Hong Kong Med J 2014 Oct;20(5):379–85 | Epub 6 Jun 2014
DOI: 10.12809/hkmj134021
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Obstructive sleep apnoea syndrome in patients
with primary open-angle glaucoma
Ege G Balbay, MD1; Oner Balbay, MD1; Ali N Annakkaya, MD1; Kezban O Suner, MD1; Harun Yuksel, MD2; Murat Tunç, MD2; Peri Arbak, MD1
1 Department of Chest Diseases, Faculty of Medicine, Düzce University, 81620 Düzce, Turkey
2 Department of Ophthalmology, Faculty of Medicine, Düzce University, 81620 Düzce, Turkey
This study was presented as thematic poster in the 21st European
Respiratory Society Annual Congress in Amsterdam, The Netherlands,
24-28 Sep 2011. The abstract was published in European Respiratory
Journal 2011;38(Suppl 55):2253.
Corresponding author: Dr Ege G Balbay (egegulecbalbay@gmail.com)
Abstract
Objective: To investigate the prevalence of
obstructive sleep apnoea syndrome in patients with
primary open-angle glaucoma.
Design: Case series.
Setting: School of Medicine, Düzce University,
Turkey.
Patients: Twenty-one consecutive primary
open-angle glaucoma patients (12 females and 9
males) who attended the out-patient clinic of the
Department of Ophthalmology between July 2007
and February 2008 were included in this study. All
patients underwent polysomnographic examination.
Results: The prevalence of obstructive sleep apnoea
syndrome was 33.3% in patients with primary
open-angle glaucoma; the severity of the condition
was mild in 14.3% and moderate in 19.0% of the
subjects. The age (P=0.047) and neck circumference
(P=0.024) in patients with obstructive sleep apnoea
syndrome were significantly greater than those
without the syndrome. Triceps skinfold thickness in
glaucomatous obstructive sleep apnoea syndrome
patients reached near significance versus those
without the syndrome (P=0.078). Snoring was
observed in all glaucoma cases with obstructive
sleep apnoea syndrome. The intra-ocular pressure
of patients with primary open-angle glaucoma
with obstructive sleep apnoea syndrome was
significantly lower than those without obstructive
sleep apnoea syndrome (P=0.006 and P=0.035 for
the right and left eyes, respectively). There was no significant difference in the cup/disc ratio and visual
acuity, except visual field defect, between primary
open-angle glaucoma patients with and without
obstructive sleep apnoea syndrome.
Conclusions: Although it does not provide evidence
for a cause-effect relationship, high prevalence
of obstructive sleep apnoea syndrome in patients
with primary open-angle glaucoma in this study
suggests the need to explore the long-term results
of coincidence, relationship, and cross-interaction of
these two common disorders.
New knowledge added by this
study
- Although this study did not provide an evidence for a cause-effect relationship between obstructive sleep apnoea syndrome (OSAS) and primary open-angle glaucoma (POAG), the high prevalence of OSAS in patients with POAG might present a new perspective to ophthalmologists when managing glaucoma patients.
- In clinical practice, OSAS is often not taken into diagnostic consideration for glaucoma patients. The high prevalence of OSAS in patients with POAG might present a new perspective to ophthalmologists and encourage them to explore the long-term results of coincidence, relationship, and cross-interaction of these two common disorders. Based on data from the present study, we recommend eliciting history of sleep apnoea symptoms in patients with glaucoma, especially in those who are obese and have thick necks. We also recommend polysomnography in patients with two or more major sleep disturbance symptoms.
