Hong Kong Med J 2025 Feb;31(1):68–71 | Epub 10 Feb 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Pneumothorax associated with a displaced thoracoamniotic Somatex shunt in an infant with congenital pulmonary airway malformation: a case report
Viola YT Chan, FHKAM (Obstetrics and Gynaecology)1; WT Tse, FHKAM (Obstetrics and Gynaecology)2; MC Chan, FHKAM (Paediatrics)3; Kenneth KY Wong, PhD, FHKAM (Surgery)4; WC Leung, FHKAM (Obstetrics and Gynaecology)1; TY Leung, FHKAM (Obstetrics and Gynaecology)2
1 Department of Obstetrics and Gynaecology, Kwong Wah Hospital, Hong Kong SAR, China
2 Department of Obstetrics and Gynaecology, Prince of Wales Hospital, Hong Kong SAR, China
3 Department of Paediatrics, Kwong Wah Hospital, Hong Kong SAR, China
4 Division of Paediatric Surgery, Department of Surgery, Queen Mary Hospital, Hong Kong SAR, China
Corresponding author: Dr Viola YT Chan (cyt141@ha.org.hk)
Case presentation
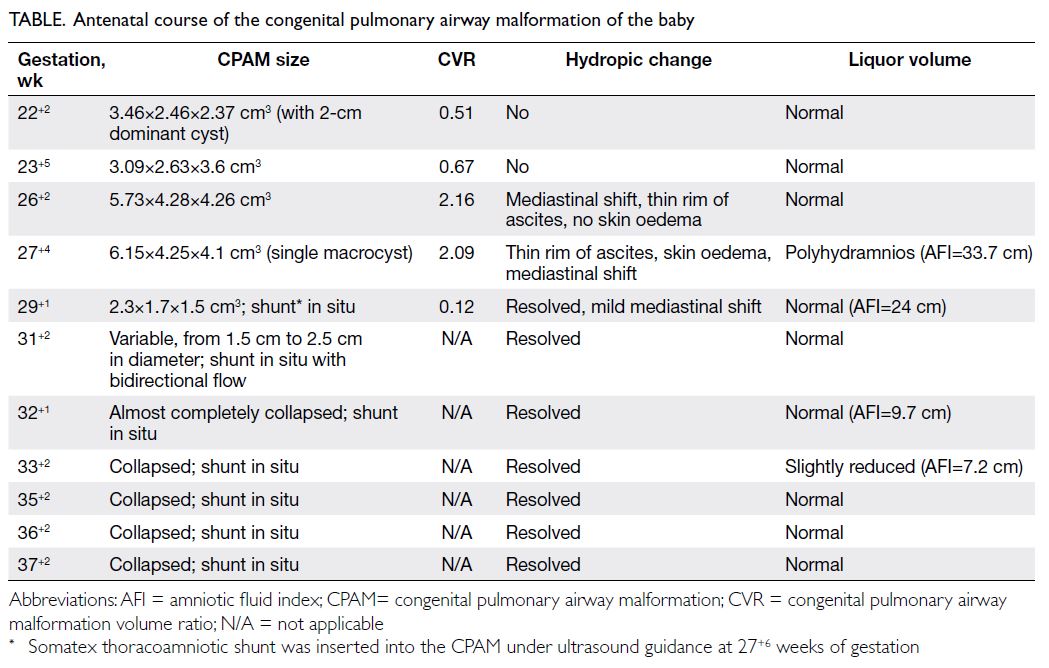
A 32-year-old nulliparous pregnant woman at 21
weeks of gestation was referred to Kwong Wah
Hospital in March 2021 for fetal right cystic lung
mass (2.16×1.99×2.50 cm3). Repeat examination at 22
weeks of gestation revealed a right multicystic lung
mass (3.46×2.46×2.37 cm3) with a dominant 2-cm
cyst, suggestive of macrocystic congenital pulmonary
airway malformation (CPAM). There was mild
mediastinal shift but no hydrops. The CPAM volume
ratio, calculated as (length×height×width×0.52)/head circumference, was 0.51. Amniocentesis with
chromosomal microarray analysis showed no copy
number variants. At 26 weeks of gestation, the lesion
had enlarged to 5.73×4.28×4.26 cm3 (CVR=2.16),
with mediastinal shift and mild ascites but no
polyhydramnios. At 27 weeks of gestation, the
lesion was dominated by a single cyst that measured
6.15×4.25×4.1 cm3 (CPAM volume ratio=2.09),
with moderate polyhydramnios, ascites and skin
oedema suggestive of fetal hydrops. Intramuscular
betamethasone was administered for fetal lung
maturation in view of the high risk of preterm
delivery. Fetal thoracoamniotic shunting was offered
to relieve the mass effect and fetal hydrops. The
next day, a Somatex shunt (SOMATEX Medical
Technologies, Berlin, Germany) was inserted into
the CPAM under ultrasound guidance and 800 mL
amniotic fluid was drained via the shunt cannula.
