© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Liver- and tumour-specific indicators predicting suboptimal survival following repeat transarterial chemoembolisation in patients with hepatocellular carcinoma
LM Chen, PhD1,2,3,4; Simon CH Yu, MB, BS, FHKAM (Radiology)1,2; Leung Li, MB, ChB, FRCP5; Edwin P Hui, MB, ChB, FHKAM (Medicine)5; Winnie Yeo, MB, BS, FHKAM (Medicine)5,6; Stephen L Chan, MB, BS, FHKAM (Medicine)5,6
1 Department of Imaging and Interventional Radiology, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
2 Vascular and Interventional Radiology Foundation Clinical Science
Centre, Prince of Wales Hospital, The Chinese University of Hong Kong,
Hong Kong SAR, China
3 Department of Medical Ultrasonics, The Sixth Affiliated Hospital, Sun
Yat-Sen University, Guangzhou, China
4 Biomedical Innovation Center, The Sixth Affiliated Hospital, Sun Yat-Sen
University, Guangzhou, China
5 Department of Clinical Oncology, Prince of Wales Hospital, The Chinese
University of Hong Kong, Hong Kong SAR, China
6 State Key Laboratory of Translational Oncology, China
Corresponding author: Prof Simon CH Yu (simonyu@cuhk.edu.hk)
Abstract
Introduction: This study explored liver- and
tumour-specific indicators predictive of suboptimal
survival outcomes following repeat transarterial
chemoembolisation (TACE) in intermediate-stage
hepatocellular carcinoma (HCC) patients after an
initial TACE.
Methods: This study included 300 HCC patients
who underwent TACE treatment. Based on
whether persistent albumin–bilirubin (ALBI) grade
deterioration (PABD) occurred after the initial
TACE, defining as a shift in ALBI grade to a higher
grade from baseline without recovery within 90 days,
patients were divided into PABD and non-PABD
groups. Overall survival of non-PABD and PABD
groups according to subgroups stratified by baseline
ALBI grade and tumour burden was compared
with that of patients receiving only sorafenib or
supportive care during the same period.
Results: Repeat TACE provided a survival benefit
over systemic therapy or supportive care for
patients in all post-TACE non-PABD or most PABD
subgroups, regardless of baseline liver condition
(ie, modified albumin–bilirubin [mALBI] grade
and tumour burden). This benefit was absent in two
subgroups among patients who developed PABD
after the initial TACE, namely, (1) those with a
baseline liver condition of mALBI grade 1 or 2a and
tumour burden exceeding the up-to-11 criteria, and
(2) those with a baseline liver condition of mALBI
grade 2b, regardless of tumour burden.
Conclusion: Repeat TACE is not recommended for
patients with persistent liver function deterioration
after the initial TACE, particularly those exhibiting
suboptimal baseline liver function or excessive
tumour burden. Understanding the liver condition
and tumour burden in HCC patients may assist
clinicians in planning optimal treatment strategies,
leading to better prognosis.
New knowledge added by this study
- The identification of objective and specific indicators predictive of suboptimal survival outcomes following repeat transarterial chemoembolisation (TACE) would be clinically valuable.
- The survival benefit of repeat TACE was not significant in two subgroups of patients who developed persistent albumin–bilirubin (ALBI) grade deterioration after the initial TACE, namely, (1) those with a baseline liver condition of modified albumin–bilirubin (mALBI) grade 1 or 2a and tumour burden exceeding the up-to-11 criteria, and (2) those with a baseline liver condition of mALBI grade 2b, regardless of tumour burden.
- Liver function changes after initial TACE combined with tumour burden could serve as indicators to select patients suitable for repeat TACE.
- Repeat TACE is not recommended for patients with persistent liver function deterioration and a baseline liver condition of mALBI grade 1 or 2a and tumour burden exceeding the up-to-11 criteria, or for those with a baseline liver condition of mALBI grade 2b, regardless of tumour burden.
Introduction
Hepatocellular carcinoma (HCC) imposes a substantial cancer burden worldwide; its incidence
rate in 2020 was ranked seventh, whereas its
mortality rate was ranked second.1 Transarterial
chemoembolisation (TACE) is commonly used as a
first-line treatment for patients with intermediate-stage
HCC, preserved liver function, and good
performance status.2 3
Liver function deterioration occurs in 15.1% to
52% of patients after TACE4 5 6 7 8 9; among these patients,
3% to 31% experience chronic or irreversible liver
function deterioration.5 6 7 9 Patients with post-TACE
liver function deterioration may have a suboptimal
long-term prognosis.5 8 10 Repeat TACE is indicated
when residual tumour remains or when a new
tumour is detected after the initial TACE.2 Patients
with tumours refractory to TACE are preferably
treated with systemic therapy; switching to such
therapy has demonstrated a survival benefit and
better liver function preservation relative to
continued TACE.11 12
Liver condition is crucial to the clinical
outcome of repeat TACE. Patients with suboptimal
liver function are more likely to experience
irreversible liver function deterioration after repeat
TACE, leading to suboptimal survival outcomes.