Introduction
Obstructive sleep apnoea syndrome (OSAS) is
characterised by repetitive, complete, or partial
collapse of the pharyngeal airway during sleep and, generally, reduction in oxygen desaturation.1 The
prevalence of OSAS is estimated to be 1% to 2% in
men and 1.2% to 2.5% in women.2 The prevalence
of OSAS in Turkey was reported as 1.8% in
epidemiological studies.3
Sleep-disordered diseases are associated with
a number of eye disorders including floppy eyelid
syndrome, optic neuropathy, keratoconus, retinal
vascular tortuosity and congestion, retinal bleeding,
non-arteritic anterior ischaemic optic neuropathy
and papilloedema secondary to increased
intracranial pressure, normal tension glaucoma, and
primary open-angle glaucoma (POAG).4 5 6 7 Sleep-disordered
breathing may impair autoregulation
of optic nerve perfusion due to the direct effect of
hypoxia. Glaucoma is a multifactorial and specific
optic neuropathy often characterised by increased
intra-ocular pressure (IOP) that results in typical
and progressive visual field loss.8
The prevalence of glaucoma in the general
population is between 1% and 2%.9 While the
aetiology of POAG still remains unclear, several
risk factors have been associated with the
condition. It was known that OSAS effects the
oxygenation, neurohumoral factors, and vascular
haemodynamics.4 It has been suggested that OSAS
aggravates or even causes glaucoma by impaired
optic nerve head blood flow and tissue atrophy,
infarction due to vascular dysregulation or by direct
damage to the optic nerve secondary to prolonged
hypoxia.4 9
In addition to elevated IOP, cardiovascular risk factors—such as arterial hypotension and
hypertension, vasospasms, autoregulatory defects,
and atherosclerosis—are of increasing importance
in the pathogenesis of glaucoma, especially in
normal-tension glaucoma (NTG). Several recent
reports4 5 10 11 suggest that OSAS may be an
additional risk factor for glaucoma. Some of the
possible causes of glaucoma-like abnormal blood
coagulation, vasospastic disease, and optic nerve
vascular dysregulation are also consequences of
OSAS. It is not surprising, therefore, that some
studies4 5 10 11 show an increased prevalence of OSAS
in patients with glaucoma and vice versa. In clinical
practice, OSAS is often not taken into diagnostic
consideration in glaucoma patients.11
The association between glaucoma and OSAS
has been reported in many studies. Most of them
focused on the prevalence of glaucoma in OSAS
patients and indicated it as a risk factor. Some of
these articles have been observational case reports
or case series.4 12 13 14 15 The objective of this study was to
investigate the prevalence of OSAS in patients with
POAG.
Methods
Study group
This was a prospective case series that included 30
consecutive adult POAG patients who attended the
out-patient clinic of the Düzce University, School
of Medicine, Turkey between July 2007 and February 2008.
Informed consent was obtained from the study
participants.
Exclusion criteria
Individuals with diabetes mellitus (n=4), thyroid
function disorders (n=2), hyperlipidaemia (n=2),
and who refused to participate in the study (n=1)
were excluded.
Ophthalmological examination
All patients underwent routine eye examination,
including Snellen visual acuity, manifest refraction,
slit-lamp examination of the anterior eye segment,
IOP measurement, gonioscopy, and binocular
examination of the optic disc.
Patients were considered to have POAG if
they had untreated IOP of ≥21 mm Hg, an open
anterior chamber angle, glaucomatous visual field
defects or glaucomatous cupping of the optic disk,
and no fundus or neurological lesion other than
glaucomatous cupping to account for the visual field
defect.
Data collection
Prior to the sleep test, all POAG patients completed
a questionnaire about sleep disturbance. Data from the questionnaire were used to evaluate basic
OSAS symptoms such as snoring (presence of
snoring for at least five nights per week), witnessed
apnoea (spouse or relatives of patients with OSAS,
identifying noisy and irregular snoring, and arrested
respiration through the mouth and nose), and
daytime sleepiness. The Epworth Sleepiness Scale
was used to objectively evaluate excessive daytime
sleepiness. If the score obtained on this scale
was above 10, excessive daytime sleepiness was
considered present.16 All POAG patients received an
otorhinolaryngeal examination.
In addition, polysomnography (PSG; Somno-Medics Gmbh-8 Co. KG, Nonnengarten 8, D-97270
Kist Germany. Model: Somnoscreen-PSG, Ser-No:
0372 CAA5-OJ), electroencephalography, electro-oculography,
chin electromyography, oral and nasal
airflow (nasal-oral ‘thermistor’ and nasal cannula),
thorax movements, abdominal movements, arterial
oxygen saturation (pulse oximetry instrument),
electrocardiogram, and snoring recordings (>6
hours) were obtained from all patients. All records
were scored manually in a computer environment.