Examination 9 days later showed CPAM with
reduced size (2.3×1.7×1.5 cm3; CVR=0.12), shunt in
situ, and no hydrops (Table).
At 29 weeks of gestation, the patient went
into preterm prelabour with rupture of membrane
that sealed off spontaneously. Serial ultrasound
examinations showed satisfactory fetal growth,
collapsed CPAM with shunt in situ, normal liquor
volume and no hydrops (Table). Labour was induced at 38 weeks of gestation for oligohydramnios. A
2.75-kg female baby was delivered by vacuum
extraction for maternal exhaustion, with paediatrician
standby. The Apgar scores of the baby were 8 at
1 minute and 10 at 5 minutes and the arterial cord
blood pH was 7.31, with base excess of -7.1 mmol/L.
The shunt was specifically searched for immediately
after delivery but the skin was intact (Fig a). The
baby was given continuous positive airway pressure
because of respiratory distress and was transferred
to the neonatal intensive care unit. Urgent chest
X-ray revealed the shunt in the right chest with right
pneumothorax (Fig b). A chest drain was inserted
and the baby was intubated. Computed tomography
of the thorax of the baby on day 1 of life showed
an irregular 4.2×3.5×2.5 cm3 cystic lesion in the
right lung with the distal end of the shunt migrated
between the chest wall and the scapular, abutting
the right subscapularis muscle. Thoracoscopy on
day 6 of life confirmed that one end of the shunt was
within the CPAM in the right middle lobe, while the
other end was at the subscapular space. The shunt
was removed intact and near-total right middle
lobe excision was performed thoracoscopically. The
baby was successfully weaned off oxygen 3 weeks
postoperatively and discharged 5 weeks later.
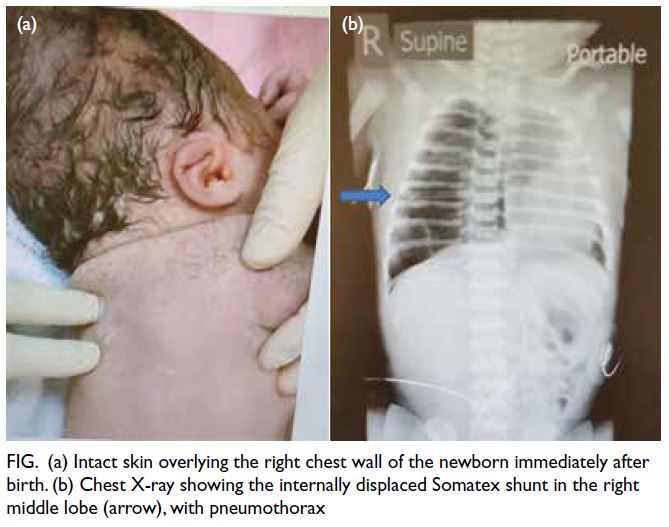
Figure. (a) Intact skin overlying the right chest wall of the newborn immediately after birth. (b) Chest X-ray showing the internally displaced Somatex shunt in the right middle lobe (arrow), with pneumothorax
Discussion
Congenital pulmonary airway malformations
are uncommon lung lesions characterised by an
overgrowth of terminal respiratory bronchioles that
are often immature and non-functioning. A CPAM
is considered macrocystic if at least one cyst is >5 mm
and microcystic if the lesion appears echogenic on
ultrasound examination. Although a microcystic
CPAM may regress spontaneously after 26 to 28
weeks of gestation, most macrocystic CPAMs do
not.1 Fetuses with large cystic lesions are also at risk of pulmonary hypoplasia and development of
fetal hydrops due to compression of lung tissue and
venous return. The survival of a hydropic fetus with
congenital lung lesion has been reported to be 38%,
compared with 87% for a fetus without hydrops.1
Studies have demonstrated a favourable outcome
following thoracoamniotic shunting for macrocystic CPAM, with reduction in lesion volume, resolution
of hydrops and improved survival.2 3 A systematic
review showed an improved survival from 3% to
62% in hydropic fetuses treated with shunting.4 In a
single-centre case series, survival was significantly
associated with gestational age at birth, hydrops
resolution and higher percent reduction in the size
of the lung lesion following shunting.3
Although thoracoamniotic shunting improves
fetal outcome, complications such as shunt
occlusion, displacement, dislodgement, bleeding
and chest wall deformation have been reported.3 5 6 7
Following successful drainage of the lesion, its
surrounding normal lung parenchyma expands and
grows. This may result in inward migration of the
shunt. In a retrospective review,5 thoracoamniotic
shunts inserted for primary pleural effusions and
macrocystic CPAMs were antenatally displaced
in 8.5% of fetuses, of which two-thirds migrated
into the thorax. Re-shunting may be required if
the displaced shunt fails to drain and fluid re-accumulates.5 Retained intrathoracic shunts may be
managed conservatively as they are well tolerated
without untoward postnatal sequalae.5 8 Nonetheless
surgical removal may be necessary if the baby
develops complications such as respiratory distress
or tension pneumothorax.7 9
The Somatex shunt is commonly used to treat
fetuses with obstructive urinary tract disorders.
