often, they have been insufficient to identify
patients who will benefit from those who may not
benefit. A number of prognostic tools have been
developed, including Oncotype DX and Adjuvant! Online that
can aid the multidisciplinary team
in making decisions on adjuvant treatment. The
additional information provided by the Oncotype
DX Recurrence Score result provides the physician
with unique information in the assessment of risk
of recurrence. In this study, cases were selected that
were deemed to have intermediate risk using clinical
factors and Adjuvant! Online, and for which there
was no unanimous agreement. This was exemplified
by inclusion of nine lymph node–positive cases.
Their disease was considered less aggressive based
on assessment of the tumour biology, creating
uncertainty about the necessity of chemotherapy
for these patients. Thus, the Oncotype DX test was
recommended so that the multidisciplinary team
would have additional information on which they
could base their adjuvant treatment decisions.
Given the high proportion of ESBC cases in Hong
Kong, such cases may be frequent and there is an
evident need for Oncotype DX testing to assist in
making treatment recommendations. The additional
information gained can help physicians and patients
avoid expensive and toxic chemotherapy.
Limitations of the study were its
retrospective
nature. In addition, selection of patients was
non-uniform; cases were selected based on their
intermediate-risk assessment in MDMs; and the
selected cases were the ones for which the physicians
had difficulty in making treatment recommendations.
Conclusion
This study demonstrated that the
distribution
of Oncotype DX Recurrence Score results in the
population of women with ESBC in Hong Kong
is similar to that reported in other geographical
regions in the world. The impact of the Recurrence
Score information on adjuvant treatment decisions
in Hong Kong was also similar to that reported by
others, with the main effect being a shift in treatment
recommendations to lower-intensity regimens.
Finally, the proportion of equipoise chemotherapy
recommendations was greatly reduced, suggesting
that the Recurrence Score can assist in making
definitive treatment recommendations in cases
for which physicians are ambivalent about using
chemotherapy.
Acknowledgements
The authors wish to thank The Hong Kong
Sanatorium
and Hospital for supporting and facilitating the
weekly Multidisciplinary Breast Meeting and the
members of the multidisciplinary breast team for
their active participation.
Declaration
No conflicts of interest were declared by
authors.
References
1. Hong Kong Cancer Registry.
Annual Report 2012. Hong Kong: Hong Kong Breast Cancer Foundation;
2012. Available from: http://www.hkbcf.org/breastcancerregistry.
Accessed 8 Apr 2013.
2. Hong Kong Breast Cancer
Registry. Report No. 5 Hong Kong Breast Cancer Foundation; 2013.
HKBCF website: http://www.hkbcf.org/breastcancerregistry. Accessed
8 Apr 2013.
3. Polychemotherapy for early
breast cancer: an overview of the randomised trials. Early Breast
Cancer Trialists' Cooperative Group. Lancet 1998;352:930-42. CrossRef
4. NCCN Clinical Practice
Guidelines in Oncology: Breast Cancer. National Comprehensive
Cancer Network, 2011. Available from:
http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
Accessed 8 Apr 2013.
5. Goldhirsch A, Winer EP, Coates
AS, et al. Personalizing the treatment of women with early breast
cancer: highlights of the St Gallen International Expert Consensus
on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol
2013;24:2206-23. CrossRef
6. Paik S, Shak S, Tang G, et al. A
multigene assay to predict recurrence of tamoxifen-treated,
node-negative breast cancer. N Engl J Med 2004;351:2817-26. CrossRef
7. Paik S, Tang G, Shak S, et al.
Gene expression and benefit of chemotherapy in women with
node-negative, estrogen receptor–positive breast cancer. J Clin
Oncol 2006;24:3726-34. CrossRef
8. Adjuvant! Online 2013 Home page.
Adjuvant website: www.adjuvantonline.com/index.jsp. Accessed 8 Apr 2013.
9. Albanell J, Gonz ález A,
Ruiz-Borrego M, et al. Prospective transGEICAM study of the impact
of the 21-gene Recurrence Score assay and traditional
clinicopathological factors on adjuvant clinical decision making
in women with estrogen receptor–positive (ER+) node-negative
breast cancer. Ann Oncol 2012;23:625-31. CrossRef
10. Geffen DB, Abu-Ghanem S,
Sion-Vardy N, et al. The impact of the 21-gene recurrence score
assay on decision making about adjuvant chemotherapy in
early-stage estrogen-receptor–positive breast cancer in an
oncology practice with a unified treatment policy. Ann Oncol
2011;22:2381-6. CrossRef
11. Klang SH, Hammerman A,
Liebermann N, Efrat N, Doberne J, Hornberger J. Economic
implications of 21-gene breast cancer risk assay from the
perspective of an Israeli-managed health-care organization. Value
Health 2010;13:381-7. CrossRef
12. Lo SS, Mumby PB, Norton J, et
al. Prospective multicenter study of the impact of the 21-gene
recurrence score assay on medical oncologist and patient adjuvant
breast cancer treatment selection. J Clin Oncol 2010;28:1671-6. CrossRef
13. Oratz R, Kim B, Chao C, et al.
Physician survey of the effect of the 21-gene recurrence score
assay results on treatment recommendations for patients with lymph
node–positive, estrogen receptor–positive breast cancer. J Oncol
Pract 2011;7:94-9. CrossRef
14. Chao C. The distribution of
Recurrence Scores(R) from the 21-gene breast cancer assay in the
Asia Pacific Region compared with the United States. Poster
presented at: The Organisation for Oncology and Translational
Research (OOTR) 6th Annual Conference in Kyoto, Japan; 2010 Feb 26.
15. Goldstein LJ, Gray R, Badve S,
et al. Prognostic utility of the 21-gene assay in hormone
receptor–positive operable breast cancer compared with classical
clinicopathologic features. J Clin Oncol 2008;26:4063-71. CrossRef
16. Tang G, Shak S, Paik S, et al.
Comparison of the prognostic and predictive utilities of the
21-gene Recurrence Score assay and Adjuvant! for women with
node-negative, ER-positive breast cancer: results from NSABP B-14
and NSABP B-20. Breast Cancer Res Treat 2011;127:133-42. CrossRef
17. Henry LR, Stojadinovic A,
Swain SM, Prindiville S, Cordes R, Soballe PW. The influence of a
gene expression profile on breast cancer decisions. J Surg Oncol
2009;99:319-23. CrossRef