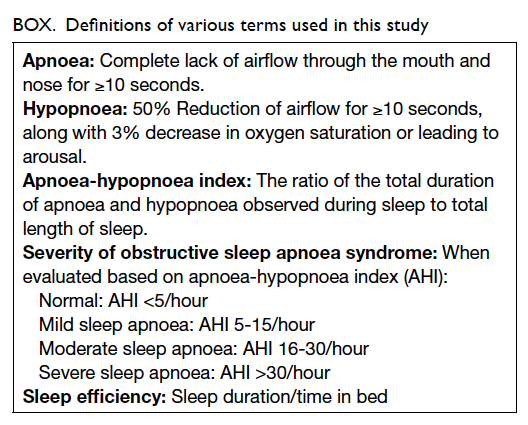
Definitions of various terms are shown in the Box.17
Statistical analysis
Data were analysed using the Statistical Package for
the Social Sciences (Windows version 10.0; SPSS Inc,
Chicago [IL], US). Mann Whitney U test was used
for comparing quantitative data. Chi squared test or
Fisher’s exact test was used to compare categorical
data. A P value of <0.05 was considered statistically
significant. Spearman’s test was used for evaluating
correlations between sample pairs.
Results
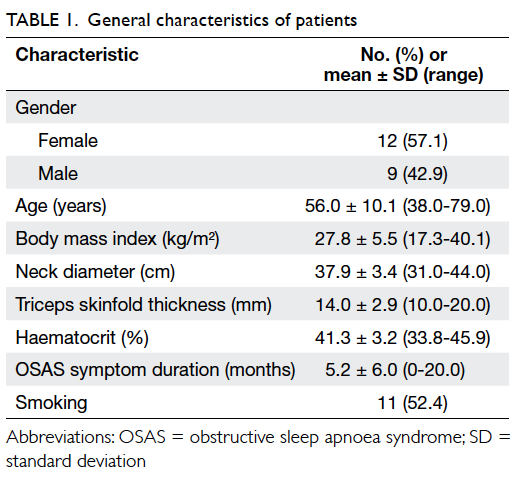
Of the 21 POAG patients, 12 were female and 9
were male. Demographic and clinical features of the
patients are summarised in Table 1.
Snoring was the most prevalent (81.0%) major
symptom of OSAS; snoring was habitual in 42.9%
of the patients. Daytime sleepiness and witnessed
apnoea were found in 23.8% and 14.3% of the
patients, respectively. While no major symptom
was present in 52.4% of POAG patients, three major
symptoms were concomitantly present in one (4.8%)
POAG patient (Table 2).
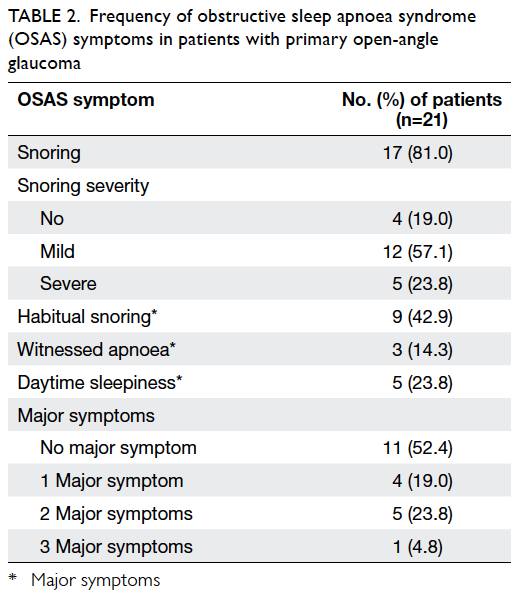
Table 2. Frequency of obstructive sleep apnoea syndrome (OSAS) symptoms in patients with primary open-angle glaucoma
Polysomnographic study showed that OSAS
was present in 33.3% (n=7) of the POAG patients
(apnoea-hypopnoea index [AHI] ≥5/hour). The
severity of OSAS was mild (AHI of 5-15/hour) in
14.3% (n=3) and moderate (AHI of 16-30/hour) in
19.0% (n=4) of the patients. Age (P=0.047) and neck circumference (P=0.024) were significantly higher
in POAG patients with OSAS versus those without
OSAS; triceps skinfold thickness was also higher
in OSAS patients, but it did not reach statistical
significance (P=0.078). No significant difference
was observed between POAG patients with and without OSAS with regard to body mass index and
the duration of one or more major OSAS symptom
(Table 3).