Such patients also exhibit risks of reduced treatment
efficacy and compromised safety during subsequent
treatment with systemic therapy. In patients with
HCC, liver condition is inevitably linked to tumour
burden; liver function deterioration occurs more
frequently in those with a high tumour burden.5 13
The identification of objective and specific
indicators predictive of suboptimal survival outcomes
following repeat TACE would be clinically valuable
because such indicators could guide decisions
regarding whether to pursue repeat TACE or switch
to systemic therapy. We hypothesised that specific
indicators based on liver condition and tumour
burden, predictive of suboptimal survival outcomes
following repeat TACE, could be identified. In this
study, we sought to identify liver- and tumour-specific
indicators predictive of suboptimal survival
outcomes with repeat TACE relative to sorafenib or
supportive care (SC) in patients who had received an
initial TACE.
Methods
All patients presenting to our institution with
unresectable HCC between January 2005 and
December 2019 who met the eligibility criteria were
recruited. Inclusion criteria consisted of treatment-naïve
unresectable HCC confirmed by biopsy or
contrast-enhanced imaging demonstrating typical
enhancement features, Barcelona Clinic Liver
Cancer stage B disease, and treatment with one of three options: TACE, sorafenib, or SC. Exclusion
criteria were age <18 years, intrahepatic tumours
with vascular invasion, extrahepatic metastases,
liver function classified as albumin–bilirubin (ALBI)
grade 3, or incomplete post-TACE liver function data.
According to standard practice at our institution
during the study period, patients with unresectable
intermediate-stage HCC and no contraindication
to TACE were prioritised for TACE. Patients who
refused TACE were treated with sorafenib; those
who declined both treatments received SC.
Liver condition indicator
Liver condition was assessed using the modified
albumin–bilirubin (mALBI) grade.14 The grade was
defined by the ALBI score, which was calculated
using the following equation: log10 (bilirubin
[in μmol/L])×0.66+albumin [in g/L]×(-0.085). Patients
were categorised into four grades: 1 (ALBI score
≤-2.60), 2a (ALBI score >-2.60 and ≤-2.27), 2b
(ALBI score >-2.27 and ≤-1.39), and 3 (ALBI score
>-1.39). Post–transarterial chemoembolisation liver
condition was classified into three categories based
on post-TACE ALBI grade deterioration, defined
as a shift to a higher grade from baseline following TACE, such as from grade 1 to grade 2-3, grade 2a to
2b-3, or grade 2b to 3. No ALBI grade deterioration
(NABD) was regarded as the lack of a shift to a
higher ALBI grade after TACE. Temporary ALBI
grade deterioration (TABD) constituted ALBI grade
deterioration that resolved within 90 days after
TACE. Persistent ALBI grade deterioration (PABD)
was defined as ALBI grade deterioration that did
not resolve within 90 days after TACE. Patients in
NABD and TABD groups were categorised as non-
PABD group.
Tumour burden indicators
Tumour burden was assessed using the up-to-7
and up-to-11 criteria, defined as the sum of the
tumour number and the largest tumour diameter
in centimetres, with thresholds set at 7 and 11,
respectively. Tumour burden was subclassified into
four categories: within or beyond the up-to-7 or up-to-11 criteria.
Study design
At our institution, it was standard practice for patients
initially treated with TACE to receive repeat TACE
if residual or recurrent intrahepatic tumours were
present, until a contraindication to TACE occurred.
Contraindications included an Eastern Cooperative
Oncology Group performance status score >2 or a
Child-Pugh score >7, regardless of liver condition
changes following the initial TACE. Assuming that
patients with PABD after the initial TACE have a
higher risk of further liver damage and worse survival
outcomes if subjected to repeat TACE, such patients
were targeted in this study. The overall survival
(OS) of patients with or without PABD after the
initial TACE was compared with the OS of patients
receiving only sorafenib or SC during the same period.
Among patients with or without post-TACE PABD,
we identified subgroups with baseline mALBI grade
and tumour burden who showed no survival benefit
over sorafenib or SC; these patients were considered
unsuitable for repeat TACE. Overall survival was
calculated from the date of TACE or sorafenib
initiation to the date of death from any cause. For
patients who received SC, OS was calculated from
the date of HCC diagnosis to the date of death from
any cause. Censoring was applied to patients who
were lost to follow-up, underwent subsequent liver
resection, or were last known to be alive.
