© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Multimodal imaging of a left anterior descending
artery fistula with a dissecting interventricular
septal aneurysm: a case report
Danling Xie, MD; Guoliang Yang, PhD; Chun Li, MD; Hui Li, MS; Xianglin Hao, PhD; Yun Zhang, MD; Mingxun Xie, MD; Yali Xu, PhD
Department of Ultrasound, The Second Affiliated Hospital of Army Medical University (Third Military Medical University), Chongqing, China
Corresponding author: Dr Yali Xu (xuyali1976@163.com)
Case presentation
A 37-year-old male attended for evaluation of an
interventricular septal (IVS) mass incidentally
discovered during a routine physical examination.
On admission he was hypertensive at 153/85 mm Hg)
with heart rate 93 bpm. Electrocardiogram
findings revealed occasional premature ventricular
contractions with horizontal ST-segment depression
and T-wave inversion, suggestive of myocardial
ischaemia. Laboratory results were normal.
Transthoracic echocardiography (TTE) identified a
well-defined, ovoid mass within the inferior segment
of the IVS, appearing as a complex solid-cystic mass
lesion. The mass measured approximately 35 × 31 ×
40 mm3 (anteroposterior × transverse × longitudinal)
[Fig 1a]. The left ventricular apical cavity was
compressed by the mass. Colour Doppler imaging
showed blood flow within the lesion, with diastole
filling and systole outflow (Fig 1b). Notably, the cystic
cavity exhibited minimal changes during the cardiac
cycle. Continuous-wave Doppler at the lesion (Fig 1c) revealed bidirectional blood flow across systole
and diastole. Tracking revealed a 4.3-mm dilated
coronary artery branch at the heart’s apex as the
flow’s source, with no proximal stenosis or dilation
in the coronary arteries. Contrast TTE (cTTE)
detected a small (1-2 mm) left anterior descending
(LAD) artery fistula within the IVS, with delayed
cystic enhancement relative to the left ventricle