Recently, thoracoamniotic shunting with a Somatex shunt has been reported effective in relieving fetal
pleural effusions with good survival rate although
shunt dislodgement and entrapment has been
reported in four of eight cases.10 Thoracoscopic
removal of a displaced Somatex shunt has been
reported necessary in a newborn with respiratory
distress and progressive pleural effusion.7 In
comparison with other commonly used shunts,
such as Harrison and Rocket, Somatex insertion
has multiple advantages including a finer introducer
(1.2 mm) but a bigger shunt lumen (2.4 mm). It is
also made of metal facilitating its easy identification
antenatally on ultrasound or postnatally with X-rays
or computed tomography.
A thoracoamniotic shunt should be clamped
immediately following delivery to prevent air from
entering the thorax and causing pneumothorax.2 In
the current case, we did not expect air to enter the
pleural cavity because the shunt was buried inside the
skin of the baby. Our hypothesis is that air may have
entered from the lung tissue into the pleural space via
the displaced shunt. This is similar to the reported
case of tension pneumothorax due to an internally
displaced thoracoamniotic shunt communicating
between the CPAM and the pleural cavity and
diagnosed following neonatal resuscitation for
apnoea.9 Both cases illustrate that pneumothorax
is a possible and potentially life-threatening
complication of an internally displaced shunt. It
should be anticipated at birth and preparations made
for emergency needle thoracocentesis. Obstetricians
should be aware of the possible complications of
thoracoamniotic shunts, and paediatricians should
be alerted so that the newborn can receive prompt
assessment and treatment.
Author contributions
Concept or design: VYT Chan, WC Leung, TY Leung.
Acquisition of data: VYT Chan, WT Tse, MC Chan, KKY Wong.
Analysis or interpretation of data: VYT Chan.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: KKY Wong, WC Leung, TY Leung.
Acquisition of data: VYT Chan, WT Tse, MC Chan, KKY Wong.
Analysis or interpretation of data: VYT Chan.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: KKY Wong, WC Leung, TY Leung.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
As an editor of the journal, KKY Wong was not involved in the peer review process. Other authors have disclosed no
conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient and her baby were treated in accordance with the
Declaration of Helsinki. Parental consent was obtained for the
patient’s baby, and informed consent was obtained from the
patient for all treatments and procedures, and publication of
the case report.
References
1. Walker L, Cohen K, Rankin J, Crabbe D. Outcome of
prenatally diagnosed congenital lung anomalies in the
North of England: a review of 228 cases to aid in prenatal
counselling. Prenat Diagn 2017;37:1001-7. Crossref
2. Schrey S, Kelly EN, Langer JC, et al. Fetal thoracoamniotic
shunting for large macrocystic congenital cystic
adenomatoid malformations of the lung. Ultrasound
Obstet Gynecol 2012;39:515-20. Crossref
3. Peranteau WH, Adzick NS, Boelig MM, et al.
Thoracoamniotic shunts for the management of fetal lung
lesions and pleural effusions: a single-institution review
and predictors of survival in 75 cases. J Pediatr Surg
2015;50:301-5. Crossref
4. Knox EM, Kilby MD, Martin WL, Khan KS. In-utero
pulmonary drainage in the management of primary
hydrothorax and congenital cystic lung lesion: a systematic
review. Ultrasound Obstet Gynecol 2006;28:726-34. Crossref
5. Abbasi N, Windrim R, Keunen J, et al. Perinatal outcome
in fetuses with dislodged thoraco-amniotic shunts. Fetal
Diagn Ther 2021;48:430-9. Crossref
6. Makishi A, Kiyoshi K, Funakoshi T. EP21.22: Fetal chest
wall deformity after thoracoamniotic shunting using a
double-basket catheter for chylothorax: a case report.
Ultrasound Obstet Gynecol 2016;48:362-3. Crossref
7. Sham GT, Chung PH, Chan IM, Leung WC, Wong KK.
Thoracoscopic removal of a displaced thoracoamniotic
shunt in a newborn with antenatal pleural effusion—a case
report. Transl Pediatr 2020;9:702-6. Crossref
8. