© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE (HEALTHCARE IN CHINA)
Use of pronase in screening for early cancers of
the upper gastrointestinal tract
Zhengqi Wu, BSc1; Shihua Li, BSc2; Linzhi Lu, BSc2; Zhiyi Zhang, BSc2; Guiqi Wang, BSc, MD3; Tianyan Qin, MSc2; Guangyuan Zhao, MSc2; Jindian Liu, MSc2
1 Department of Gastroenterology, Wuwei Liangzhou Hospital, Wuwei, China
2 Department of Gastroenterology, Wuwei Tumor Hospital, Wuwei, China
3 Department of Endoscopy, Cancer Hospital, Chinese Academy of Medical Sciences, Beijing, China
Corresponding author: Prof Zhengqi Wu (wzqwwzl@163.com)
Abstract
Introduction: This study aimed to investigate the
effectiveness of pronase in improving the detection
rate of early cancer and enhancing visual field clarity
during gastroscopy in China.
Methods: In total, 1450 patients who participated
in an early diagnosis and treatment programme
of upper gastrointestinal cancer in Wuwei, Gansu
Province between 2020 and 2021 were enrolled.
Cluster randomisation was utilised at the community
level. All patients underwent endoscopy and biopsy.
The experimental group (n=725) received pronase
granules and dimethicone prior to gastroscopy; the
control group (n=725) received dimethicone alone.
Endoscopic visibility scores, examination durations,
and lesion detection rates were recorded for both
groups.
Results: Visibility scores for all regions of the
stomach were significantly lower in the experimental
group than in the control group (P<0.001). This
finding remained consistent after adjustment for
confounding factors in multiple linear regression
analysis. The detection rate of precancerous lesions
and early cancer was significantly higher in the
experimental group than in the control group (77.5%
vs 62.5%; P<0.001). Binary logistic regression analysis
indicated that the likelihood of detecting early cancer
was greater in the experimental group, with an odds ratio of 3.840 (95% confidence interval=1.204-12.241;
P=0.023). Also, average gastroscopy time was
significantly shorter in the experimental group than
in the control group (6.52±2.51 min vs 10.03±1.23
min, t=33.81; P=0.001).
Conclusion: The administration of pronase prior
to gastroscopy enhances visual field clarity, reduces
examination time, and increases the detection rates
of precancerous lesions and early cancer.
New knowledge added by this study
- Pronase enhances visual field clarity during gastroscopy and reduces examination time.
- Pronase can enhance diagnostic precision by minimising misdiagnoses and missed lesions.
- Pronase improves the detection rates of precancerous lesions and early cancer. The results provide a strong scientific foundation for using pronase in endoscopic screening during clinical diagnostic examinations.
- The findings support adoption of pronase as a standard adjunct in gastroscopy to improve diagnostic accuracy and procedural efficiency.
Introduction
The implementation of early gastric cancer screening
in community populations and performance of
endoscopic examinations in high-risk groups
represents a feasible, cost-effective, and efficient
strategy to address the challenges of gastric cancer
diagnosis and treatment in China.1 More than 80%
of early-stage gastric cancer cases are identified
in asymptomatic community populations aged ≥40 years. Thus, community-based screening
programmes are important for increased detection
of early-stage cancer. Gastroscopy remains the
gold standard for diagnosing upper gastrointestinal
diseases. High-quality intragastric visibility
is essential for ensuring diagnostic accuracy,
minimising the risks of misdiagnosis and missed
diagnosis, and improving the detection of minimal-change
gastric lesions. However, air bubbles and mucus in the stomach often reduce gastroscopic
field visibility, leading to missed diagnoses and
prolonged examination times. Pretreatment with
defoaming agents and mucolytic agents enhances
gastroscopic field visibility.2 Pronase, a proteolytic
enzyme isolated from the culture filtrate of
Streptomyces griseus, effectively cleaves the peptide
bonds of glycoproteins, thereby dissolving and
eliminating gastric mucus.3 This study aimed to
evaluate the impact of pronase on the detection rate
of precancerous lesions and early cancer, clarifying
its utility in early gastric cancer screening. The
findings will provide foundational evidence for the
incorporation of pronase in endoscopic screening
for upper gastrointestinal tract cancers and clinical
diagnostic examinations.
Methods
Participants
This study enrolled 1450 individuals aged 40 to 70
years from a community population who participated
in the 2020-2021 Upper Gastrointestinal Cancer
Screening Programme in Wuwei, Gansu Province,
China. The inclusion criteria were: (1) ability to
cooperate with the gastroscopic procedure; (2) ability
to discontinue anticoagulant medications 1 week
prior to endoscopy; and (3) voluntary participation
and provision of written informed consent. The
exclusion criteria were: (1) contraindications to
gastroscopy; (2) severe heart disease or heart
failure; (3) severe respiratory disease; (4) posterior pharyngeal abscess or severe spinal deformity; (5)
other serious illnesses or physical conditions that
precluded tolerance of endoscopy; and (6) bleeding
tendency.
Gastroscopy examinations
Using a random number table, all 1450 participants
from the community population were randomly
assigned to either an experimental group (n=725) or
a control group (n=725). All participants underwent
gastroscopy and tissue biopsy. In the experimental
group, 1 sachet (20 000 U) of pronase (Beijing Tide
Pharmaceutical, Beijing, China) and 1 g of sodium
bicarbonate were dissolved in 50 to 80 mL of drinking
water (20-40°C) by shaking. The solution was orally
administered 15 to 30 minutes before gastroscopy
(GIF-H290; Olympus, Tokyo, Japan). Dimethicone
was also given orally to lubricate the cavity and
remove gastric bubbles. To ensure that pronase
reached all areas of the stomach, participants laid
flat on a bed under a nurse’s guidance, then turned
sideways three to five times. Subsequently, routine
gastroscopy was performed. In the control group,
participants received oral dimethicone 15 to 30
minutes before routine gastroscopy (GIF-H290).
The gastroscopy examinations were performed
by two physicians holding the title of associate
chief physician or higher, each having >10 years of
experience in gastroscopy. The visibility of each part
of the visual field was evaluated during the procedure;
pathological examinations were conducted on tissue
biopsies collected from minimal-change lesions.
Observation indicators
Endoscopic visibility scores were compared between
the two groups. Scoring criteria were as follows4:
1 point, no mucus; 2 points, a small amount of
mucus but no blurring of the visual field; 3 points,
a large amount of mucus with a blurred visual field,
requiring <30 mL of water for rinsing; and 4 points,
very thick mucus with a blurred visual field, requiring
≥30 mL of water for rinsing. Lower scores indicated
better endoscopic visibility. To minimise errors
during the scoring process, each visibility score was
recorded as the average of scores assigned by the
two physicians who performed gastroscopy. The
lesion detection rate was defined as the percentage
of subjects within a group in whom lesions were
identified. Gastroscopy time was measured from
entry of the gastroscope into the oesophagus until
its removal. Adverse reactions included nausea,
vomiting, difficulty breathing, facial flushing, and
other symptoms.
Statistical analyses
R software (version 4.0.5) was used for statistical
analysis. Quantitative data were expressed as mean±standard deviation; intergroup differences
were analysed using independent sample t tests.
Qualitative data were expressed as frequency and
percentage; intergroup differences were assessed
using the Chi squared test or Fisher’s exact test.
Multivariable linear regression analysis was
performed to evaluate the effect of group assignment
on visibility scores after adjustment for confounding
factors. Differences in early cancer detection
rates between the two groups were analysed using
multivariable binary logistic regression analysis. All
statistical tests were two-sided, and P values <0.05
were considered statistically significant.
Results
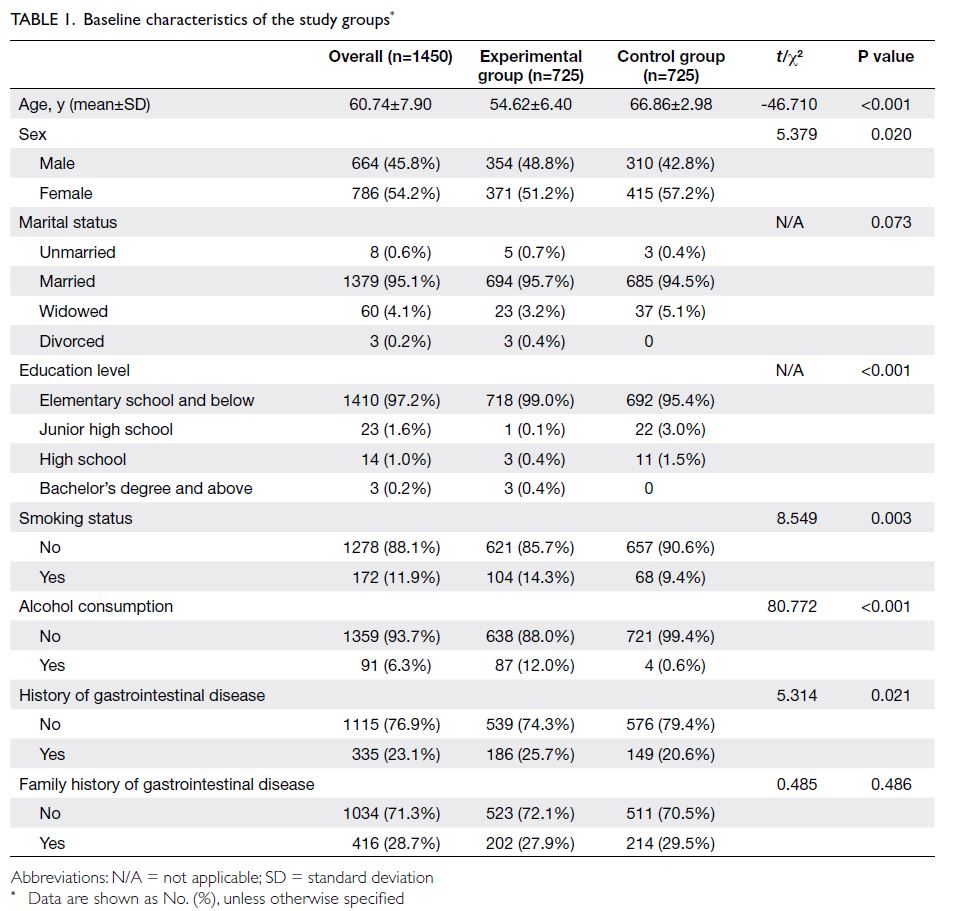
A summary of the baseline characteristics of the
experimental and control groups is provided in
Table 1. Among the 1450 patients in the cohort,
416 (28.7%) had a family history of gastrointestinal disease, 172 (11.9%) had a history of smoking, 91
(6.3%) had a history of alcohol consumption, and
335 (23.1%) had a history of gastrointestinal disease.
Significant differences between the two groups
were observed in the proportions of patients with
a history of smoking, alcohol consumption, and
gastrointestinal disease.
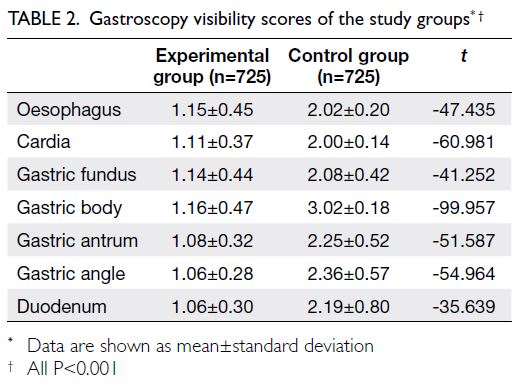
Average visibility scores for the oesophagus,
cardia, gastric fundus, gastric body, gastric antrum,
gastric angle, and duodenum were significantly
lower in the experimental group than in the control
group (P<0.001 for all comparisons) [Table 2]. The
visibility of different regions of the stomach under
gastroscopy substantially differed between the two
groups (Fig).
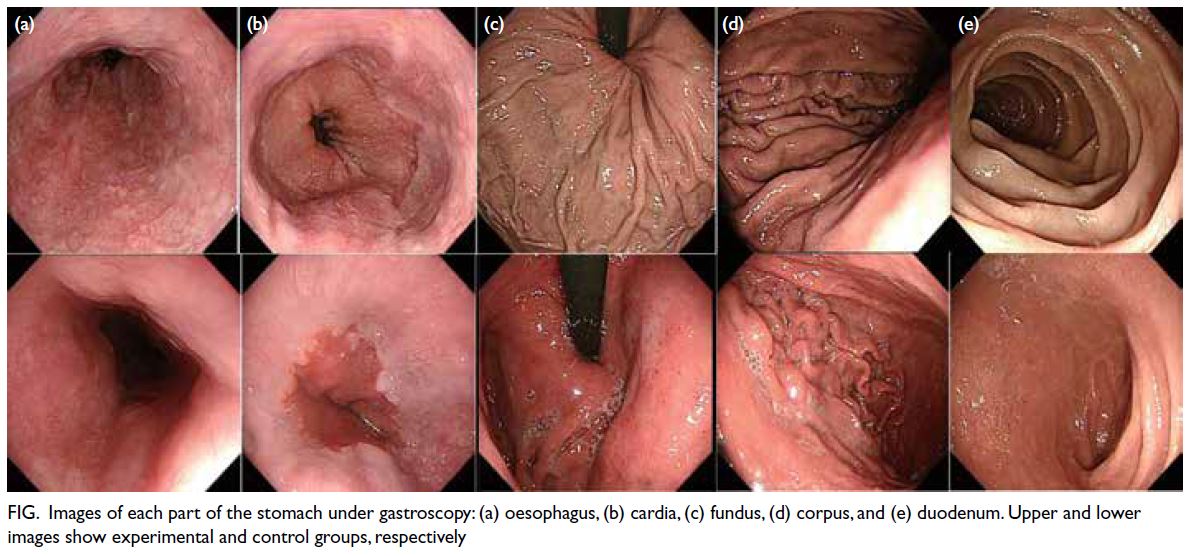
Figure. Images of each part of the stomach under gastroscopy: (a) oesophagus, (b) cardia, (c) fundus, (d) corpus, and (e) duodenum. Upper and lower images show experimental and control groups, respectively
Effect of pronase on visibility score
Multiple linear regression analysis was performed
with the visibility score for each site as the dependent variable and group assignment as the independent
variable; adjustments were conducted for sex, age,
marital status, education level, smoking status,
alcohol consumption, history of gastrointestinal
disease, and family history of gastrointestinal
disease. After adjustment for these confounding
factors, the visibility scores for all regions of the
stomach remained significantly higher in the control
group than in the experimental group (P<0.001 for
all visibility scores) [Table 3].
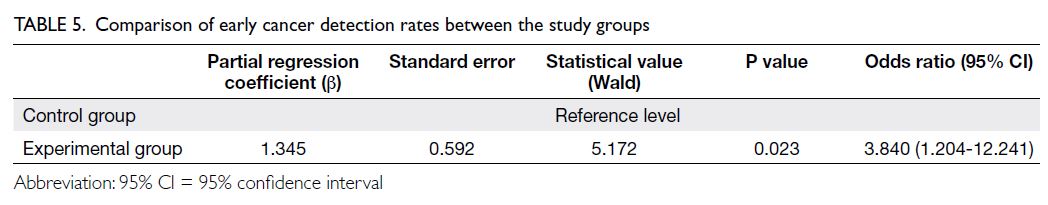
Lesion and early cancer detection rates
Chi squared test analyses revealed that the detection
rates of precancerous lesions (including atrophic
gastritis, intestinal metaplasia, and low-grade
intraepithelial neoplasia5) and early cancer were
significantly higher in the experimental group than in the control group (77.5% vs 62.5%; P<0.001) [Table 4].
Multivariable binary logistic regression
analysis was performed with early cancer detection
as the dependent variable and group assignment
as the independent variable; adjustments were
conducted for sex, age, marital status, education
level, smoking status, alcohol consumption, history
of gastrointestinal disease, and family history of
gastrointestinal disease. The likelihood of early
cancer detection was significantly higher in the
experimental group compared with the control
group, with an odds ratio of 3.840 (95% confidence
interval=1.204-12.241; P=0.023) [Table 5].
Examination time
Average gastroscopy times were 6.52±2.51 minutes in the experimental group and 10.03±1.23 minutes
in the control group. Gastroscopy time significantly
differed between the two groups (t=33.81; P=0.001).
Adverse reactions
No adverse reactions, such as nausea, vomiting, dyspnoea, or facial flushing, were reported in either
group.
Discussion
Currently, approximately 90% of primary gastric
cancers in China are diagnosed at an advanced
stage.6 The prognosis of affected patients is closely
related to the timing of diagnosis and treatment.
Despite surgical intervention, the 5-year survival rate
for patients with advanced gastric cancer remains
<30%.7 After treatment, the 5-year survival rate for
patients with early gastric cancer exceeds 90%, and
cure may be achieved.8 However, the rates of early
diagnosis and treatment of gastric cancer in China
are <10%, substantially lower than rates reported
in Japan (70%) and South Korea (50%).9 In Wuwei,
the incidence and mortality rates of gastric cancer
remain among the highest in the country; gastric
cancer ranks first among malignant tumours in the
city.10 Screening for upper gastrointestinal cancer is
one of the most effective methods for population-level
detection of early-stage cancer. Since 2010,
Wuwei Tumour Hospital has implemented an
upper gastrointestinal cancer screening programme (endoscopy combined with tissue biopsy) in Wuwei.
Improvements in the detection rates of precancerous
lesions and upper gastrointestinal cancer are key
objectives of this screening initiative.
Gastroscopy is currently a widely used
method for the clinical diagnosis and treatment of
gastrointestinal diseases. A clear endoscopic field
of vision is essential for accurate diagnosis and
effective treatment by endoscopists. To optimise
gastroscopy outcomes and enhance visibility within
the stomach, bubbles and mucus must be removed.
The use of pronase in combination with defoaming
agents is recommended by the Consensus on Early
Gastric Cancer Screening and Endoscopic Diagnosis
and Treatment in China11 and the Guidelines for
Endoscopic Diagnosis of Early Gastric Cancer (2019
edition) developed by the Japan Gastroenterological
Endoscopy Society.12
Lee et al13 demonstrated that administering
pronase 10 to 20 minutes before gastroscopy
significantly improved the visibility of the endoscopic
visual field and reduced the number of water washes
required. Similarly, a multicentre randomised
controlled study by Liu et al14 indicated that the
combination of pronase and dimethicone significantly
enhanced the visibility of the upper gastrointestinal
mucosa. Pronase has also been utilised in narrow-band
imaging endoscopy. A randomised controlled
study by Cha et al15 compared the effects of orally
administering pronase and simethicone 10 minutes
before narrow-band imaging endoscopy on mucosal visibility and diagnostic performance. The results
showed that mucosal visibility within the proximal
stomach was significantly better in the pronase
group than in the simethicone group.15 In the
present study, the visibility scores for all sites in
patients who received pronase were approximately
1 point, indicating minimal mucus adhesion. After
adjustment for confounding factors, multiple linear
regression analysis confirmed that visibility scores
remained significantly lower in the pronase group
than in the control group at all sites; this finding
further validated the effectiveness of pronase.
The present study also revealed that the average
endoscopic examination time was significantly
shorter (approximately 5 minutes) in the pronase
group than in the control group. This reduced
examination time was attributed to the near-complete
absence of mucus adhesion after pronase
administration, which decreased the number of
rinses needed during the procedure. The shorter
examination also enhanced patient comfort and
increased compliance for subsequent screenings.
Zhang et al16 and Gao et al17 conducted
retrospective analyses of 25 314 patients who
underwent gastroscopy at Nanfang Hospital of
Southern Medical University and 166 260 patients
at Bazhong Central Hospital, revealing early cancer
detection rates of 0.2% and 0.62%, respectively.
Zhang et al1 performed a follow-up analysis of
individuals in Liangzhou District in Wuwei who
underwent upper gastrointestinal cancer screening
in 2017; they observed an early cancer detection
rate of 2.8%.1 In the present study, lesion detection
rates for the experimental and control groups were
77.5% and 62.5%, respectively; corresponding early
cancer detection rates were 3.0% and 2.1%. These
percentages align with findings from the previous
study in Wuwei1 and are substantially higher than
those reported for other regions.16 17 The present
results suggest that in Wuwei, a region displaying one
of the highest incidences of upper gastrointestinal
cancer in China, early cancer screening should be
actively promoted. Furthermore, the detection rates
of precancerous lesions and early cancer can be
improved by using endoscopy combined with tissue
biopsy.
The efficacy of pronase in improving the
endoscopic visual field is well established, but
studies investigating its impacts on the detection
rates of precancerous lesions and early cancer
have yielded inconsistent results.14 18 19 Chen et al18
conducted a randomised controlled trial that
enrolled older patients undergoing gastroscopy; they
found that the detection rate of minimal-change
lesions was higher in the pronase group than in the
control group (45.2% vs 27.5%; P<0.05).18 Lee et al19
demonstrated that the use of pronase when rinsing a
lesion during endoscopy significantly increased the tissue depth of endoscopic biopsies and improved
the anatomical localisation of biopsy sites, thereby
enhancing the accuracy of disease diagnosis. In the
present study, the detection rates of precancerous
lesions and early cancer were significantly higher in
the experimental group than in the control group
(P<0.001). After adjustment for confounding factors,
multivariable logistic regression showed that the
likelihood of detecting early cancer was significantly
greater in the experimental group than in the
control group (odds ratio=3.840; P=0.023) [Table 5]. This finding indicates that pronase pretreatment
before gastroscopy can enhance the detection
rates of precancerous lesions and early cancer. The
enhancement may be attributed to the clear visual
field provided by pronase, which facilitates accurate
selection of biopsy sites and improves recognition
of minimal-change lesions. Gastroscopy physicians
have substantial daily workloads and manage large
numbers of patients requiring treatment. The use of
pronase reduced the time required for endoscopy,
potentially improving patient compliance with
clinical microscopy.
Limitations
As an early cancer screening study, this investigation
had a relatively small sample size; therefore, the
findings require further validation in large-scale
clinical studies. Cluster randomisation was used in
this study, leading to baseline differences between
groups; however, adjustments for these factors were
included in the statistical analyses. The gastroscopy
procedures were performed by highly skilled
endoscopists. The generalisability of the findings to
all endoscopists warrants additional investigation.
Conclusion
Pronase pretreatment before gastroscopy improves
visual field clarity, reduces examination time,
increases the detection rates of precancerous
lesions and early cancer, and demonstrates good
safety. This approach is beneficial for early cancer
screening in regions with a high incidence of upper
gastrointestinal cancer. The practical value of this
method requires confirmation in large-scale clinical
studies.
Author contributions
Concept or design: Z Wu, S Li, G Wang.
Acquisition of data: L Lu, G Zhao, J Liu, S Li.
Analysis or interpretation of data: T Qin.
Drafting of the manuscript: Z Zhang.
Critical revision of the manuscript for important intellectual content: Z Wu.
Acquisition of data: L Lu, G Zhao, J Liu, S Li.
Analysis or interpretation of data: T Qin.
Drafting of the manuscript: Z Zhang.
Critical revision of the manuscript for important intellectual content: Z Wu.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This research was supported by the National Key Research and
Development Program of China (Ref No.: 2017YFC0908302).
The funder had no role in study design, data collection,
analysis, interpretation, or manuscript preparation.
Ethics approval
This research was approved by the Medical Ethics Committee
of Wuwei Cancer Hospital, Wuwei, Gansu, China (Ref No.:
2019-Ethical review-11). The trial was registered with the
Chinese Clinical Trial Registry (Ref No.: ChiCTR2200064855).
Informed consent was obtained from all study participants,
including consent for the publication of their anonymised
data and clinical photos.
References
1. Zhang Z, Wu Z, Lu L, et al. Analysis of the upper
gastrointestinal cancer screening and follow-up results in
Liangzhou District of Wuwei City from 2009 to 2017 [in
Chinese]. Chin J Cancer Prev Treat 2019;23:1750-5.
2. Choi IJ. Gastric preparation for upper endoscopy. Clin
Endosc 2012;45:113-4. Crossref
3. Kim GH, Cho YK, Cha JM, Lee SY, Chung IK. Effect
of pronase as mucolytic agent on imaging quality
of magnifying endoscopy. World J Gastroenterol
2015;21:2483-9. Crossref
4. Beg S, Ragunath K, Wyman A, et al. Quality standards in
upper gastrointestinal endoscopy: a position statement
of the British Society of Gastroenterology (BSG) and
Association of Upper Gastrointestinal Surgeons of Great
Britain and Ireland (AUGIS). Gut 2017;66:1886-99. Crossref
5. Gomceli I, Demiriz B, Tez M. Gastric carcinogenesis.
World J Gastroenterol 2012;18:5164-70. Crossref
6. Committee of Laboratory Medicine of Chinese Association
of Integrative Medicine. Chinese Expert Consensus on
Detection Technologies for Early-stage Gastric Cancer
Screening [in Chinese]. Chin J Lab Med 2023;46:347-59.
7. Katai H, Ishikawa T, Akazawa K, et al. Five-year survival
analysis of surgically resected gastric cancer cases in Japan:
a retrospective analysis of more than 100,000 patients from
the nationwide registry of the Japanese Gastric Cancer
Association (2001-2007). Gastric Cancer 2018;21:144-54. Crossref
8. Sumiyama K. Past and current trends in endoscopic
diagnosis for early-stage gastric cancer in Japan. Gastric
Cancer 2017;20(Suppl 1):20-7. Crossref
9. Ren W, Yu J, Zhang Z, Song Y, Li Y, Wang L. Missed diagnosis
of early gastric cancer or high-grade intraepithelial
neoplasia. World J Gastroenterol 2013;19:2092-6. Crossref
10. Lu L, Nie P, Zhang Z. Analysis of incidence and mortality
of stomach cancer from 2011 to 2015 in Wuwei City, Gansu
Province [in Chinese]. China Cancer 2020;29:677-81.
11. Chinese Society of Digestive Endoscopy; Chinese Anti-Cancer Association The Society of Tumor Endoscopy.
Chinese Consensus on Screening and Endoscopic
Diagnosis and Management of Early Gastric Cancer
(Changsha, April 2014) [in Chinese]. Chin J Gastroenterol
2014;19:408-27.
12. Yao K, Uedo N, Kamada T, et al. Guidelines for endoscopic
diagnosis of early gastric cancer. Dig Endosc 2020;32:663-98. Crossref
13. Lee GJ, Park SJ, Kim SJ, Kim HH, Park MI, Moon W.
Effectiveness of premedication with pronase for
visualization of the mucosa during endoscopy: a
randomized, controlled trial. Clin Endosc 2012;45:161-4. Crossref
14. Liu X, Guan CT, Xue LY, et al. Effect of premedication
on lesion detection rate and visualization of the mucosa
during upper gastrointestinal endoscopy: a multicenter
large sample randomized controlled double-blind study.
Surg Endosc 2018;32:3548-56. Crossref
15. Cha JM, Won KY, Chung IK, Kim GH, Lee SY, Cho YK.
Effect of pronase premedication on narrow-band imaging
endoscopy in patients with precancerous conditions of
stomach. Dig Dis Sci 2014;59:2735-41. Crossref
16. Zhang Q, Chen Z, Chen C, et al. Training in early gastric
cancer diagnosis improves the detection rate of early
gastric cancer: an observational study in China. Medicine
(Baltimore) 2015;94:e384. Crossref
17. Gao Z, Liang S, Li M, et al. Clinicopathological features
and trends of 1025 cases of early gastric cancer, 2006-2020
[in Chinese]. J Cancer Control Treat 2021;34:649-54.
18. Chen L, Feng Y, Wang W, Zheng P. Clinical value of
pronase combined with sodium bicarbonate in gastroscopy
of elderly patients [in Chinese]. Zhejiang JITCWM
2018;28:225-7.
19. Lee SY, Han HS, Cha JM, Cho YK, Kim GH, Chung IK.
Endoscopic flushing with pronase improves the quantity
and quality of gastric biopsy: a prospective study.
Endoscopy 2014;46:747-53. Crossref