but simultaneous with the IVS myocardium post-contrast,
and no infiltration of the solid component
(Fig 1d-e, SV1). These findings indicated the presence
of a coronary artery fistula originating from the LAD
artery that drains into an IVS forming a dissecting
aneurysm.
Coronary computed tomography angiography
showed no proximal left coronary artery dilation
but mild distal dilation. The aneurysm showed
significant enhancement and slight calcification,
with no enhancement observed in the surrounding
myocardium (Fig 1f). Cardiac magnetic resonance
confirmed the findings, showing the mass with
slightly high T1-weighted and predominantly high
T2-weighted signal intensities, encircled by a low-signal intensity ring, with no significant myocardial enhancement post-contrast (Fig 1).
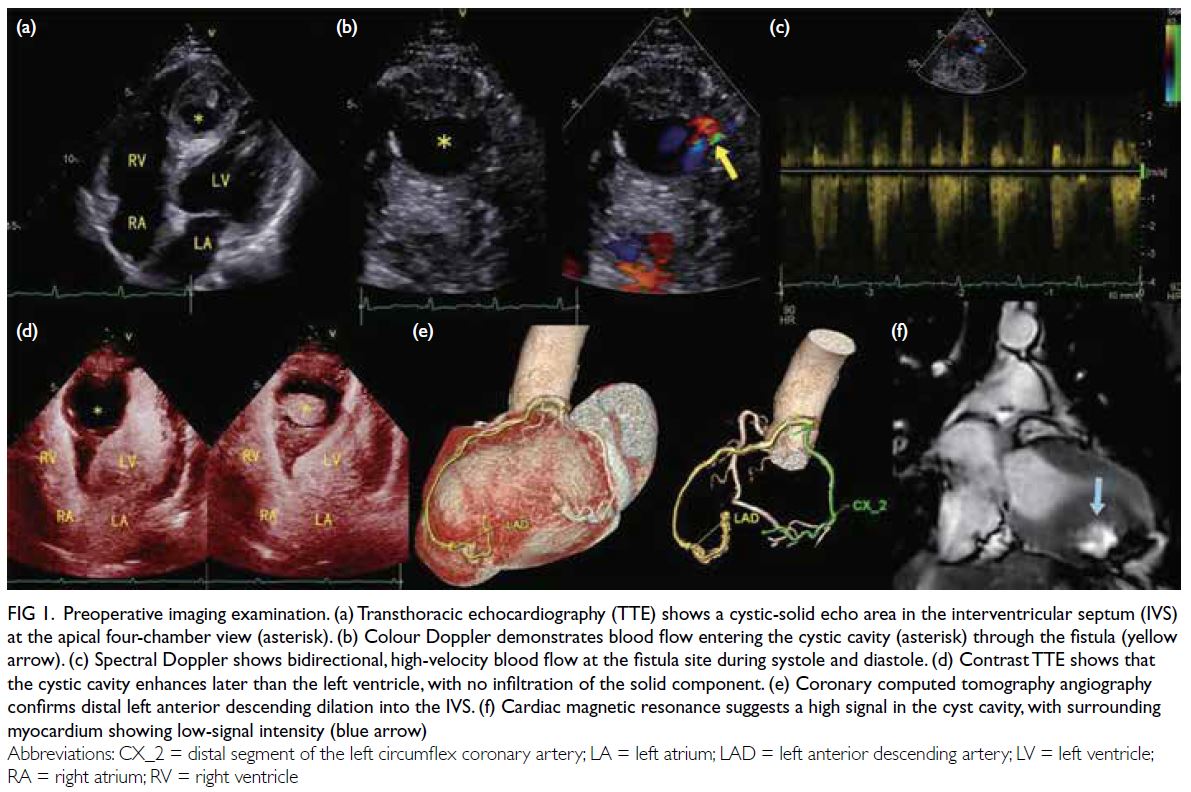
Figure 1. Preoperative imaging examination. (a) Transthoracic echocardiography (TTE) shows a cystic-solid echo area in the interventricular septum (IVS) at the apical four-chamber view (asterisk). (b) Colour Doppler demonstrates blood flow entering the cystic cavity (asterisk) through the fistula (yellow arrow). (c) Spectral Doppler shows bidirectional, high-velocity blood flow at the fistula site during systole and diastole. (d) Contrast TTE shows that the cystic cavity enhances later than the left ventricle, with no infiltration of the solid component. (e) Coronary computed tomography angiography confirms distal left anterior descending dilation into the IVS. (f) Cardiac magnetic resonance suggests a high signal in the cyst cavity, with surrounding myocardium showing low-signal intensity (blue arrow)
The patient underwent surgical correction
of the coronary artery fistula and reduction of the
dissecting aneurysm of the interventricular septum
(DAIS). Intraoperatively, the LAD was observed
penetrating the myocardium, forming a sac-like
cavity due to the convergence of the coronary fistula