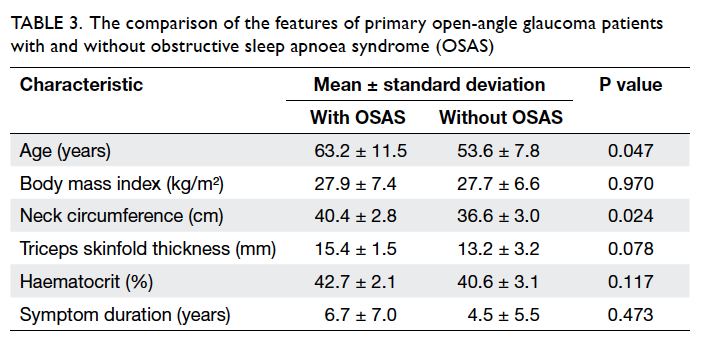
Table 3. The comparison of the features of primary open-angle glaucoma patients with and without obstructive sleep apnoea syndrome (OSAS)
Primary open-angle glaucoma patients with
and without OSAS did not differ significantly in
terms of gender, smoking, hypertension, cup/disc
ratio, and visual acuity. Intra-ocular pressure in
POAG patients with OSAS was significantly lower
than that in patients without OSAS (P=0.006 and
P=0.035 for the right and left eyes, respectively).
Apnoea-hypopnoea index was significantly higher
(P<0.001) and the lowest desaturation on PSG was
significantly lower (P=0.043) in POAG patients with
OSAS than those without OSAS. Visual field defects
were significantly more common in POAG patients
with OSAS (P=0.038) [Table 4].
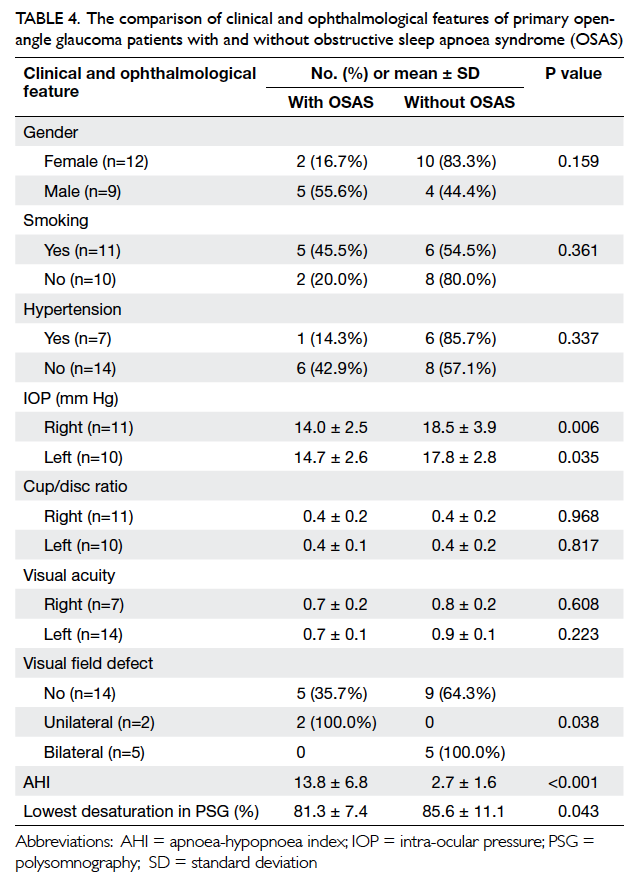
Table 4. The comparison of clinical and ophthalmological features of primary open-angle glaucoma patients with and without obstructive sleep apnoea syndrome (OSAS)
Obstructive sleep apnoea syndrome was
not observed in POAG patients with no snoring
(including simple snoring). As the degree of snoring
increased, OSAS prevalence reached almost
statistical significance. The symptoms of habitual
snoring (P<0.001) and witnessed apnoea (P=0.026)
were significantly more frequent in POAG patients
with OSAS versus those without OSAS (Table 5).