Transarterial chemoembolisation
The TACE procedure was performed under local
anaesthesia and guided by digital subtraction
angiography. An emulsion consisting of aqueous
cisplatin (Platosin; Pharmachemie BV, Haarlem, the Netherlands) and ethiodised oil in a 1:1 volume
ratio was delivered transarterially into the tumour
vasculature until flow stagnation occurred or a
maximum dose of 40 mL emulsion was reached.
Tumour-feeding arteries were subsequently
embolised using 5 to 10 mL of gelatin sponge. The
completeness of the procedure was verified using
digital subtraction angiography, with or without non-contrast
multiplanar computed tomography (CT).
Systemic therapy
Oral sorafenib was administered twice daily at a
standard dose of 400 mg. Dose adjustments or drug
discontinuation were performed at the discretion of
the oncologist based on patient tolerance.
Statistical analysis
Categorical variables are presented as numbers
(percentages) and continuous variables are
presented as medians (interquartile ranges). The
Chi squared test was used to compare categorical
data. The Mann-Whitney U test or Kruskal–Wallis
test was performed for comparisons of continuous
data. Differences in OS between subgroups were
analysed using the log-rank test and hazard
ratios (HRs) with 95% confidence intervals (CIs).
Interaction terms were included to evaluate whether
the survival benefit of the post-TACE PABD or non-PABD group over the sorafenib or SC group varied
across subgroups. P values <0.05 were considered
statistically significant. Data analysis was performed
using SPSS (Windows version 25.0; IBM Corp,
Armonk [NY], United States).
Results
Study participants
In total, 300 treatment-naïve patients with HCC
received TACE. The median age was 65 years
(interquartile range, 56-72); the cohort included
255 men and 45 women. After the first TACE, 235
of 300 patients experienced ALBI deterioration:
154 exhibited TABD and 81 displayed PABD. The
demographics of patients with NABD, TABD, and
PABD are listed in Table 1. The OS was similar for
patients with NABD and TABD (22.40 vs 23.83
months), indicating that TABD did not adversely
affect treatment outcomes. Therefore, patients
with NABD and TABD were combined into the
non-PABD group. The demographics of patients in
non-PABD group and PABD group were compared
to sorafenib group and SC group, as listed in Table 2. Patients in non-PABD group and PABD group
had significantly better OS than those in sorafenib
and SC group (23.13, 8.03, 5.11, and 2.57 months,
respectively).
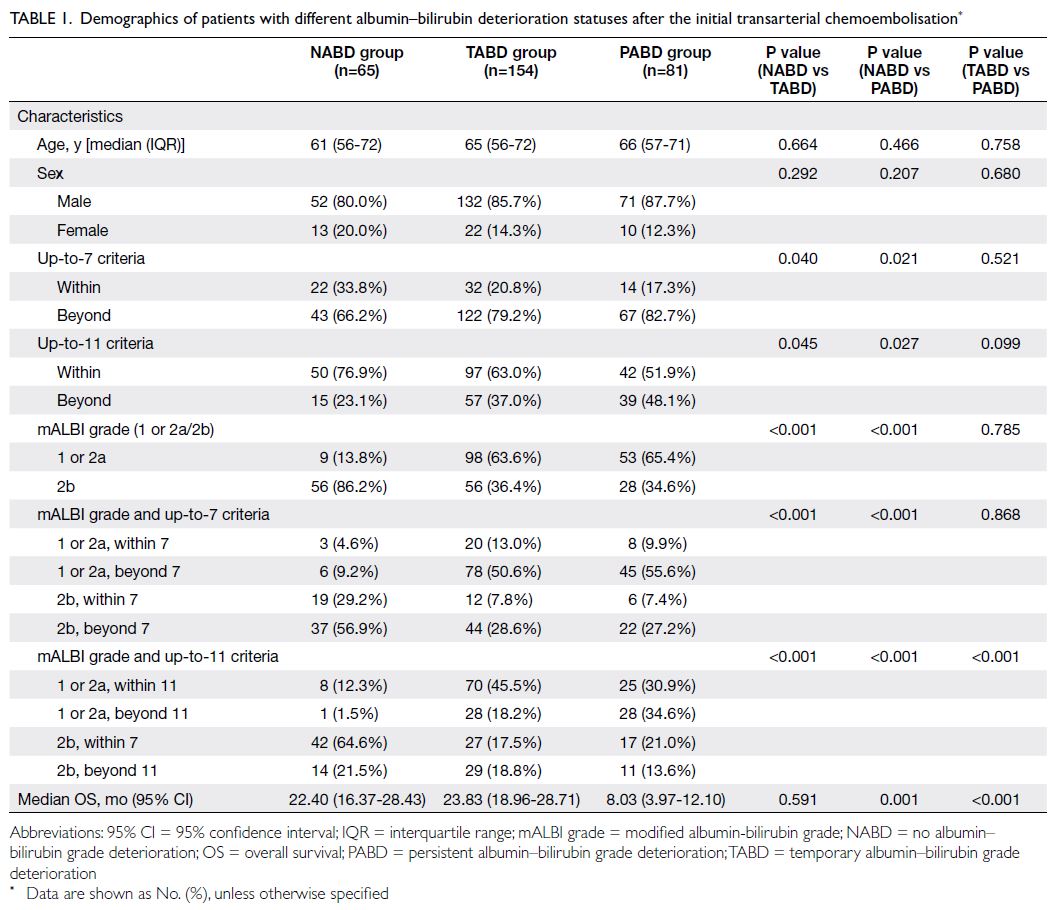
Table 1. Demographics of patients with different albumin–bilirubin deterioration statuses after the initial transarterial chemoembolisation
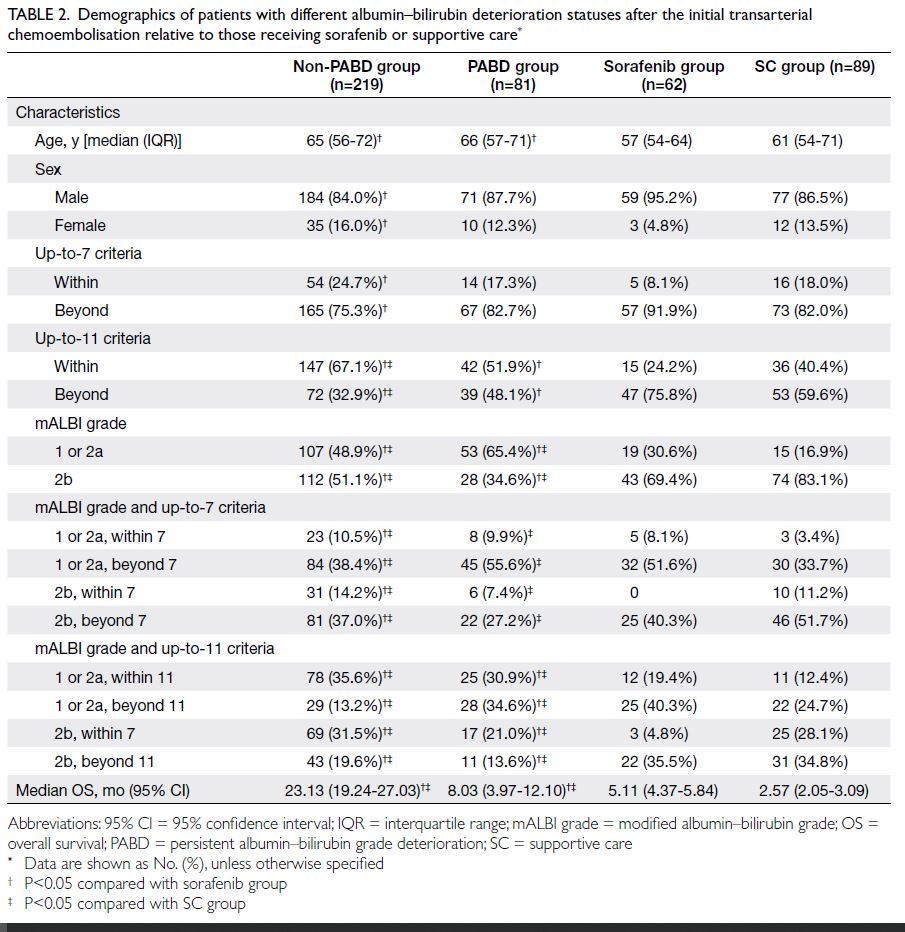
Table 2. Demographics of patients with different albumin–bilirubin deterioration statuses after the initial transarterial chemoembolisation relative to those receiving sorafenib or supportive care
Overall survival
Patients with post–transarterial
chemoembolisation persistent albumin–bilirubin
grade deterioration versus sorafenib in subgroups
Online supplementary Figure 1 illustrates the
median OS of patients with post-TACE PABD
relative to patients treated with sorafenib. Patients
receiving TACE who developed post-TACE PABD
had significantly longer median OS than those
receiving sorafenib in subgroups within and beyond
the up-to-7 criteria (19.63 vs 5.17 months; P=0.019
and 7.63 vs 5.11 months; P=0.030, respectively).
A significantly longer median OS was observed
in patients receiving TACE who developed post-TACE PABD relative to those receiving sorafenib in
the subgroup within the up-to-11 criteria (10.20 vs 5.37 months; P=0.016). However, this difference was
not significant in the subgroup beyond the up-to-11
criteria (8.00 vs 4.94 months; P=0.083). Similarly,
OS was significantly improved in the post-TACE
PABD group relative to the sorafenib group within
the mALBI grade 1 or 2a subgroup (11.50 vs 6.60
months; P=0.001). However, no significant difference
was observed in the mALBI grade 2b subgroup (3.47
vs 4.39 months; P=0.517) [online supplementary Fig 1].
Based on stratification according to mALBI
grade and the up-to-7 criteria, patients receiving
TACE who developed post-TACE PABD had
significantly longer median OS relative to those
receiving sorafenib in the subgroup with mALBI
grade 1 or 2a and within the up-to-7 criteria (29.57 vs 5.17 months; P=0.003) and the subgroup with
mALBI grade 1 or 2a and beyond the up-to-7 criteria
(10.57 vs 6.60 months; P=0.020). However, OS was
not significantly improved in the subgroup with
mALBI grade 2b and within the up-to-7 criteria (6.40
vs 4.39 months; P=0.071) or in the subgroup with
mALBI grade 2b and beyond the up-to-7 criteria
(3.07 vs 4.39 months; P=0.891). The interaction
between treatment effects in subgroups stratified
according to mALBI grade and the up-to-7 criteria
had a 5% level of significance, with a tendency of a
significant interaction that warrants further studies
(P=0.058) [online supplementary Fig 1].
Based on stratification according to mALBI
grade and the up-to-11 criteria, patients receiving
TACE who developed post-TACE PABD had significantly longer median OS relative to those
receiving sorafenib in the subgroup with mALBI
grade 1 or 2a and within the up-to-11 criteria (13.37
vs 5.76 months; P=0.004). However, OS was not
significantly improved in the subgroup with mALBI
grade 1 or 2a and beyond the up-to-11 criteria (11.50
vs 6.60 months; P=0.061), the subgroup with mALBI
grade 2b and within the up-to-11 criteria (5.07 vs
4.52 months; P=0.313), or the subgroup with mALBI
grade 2b and beyond the up-to-11 criteria (3.07 vs
4.10 months; P=0.316). The interaction between
treatment effects in subgroups stratified according
to mALBI grade and the up-to-11 criteria had a 5%
level of significance, with a tendency of a significant
interaction that warrants further studies (P=0.071)
[online supplementary Fig 1].
Patients with post–transarterial
chemoembolisation persistent albumin–bilirubin
grade deterioration versus sorafenib in subgroups
The median OS of patients who developed post-TACE PABD relative to those receiving SC is shown
in online supplementary Figure 2. Patients receiving
TACE who developed post-TACE PABD had
significantly longer median OS compared with those
receiving SC in the subgroup with mALBI grade 1
or 2a and within the up-to-7 criteria (29.57 vs 15.38
months; P=0.036) and the subgroup with mALBI
grade 1 or 2a and beyond the up-to-7 criteria (10.57
vs 3.32 months; P<0.001). However, no significant
improvement in OS was observed in the subgroup
with mALBI grade 2b and within the up-to-7 criteria
(6.40 vs 5.40 months; P=0.266) or in the subgroup
with mALBI grade 2b and beyond the up-to-7
criteria (3.07 vs 2.18 months; P=0.051).
Patients receiving TACE who developed post-
TACE PABD also had significantly longer median OS
relative to those receiving SC in the subgroup with
mALBI grade 1 or 2a and within the up-to-11 criteria
(13.37 vs 4.29 months; P=0.035) and the subgroup
with mALBI grade 1 or 2a and beyond the up-to-11
criteria (11.50 vs 3.32 months; P=0.001). However,
no significant improvement in OS was observed in
the subgroup with mALBI grade 2b and within the
up-to-11 criteria (5.07 vs 2.57 months; P=0.084) or
in the subgroup with mALBI grade 2b and beyond
the up-to-11 criteria (3.07 vs 2.08 months; P=0.269)
[online supplementary Fig 2].
Patients with post–transarterial
chemoembolisation non-persistent albumin–bilirubin grade deterioration versus sorafenib or
supportive care in subgroups
Significantly longer median OS was observed
among patients in the non-PABD group after TACE
relative to those receiving sorafenib (all P<0.001)
[online supplementary Fig 3] or SC in all subgroups
(all P<0.001, except for the subgroup with mALBI
grade 1 or 2a and within the up-to-7 criteria, which
displayed a P value of 0.012) [online supplementary Fig 4] stratified according to various criteria.
Discussion
Principal findings
This study demonstrated that repeat TACE provided
a survival benefit over systemic therapy or SC for
patients who developed TABD or PABD after the
first TACE, regardless of baseline liver condition
(according to ALBI grade, tumour burden, or liver
function). However, this benefit was absent in
the following two subgroups among patients who
developed PABD after the first TACE: (1) those with
a baseline liver condition of mALBI grade 1 or 2a and tumour burden exceeding the up-to-11 criteria, and
(2) those with a baseline liver condition of mALBI
grade 2b, regardless of tumour burden. These two
subgroups could serve as specific indicators to guide
the decision against prescribing repeat TACE for
individual patients, based on their baseline liver
condition, tumour burden, and occurrence of PABD
after the initial TACE. In such cases, the treatment
outcomes of repeat TACE are unlikely to differ from
those of sorafenib or SC. Notably, there was a 5%
level of significance, with a tendency of a significant
interaction that warrants further studies.
Current knowledge of previous studies
Liver function deterioration after TACE is
associated with worsened long-term survival.5 8 10
Patients with no increase in Child-Pugh score 1
month after TACE had significantly better survival
rates than those with an increased Child-Pugh score
at the same time point (84.5% vs 44.4%, 43.75% vs
18.5%, and 8.3% vs 0% for 1-year, 2-year, and 3-year
survivals, respectively).8 The extent of liver function
deterioration after TACE also impacts survival
outcomes. The median OS was significantly longer in
patients with ALBI grade migration to grade 2 than
in patients with migration to grade 3 during both the
acute phase (30.9 months vs 8.9 months; P<0.001)
and the chronic phase (30.9 months vs 5.7 months;
P<0.001).5 Higher tumour burden is linked to liver
function deterioration and worse survival outcomes
after TACE.15 16 17 Based on the 7-11 criteria, patients
with high tumour burden experienced significantly
higher rates of liver function deterioration (24.4% vs
14.9% or 14.4%) and shorter median survival (11.9
vs 22.3 or 33.1 months) relative to those with low or
intermediate tumour burden.17 Currently, there are
no reports in the literature concerning studies that
identified liver- and tumour-specific indicators to
predict survival benefits of repeat TACE.
Implications for clinical practice
Repeat TACE can damage liver function and worsen
long-term survival. If a patient’s liver function is
irreversibly and severely impaired by repeat TACE,
the opportunity to switch to systemic therapy may be
missed. To maximise survival benefits, the decision
to repeat TACE, discontinue TACE, or transition
to systemic therapy should be carefully considered
and individualised. Two scoring systems have been
developed to guide retreatment strategies,18 19 but
universal validation of their predictive value is
needed. Studies have shown that these systems are
ineffective in terms of supporting decision-making
for sequential treatment.20 21
Most patients who develop TABD are able to
spontaneously recover their baseline liver function.
In this study, similar median OS was observed among
patients with TABD and NABD (23.83 vs 22.40 months). Transarterial chemoembolisation provided
a statistically significant survival benefit for patients
within the non-PABD group, regardless of tumour
burden, relative to those receiving sorafenib or SC.
This finding suggests that TABD has minimal impact
on survival benefit or long-term prognosis after
TACE, and repeat TACE remains feasible in these
patients with reversed or reversible liver function.
Based on the present findings, repeat TACE is not
recommended for patients with PABD and a baseline
liver condition of mALBI grade 2b, regardless of
tumour burden, because survival outcomes in
this subgroup are unlikely to be superior to those
achieved with sorafenib or SC. For the same reason,
repeat TACE is not recommended for patients with
PABD, a baseline liver condition of mALBI grade 1 or
2a, and tumour burden beyond the up-to-11 criteria.
Systemic therapy is preferred for this subgroup,
considering that its effectiveness is likely maximised
in patients with better liver function (eg, those with
ALBI grade 1 or mALBI grade 2a, as stated in an
expert consensus).22
Limitations
We acknowledge that sorafenib is no longer first-line
systemic therapy for HCC. Regimens such as
lenvatinib23 or atezolizumab-bevacizumab24 have
been associated with significantly better OS relative
to sorafenib. We recognise that the use of sorafenib as
a control was a limitation of this study. However, no
alternative was available because a sufficiently large
database with long-term clinical outcomes for newer
systemic therapies was not accessible for the local
population. The primary objective of this study was
not to evaluate the role of sorafenib compared with
TACE, but to use sorafenib as a control to identify
specific liver and tumour indicators predictive
of suboptimal survival outcomes after repeat
chemoembolisation. These indicators are intended
to serve as contraindications for repeat TACE in
patients with the corresponding liver and tumour
conditions. The use of a systemic drug with lower
OS benefit, such as sorafenib, as a control might
lead to overestimation of the value of repeat TACE
and, consequently, to the identification of indicators
under worse liver and tumour conditions. However,
this observation does not compromise the validity
of these indicators as criteria for contraindicating
repeat TACE.
Other limitations of the study include the
relatively small sample size in patient groups
receiving sorafenib or SC. Patient numbers
were further reduced in some subgroups after
stratification according to liver function and tumour
burden, which could introduce bias in survival
comparisons. Serum alpha-fetoprotein (AFP) levels
and tumour response after TACE were not analysed
in this study. Considering that elevated AFP levels have been associated with ALBI deterioration, AFP
may be partially represented in the baseline ALBI
grade. The median time to Child-Pugh deterioration
was significantly longer in patients who responded to
the initial TACE than in those who were refractory
to the initial TACE (55.9 vs 19.6 months).25 Most
patients (22/27, 81.5%) ineligible for repeat TACE
due to hepatic decompensation exhibited tumour
progression at the time of TACE discontinuation.26
Target lesion progression has been associated with
no survival improvement and an increased risk of
liver dysfunction after repeat TACE.27 Based on
findings in the above studies, poor tumour response
may eventually lead to liver function deterioration.
Although tumour response was not analysed in
this study, it is reasonable to assume that tumour
response varies according to treatment effectiveness.
Given that treatment effectiveness is assumed
to remain consistent under the same treatment
protocol within a single centre, it may be argued that
the overall effect of tumour response in individual
patients was reflected in liver function deterioration.
Conclusion
This study found that repeat TACE is not
recommended for patients with persistent liver
function deterioration after the initial TACE,
particularly those exhibiting suboptimal baseline
liver function or excessive tumour burden.
Understanding the liver condition and tumour
burden in HCC patients may assist clinicians in
planning optimal treatment strategies and improving
patient prognosis.
Author contributions
Concept or design: SCH Yu.
Acquisition of data: LM Chen, L Li, EP Hui, W Yeo, SL Chan.
Analysis or interpretation of data: LM Chen, SCH Yu.
Drafting of the manuscript: LM Chen, SCH Yu.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: LM Chen, L Li, EP Hui, W Yeo, SL Chan.
Analysis or interpretation of data: LM Chen, SCH Yu.
Drafting of the manuscript: LM Chen, SCH Yu.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This research was funded by the Vascular and Interventional
Radiology Foundation. The funding body was not involved in
the study design, data collection, analysis, interpretation, or
manuscript preparation.
Ethics approval
This research was approved by the Joint Chinese University of
Hong Kong–New Territories East Cluster Clinical Research Ethics Committee, Hong Kong (Ref No.: 2020.672). The
research was conducted in accordance with the Declaration of
Helsinki and the International Conference on Harmonisation,
Good Clinical Practice. The requirement for written informed
patient consent was waived by the Committee due to the
retrospective nature of the research.
Supplementary material
The supplementary material was provided by the authors, and
some information may not have been peer reviewed. Accepted
supplementary material will be published as submitted by the
authors, without any editing or formatting. Any opinions
or recommendations discussed are solely those of the
author(s) and are not endorsed by the Hong Kong Academy
of Medicine and the Hong Kong Medical Association.
The Hong Kong Academy of Medicine and the Hong Kong
Medical Association disclaim all liability and responsibility
arising from any reliance placed on the content.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics
2020: GLOBOCAN estimates of incidence and mortality
worldwide for 36 cancers in 185 countries. CA Cancer J
Clin 2021;71:209-49. Crossref
2. Cheung TT, Kwok PC, Chan S, et al. Hong Kong
consensus statements for the management of unresectable
hepatocellular carcinoma. Liver Cancer 2018;7:40-54. Crossref
3. European Association for the Study of the Liver. EASL
Clinical Practice Guidelines: management of hepatocellular
carcinoma. J Hepatol 2018;69:182-236. Crossref
4. Min YW, Kim J, Kim S, et al. Risk factors and a predictive
model for acute hepatic failure after transcatheter arterial
chemoembolization in patients with hepatocellular
carcinoma. Liver Int 2013;33:197-202. Crossref
5. Chi CT, Lee IC, Lee RC, et al. Effect of transarterial
chemoembolization on ALBI grade in intermediate-stage
hepatocellular carcinoma: criteria for unsuitable cases
selection. Cancers (Basel) 2021;13:4325. Crossref
6. Hiraoka A, Kumada T, Kudo M, et al. Hepatic function
during repeated TACE procedures and prognosis after
introducing sorafenib in patients with unresectable
hepatocellular carcinoma: multicenter analysis. Dig Dis
2017;35:602-10. Crossref
7. Miksad RA, Ogasawara S, Xia F, Fellous M, Piscaglia F. Liver
function changes after transarterial chemoembolization in
US hepatocellular carcinoma patients: the LiverT study.
BMC Cancer 2019;19:795. Crossref
8. Kohla MA, Abu Zeid MI, Al-Warraky M, Taha H, Gish RG.
Predictors of hepatic decompensation after TACE for
hepatocellular carcinoma. BMJ Open Gastroenterol
2015;2:e000032. Crossref
9. Park KH, Kim JH, Choe WH, et al. Risk factors
for liver function deterioration after transarterial
chemoembolization refractoriness in Child-Pugh class A
hepatocellular carcinoma patients. Korean J Gastroenterol
2020;75:147-56. Crossref
10. Sun Z, Li G, Ai X, et al. Hepatic and biliary damage after
transarterial chemoembolization for malignant hepatic
tumors: incidence, diagnosis, treatment, outcome and
mechanism. Crit Rev Oncol Hematol 2011;79:164-74. Crossref
11. Ogasawara S, Ooka Y, Koroki K, et al. Switching to systemic
therapy after locoregional treatment failure: definition and
best timing. Clin Mol Hepatol 2020;26:155-62. Crossref
12. Piscaglia F, Ogasawara S. Patient selection for transarterial
chemoembolization in hepatocellular carcinoma:
importance of benefit/risk assessment. Liver Cancer
2018;7:104-19. Crossref
13. Yasui Y, Tsuchiya K, Kurosaki M, et al. Up-to-seven criteria
as a useful predictor for tumor downstaging to within
Milan criteria and Child-Pugh grade deterioration after
initial conventional transarterial chemoembolization.
Hepatol Res 2018;48:442-50. Crossref
14. Hiraoka A, Michitaka K, Kumada T, et al. Validation and
potential of albumin–bilirubin grade and prognostication
in a nationwide survey of 46,681 hepatocellular carcinoma
patients in Japan: the need for a more detailed evaluation
of hepatic function. Liver Cancer 2017;6:325-36. Crossref
15. Khisti R, Patidar Y, Garg L, Mukund A, Thomas SS, Sarin SK.
Correlation of baseline portal pressure (hepatic venous
pressure gradient) and indocyanine green clearance test
with post–transarterial chemoembolization acute hepatic
failure. J Clin Exp Hepatol 2019;9:447-52. Crossref
16. Siriwardana RC, Niriella MA, Dassanayake AS, et al.
Factors affecting post-embolization fever and liver failure
after trans-arterial chemo-embolization in a cohort
without background infective hepatitis–a prospective
analysis. BMC Gastroenterol 2015;15:96. Crossref
17. Hung YW, Lee IC, Chi CT, et al. Redefining tumor burden in
patients with intermediate-stage hepatocellular carcinoma:
the seven-eleven criteria. Liver Cancer 2021;10:629-40. Crossref
18. Sieghart W, Hucke F, Pinter M, et al. The ART of decision
making: retreatment with transarterial chemoembolization
in patients with hepatocellular carcinoma. Hepatology
2013;57:2261-73. Crossref
19. Adhoute X, Penaranda G, Naude S, et al. Retreatment with
TACE: the ABCR SCORE, an aid to the decision-making
process. J Hepatol 2015;62:855-62. Crossref
20. Arizumi T, Ueshima K, Iwanishi M, et al. Evaluation of
ART scores for repeated transarterial chemoembolization
in Japanese patients with hepatocellular carcinoma.
Oncology 2015;89 Suppl 2:4-10. Crossref
21. Kloeckner R, Pitton MB, Dueber C, et al. Validation of
clinical scoring systems ART and ABCR after transarterial
chemoembolization of hepatocellular carcinoma. J Vasc
Interv Radiol 2017;28:94-102. Crossref
22. Kudo M, Han KH, Ye SL, et al. A changing paradigm for the
treatment of intermediate-stage hepatocellular carcinoma:
Asia-Pacific primary liver cancer expert consensus
statements. Liver Cancer 2020;9:245-60. Crossref
23. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib
in first-line treatment of patients with unresectable
hepatocellular carcinoma: a randomised phase 3 non-inferiority
trial. Lancet 2018;391:1163-73. Crossref
24. Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety
data from IMbrave150: atezolizumab plus bevacizumab
vs. sorafenib for unresectable hepatocellular carcinoma. J
Hepatol 2022;76:862-73. Crossref
25. Maesaka K, Sakamori R, Yamada R, et al. Initial treatment
response to transarterial chemoembolization as a
predictive factor for Child-Pugh class deterioration prior
to refractoriness in hepatocellular carcinoma. Hepatol Res
2020;50:1275-83. Crossref
26. Labeur TA, Takkenberg RB, Klümpen HJ, van Delden OM. Reason of discontinuation after transarterial
chemoembolization influences survival in patients with
hepatocellular carcinoma. Cardiovasc Intervent Radiol
2019;42:230-8. Crossref
27. Zhang YF, Guo RP, Ouyang HY, et al. Target lesion response
predicts survival of patients with hepatocellular carcinoma
retreated with transarterial chemoembolization. Liver Int
2016;36:1516-24. Crossref