into the myocardial layer of the IVS. Histopathology
from a myocardial biopsy showed fibrosis (Fig 2a-d).
At 3-month postoperative follow-up, TTE showed
a reduced IVS cystic cavity (Fig 2e), and cTTE
confirmed fistula closure with no contrast entering
the cavity (Fig 2f).
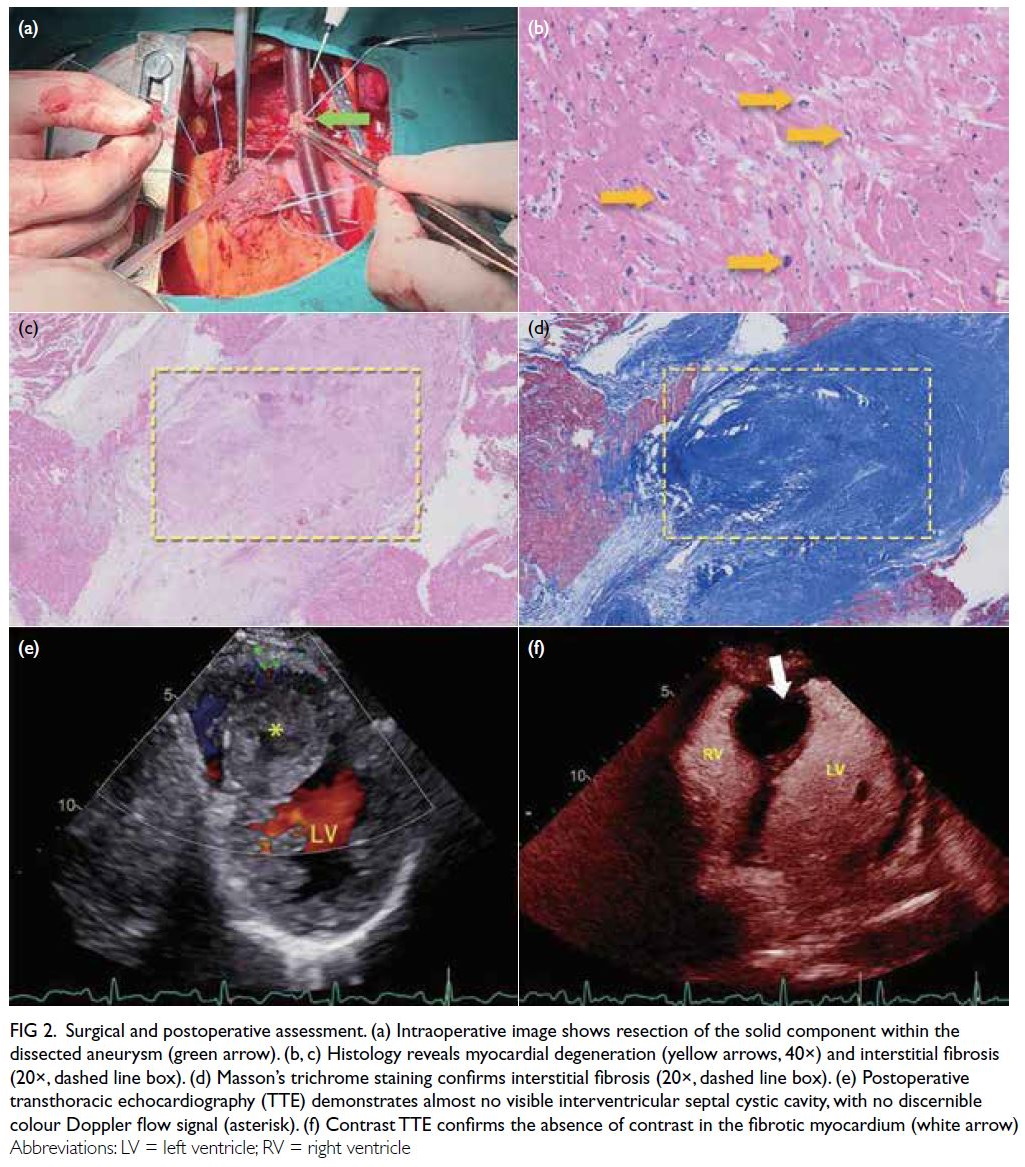
Figure 2. Surgical and postoperative assessment. (a) Intraoperative image shows resection of the solid component within the dissected aneurysm (green arrow). (b, c) Histology reveals myocardial degeneration (yellow arrows, 40×) and interstitial fibrosis (20×, dashed line box). (d) Masson’s trichrome staining confirms interstitial fibrosis (20×, dashed line box). (e) Postoperative transthoracic echocardiography (TTE) demonstrates almost no visible interventricular septal cystic cavity, with no discernible colour Doppler flow signal (asterisk). (f) Contrast TTE confirms the absence of contrast in the fibrotic myocardium (white arrow)
Discussion
Cardiac space-occupying lesions include tumours
and non-neoplastic conditions, occurring
anywhere in the heart.1 Dissecting aneurysm of
the interventricular septum often results from a
ruptured Valsalva aneurysm, myocardial infarction,
or trauma.2 Aneurysms within the IVS caused by
congenital coronary artery fistulas are rare, with only
a few reported cases.3 4 5 These cases often present
with marked dilation of the involved coronary artery
trunk and dynamic fluctuations in the cystic cavity
dimensions throughout the cardiac cycle. The cavity
typically expands during diastole and contracts
during systole.
In this case, the absence of dilation in the main
trunk of the coronary artery could be attributed to
the fistula’s origin from a small branch of the LAD
artery, with a narrow internal diameter and minimal
shunting volume. As the patient was young, the
coronary arteries exhibited greater elasticity, leading
to a reduced propensity for dilation in the main trunk.
In this case, the cystic cavity in the IVS showed
minimal size change throughout the cardiac cycle
due to a blind-ending coronary artery fistula that
prevented left ventricular communication. Chronic
shunting from the coronary artery fistula led to
the gradual enlargement of a dissecting aneurysm
within the interventricular septum, compressing
adjacent myocardium and branches of the coronary arteries. This compression resulted in localised
myocardial ischaemia and subsequent myocardial
fibrosis, as demonstrated by both the patient’s
electrocardiogram and pathological findings. The
fibrosis and high-velocity flow at the fistula site
contributed to myocardial thickening and reduced
elasticity, impairing the cavity’s expansion and
contraction, and resulting in minimal size variation.
Transthoracic echocardiography is often the
initial imaging choice for coronary artery fistulas
into the IVS, providing critical haemodynamic and
anatomical data, but its limitations may result in
misdiagnosis. Coronary computed tomography
angiography and cardiac magnetic resonance
provide detailed assessments of coronary anatomy
and myocardial fibrosis, complementing TTE.
Coronary computed tomography angiography
provides diagnostic clarity with the caveat of
radiation exposure, especially for repeated scans.
Cardiac magnetic resonance, while valuable for
its soft tissue characterisation, presents cost
considerations for patients. Contrast TTE, valued for
its safety, cost-effectiveness, and timeliness, excels
in visualising myocardial perfusion and detecting
congenital cardiac defects, enhancing diagnostic
precision when TTE results are indeterminate. In
our case, cTTE, with its high sensitivity to blood flow
signals, rapidly delineated the shunt and accurately mapped the fistula. The contrast agent, perfusing
the myocardium via the coronary arteries, resulted
in delayed opacification of the interventricular
septum compared with the left ventricle, thereby
disclosing DAIS. This comprehensive diagnostic
profiling was pivotal for tailored treatment strategies
and prognostic enhancement. This marks the first
instance, to our knowledge, where cTTE has been
utilised to diagnose DAIS.
Author contributions
Concept or design: D Xie, G Yang, C Li.
Acquisition of data: D Xie, C Li, H Li, X Hao, Y Zhang.
Analysis or interpretation of data: D Xie, H Li, X Hao, Y Zhang, M Xie, Y Xu.
Drafting of the manuscript: D Xie, G Yang, Y Xu.
Critical revision of the manuscript for important intellectual content: D Xie, G Yang, C Li, Y Xu.
Acquisition of data: D Xie, C Li, H Li, X Hao, Y Zhang.
Analysis or interpretation of data: D Xie, H Li, X Hao, Y Zhang, M Xie, Y Xu.
Drafting of the manuscript: D Xie, G Yang, Y Xu.
Critical revision of the manuscript for important intellectual content: D Xie, G Yang, C Li, Y Xu.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
The authors declared no conflicts of interest.
Acknowledgement
The authors thank Prof Yunhua Gao who provided guidance and assistance in the diagnosis.
Funding/support
This study was supported by the Individualized Training
Program for Key Supported Talents, part of the Excellent
Talents Database at the Army Medical University (Grant No.: 2019R038).
Ethics approval
The patient was treated in accordance with the Declaration of
Helsinki. The patient provided written consent for publication
of this case report.
References
1. Maleszewski JJ, Anavekar NS, Moynihan TJ, Klarich KW.
Pathology, imaging, and treatment of cardiac tumours. Nat Rev Cardiol 2017;14:536-49. Crossref
2. Zhang JP, Meng H, Wang H. Dissecting aneurysm of the
interatrial and interventricular septum with concomitant
ventricular septal defect-multimodality cardiac imaging
and surgical repair. Echocardiography 2016;33:932-5. Crossref
3. Zhi Ku L, Xia J, Lv H, Song LC, Ma XJ. Giant
interventricular septal dissecting aneurysm resulting
from congenital coronary fistula. Circ Cardiovasc Imaging
2022;15:e013861. Crossref
4. Wu Q, Jin Y, Zhou L, Liu Y, Wu D. A dissecting aneurysm
of interventricular septum resulting from congenital
coronary artery fistula. J Clin Ultrasound 2019;47:55-8. Crossref
5. Tekinhatun M, Cihan F, Demir M. Interventricular septal
dissecting aneurysm resulting from congenital coronary
fistula: a case report. Echocardiography 2023;40:1140-3. Crossref