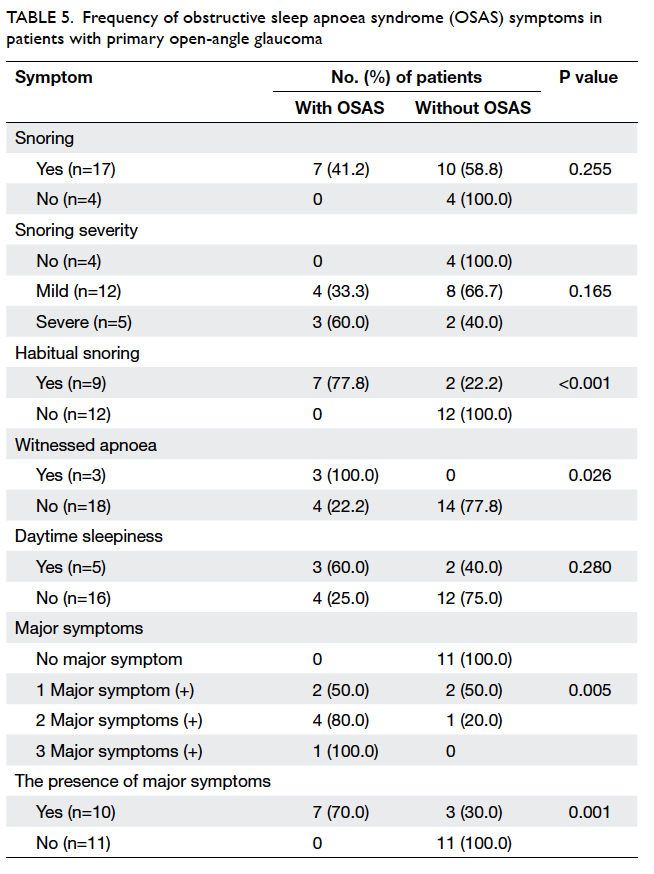
Table 5. Frequency of obstructive sleep apnoea syndrome (OSAS) symptoms in patients with primary open-angle glaucoma
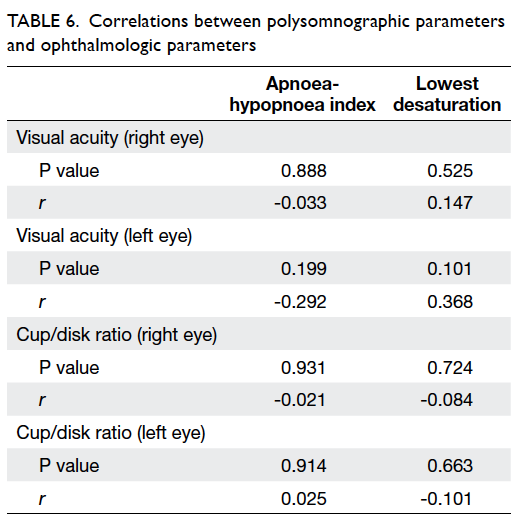
No correlation was detected between PSG
parameters (AHI, lowest desaturation in PSG) and
ophthalmologic parameters (cup/disc ratio, visual
acuity) in POAG patients (Table 6).
Discussion
In this study, the prevalence of OSAS and the
associated symptoms were higher in POAG patients
than that in the general population.2 The prevalence
of OSAS of at least mild severity was even higher
compared with that in middle-aged adults (9% in
women and 24% in men).3 Intra-ocular pressure
levels in patients with OSAS were significantly lower
than in those without OSAS. Another important
finding of the present study was that there was a
statistically significant but clinically insignificant
difference between OSAS and non-OSAS patients
regarding visual field defect.
Vascular risk factors for POAG have
been hypothesised and researched. It has been
reported that potential cardiovascular risk factors
including systemic hypertension, atherosclerosis,
vasospasm, and acute hypotension are associated
with glaucoma.5 Nevertheless, some patients may
experience progression of their neuropathy even
though their IOP seems appropriately controlled.
Obstructive sleep apnoea syndrome could be
considered one of the risk factors for POAG. Since
glaucomatous optic neuropathy is multifactorial,
treatment of OSAS—which is currently a known and
modifiable risk factor—may help the control of IOP
and management of glaucoma.18
There are a few studies examining the
correlation between POAG and OSAS. A recent study19 determined the prevalence of OSAS in
POAG associated with snoring. Thirty-one snoring
glaucomatous patients prospectively underwent
PSG. Of these, 49% were diagnosed to have OSAS.19
Mojon et al4 performed overnight transcutaneous
finger oximetry in 30 consecutive patients having
POAG (mean age, 76.0 ± 7.9 years) and found that the
oximetry disturbance index (ODI) was significantly
higher (11%) in these patients compared with normal
controls of the same age and sex distribution. They
reported OSAS prevalence as 20% (n=6/30) in POAG
patients according to ODI.4 In a group of 16 NTG
patients, the OSAS prevalence was 50% in patients
aged 45 to 64 years, and 63% in patients older than
64 years.10 We found an OSAS prevalence of 33.3% in
POAG patients according to AHI.
Mojon et al5 reported a 7.2% prevalence of
NTG among 69 white patients with OSAS (mean
age, 52.6 ± 9.7 years), and it was significantly higher
than that expected in general white population (2%).5
In another study, Sergi et al20 found a 59% prevalence
of NTG in 51 OSAS patients (mean age, 64 ± 10
years). Contrary to the other studies, the prevalence
of glaucoma in a study involving 228 patients with
OSAS was reported to be the same as in the general
population.21
Age is a common risk factor of both OSAS and
POAG; the latter itself is an ageing-associated disease.
The incidence of OSAS in the general population has
been shown to be the highest between 45 and 65
years of age.22 Thus, high mean age (56.0 years) in
our study might have contributed to the observed high
prevalence of OSAS.
Snoring is known to be the most common
symptom in OSAS.23 A group of out-patients,
including those with POAG and without POAG,
was recruited for evaluation of sleep-disordered
breathing symptoms such as snoring, excessive
daytime sleepiness, and insomnia with the help of a
questionnaire.9 The authors reported high prevalence
of sleep-disordered breathing in POAG patients.
Compared with those without POAG, POAG patients
showed a higher prevalence of snoring (47.6% vs
38.0%), snoring plus excessive daytime sleepiness
(27.3% vs 17.3%), and snoring plus excessive
daytime sleepiness plus insomnia (14.6% vs 7.8%).9
The authors speculated that the large nocturnal
fluctuations in blood pressure of OSAS patients may
have interfered with normal ocular haemodynamics,
making the eye vulnerable to glaucoma.9 In the
logistic regression model, snoring was significantly
associated with glaucoma. However, that study was
not a follow-up study of glaucomatous patients
and snoring could not be accepted as a prognostic
factor of POAG.9 Moreover, their study did not use
objective measures such as overnight PSG for the
diagnosis; instead, they only relied on self-reported
symptoms.9 In another study,7 the prevalence of sleep-disordered breathing symptoms was higher in
patients with NTG versus those without NTG (57%
vs 3%). However, contrary to our study, they only
offered PSG to patients with a positive sleep history.7
In our study, the prevalence rates of snoring, habitual snoring,
witnessed apnoea, excessive daytime sleepiness were
81.0%, 42.9%, 14.3%, and 23.8%, respectively. The concomitant presence of two or three
major OSAS symptoms was observed in 23.8% and
4.8% of our POAG patients, respectively. Blumen
Ohana et al19 reported high prevalence of OSAS in
patients with POAG and suggested that presence of
snoring should be explored at interview. Conversely,
patients who snore should be asked whether they
have POAG, and if so, should undergo all-night sleep
recording for the presence of OSAS.19 Mojon et al5
also found that respiratory disturbance index (RDI)
was positively correlated with IOP in 114 OSAS
patients. Because of the observational nature of that
study, they concluded only an association between
glaucoma and OSAS rather than a direct causal
relationship.5 A study by Karakucuk et al15 found that
the prevalence of glaucoma in patients with OSAS
was 12.9% (n=4/31); all these four patients with
glaucoma were in the severe OSAS group. There
was also a positive correlation between IOP and
AHI, and they suggested that increased IOP values
may reflect the severity of OSAS.15 In another cross-sectional
study, there was no correlation between
IOP and RDI.21 In the present study, IOP level in
patients with OSAS was significantly lower than that
in those without OSAS. Intra-ocular pressure shows
diurnal variation and patients with OSAS may have
elevated IOP and perfusional disturbance of retinal
nerve fibres during sleep. Therefore, these patients
may have completely normal or low IOP during the
daytime. On the other hand, most patients with
OSAS were regularly under glaucoma medication
which lowers the IOP to within normal limits.
Sergi et al20 did not find any difference in the
cup/disk ratio between the study patients and the
control group. They found a significant correlation
between AHI and the cup/disk ratio but none between
awake arterial blood gases and the ophthalmologic
examination data.20 They speculate that POAG could
be a consequence of changes in vascular tone and of
the increased platelet aggregability which frequently
occur in OSAS patients. In contrast with their study,
our study shows that the cup/disk ratio did not differ
between POAG patients with and without OSAS.
A study in Hong Kong24 examined the
computerised visual fields and optic discs of OSAS
patients with normal IOP and compared these with
non-OSAS population. Visual field indices were
significantly lower and the incidence of suspicious
glaucomatous disc changes was higher versus the
control arm.24 A variety of visual field defects in OSAS
patients were also reported by Mojon et al25 in nine patients; the field defects stabilised in two of these
after 18 months following continuous positive airway
pressure (CPAP). Kremmer et al11 have also reported
patients with NTG and progressive field loss despite
IOP-lowering eye drops and surgery. Nevertheless,
they stabilised field loss of patients after diagnosis
of OSAS and treatment with CPAP.11 Although there
was statistically significant difference in the visual
field defects of POAG patients with and without
OSAS in our study, it was clinically insignificant.
Only significant glaucomatous visual field defects
were considered in our evaluation. Therefore, minor
changes due to lenticular opacifications or other
aetiology were not taken into account as major
glaucomatous visual field changes.
Hypoxaemia and haemodynamic changes
resulting from intermittent apnoea and hypopnoea
during sleep are believed to play a role in
glaucomatous optic neuropathy.21 Although there is
no clear evidence for a cause-effect relationship in
the present study, the high prevalence of OSAS in
patients with POAG suggests a possible relationship.
Conclusions
In this study, the prevalence of OSAS was higher
in POAG patients versus the general population.
In clinical practice, OSAS is often not taken into
diagnostic consideration in glaucoma patients. The
high prevalence of OSAS in patients with POAG
suggests the need to explore the long-term results
of coincidence, relationship, and cross-interaction
between these two common disorders. Based on
data from the present study, we recommend that
the history of sleep apnoea symptoms be asked in
patients with glaucoma, especially in those who are
obese and have thick necks. In addition, PSG should
be performed in those patients with two or more
major sleep disturbance symptoms. Further large-scale studies are required to explore the
long-term results of these two common disorders, particularly in patients who
have been treated with CPAP therapy.
References
1. Sleep-related breathing disorders in adults:
recommendations for syndrome definition and
measurement techniques in clinical research. The Report
of an American Academy of Sleep Medicine Task Force.
Sleep 1999;22:667-89.
2. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr
S. The occurrence of sleep-disordered breathing among
middle-aged adults. N Engl J Med 1993;328:1230-5. CrossRef
3. Köktürk O. Epidemiology of sleep apnea syndrome. Tuberk
Toraks 1998;46:193-201.
4. Mojon DS, Hess CW, Goldblum D, Böhnke M, Körner F,
Mathis J. Primary open-angle glaucoma is associated with
sleep apnea syndrome. Ophthalmologica 2000;214:115-8. CrossRef
5. Mojon DS, Hess CW, Goldblum D, et al. High prevalence
of glaucoma in patients with sleep apnea syndrome.
Ophthalmology 1999;106:1009-12. CrossRef
6. McNab AA. The eye and sleep apnea. Sleep Med Rev
2007;11:269-76. CrossRef
7. Marcus DM, Costarides AP, Gokhale P, et al. Sleep
disorders: a risk factor for normal-tension glaucoma? J
Glaucoma 2001;10:177-83. CrossRef
8. Dhillon S, Shapiro CM, Flanagan J. Sleep-disordered
breathing and effects on ocular health. Can J Ophthalmol
2007;42:238-43. CrossRef
9. Onen SH, Mouriaux F, Berramdane L, Dascotte JC,
Kulik JF, Rouland JF. High prevalence of sleep-disordered
breathing in patients with primary open-angle glaucoma.
Acta Ophthalmol Scand 2000;78:638-41. CrossRef
10. Mojon DS, Hess CW, Goldblum D, et al. Normal-tension
glaucoma is associated with sleep apnea syndrome.
Ophthalmologica 2002;216:180-4. CrossRef
11. Kremmer S, Selbach JM, Ayertey HD, Steuhl KP. Normal
tension glaucoma, sleep apnea syndrome and nasal
continuous positive airway pressure therapy—case report
with a review of literature [in German]. Klin Monbl
Augenheilkd 2001;218:263-8.
12. Grieshaber MC, Flammer J. Blood flow in glaucoma. Curr
Opin Ophthalmol 2005;16:79-83. CrossRef
13. Grieshaber MC, Mozaffarieh M, Flammer J. What is the
link between vascular dysregulation and glaucoma? Surv
Ophthalmol 2007;52 Suppl 2:S144-54.
CrossRef
14. Hayreh SS. The 1994 Von Sallman Lecture. The optic
nerve head circulation in health and disease. Exp Eye Res
1995;61:259-72. CrossRef
15. Karakucuk S, Goktas S, Aksu M, et al. Ocular blood flow
in patients with obstructive sleep apnea syndrome (OSAS).
Graefes Arch Clin Exp Ophthalmol 2008;246:129-34. CrossRef
16. Johns MW. A new method for measuring daytime
sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-5.
17. International classification of sleep disorders, version 2:
diagnostic and coding manual. Rochester, MN: American
Academy of Sleep Medicine; 2005.
18. Blumen-Ohana E, Blumen M, Aptel F, Nordmann JP.
Glaucoma and sleep apnea syndrome [in French]. J Fr
Ophtalmol 2011;34:396-9. CrossRef
19. Blumen Ohana E, Blumen MB, Bluwol E, Derri M, Chabolle
F, Nordmann JP. Primary open angle glaucoma and
snoring: prevalence of OSAS. Eur Ann Otorhinolaryngol
Head Neck Dis 2010;127:159-64. CrossRef
20. Sergi M, Salerno DE, Rizzi M, et al. Prevalence of normal
tension glaucoma in obstructive sleep apnea syndrome
patients. J Glaucoma 2007;16:42-6. CrossRef
21. Geyer O, Cohen N, Segev E, et al. The prevalence of
glaucoma in patients with sleep apnea syndrome: same as in
the general population. Am J Ophthalmol 2003;136:1093-6. CrossRef
22. Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A.
Effects of age on sleep apnea in men: I. Prevalence and
severity. Am J Respir Crit Care Med 1998;157:144-8. CrossRef
23. Köktürk O. The clinical features of OSAS. Tuberk Toraks
1999;47:117-26.
24.
Tsang CS, Chong SL, Ho CK, Li MF. Moderate to severe
obstructive sleep apnoea patients is associated with a higher
incidence of visual field defect. Eye (Lond) 2006;20:38-42. CrossRef
25.
Mojon DS, Mathis J, Zulauf M, Koerner F, Hess CW.
Optic neuropathy associated with sleep apnea syndrome.
Ophthalmology 1998;105:874-7. CrossRef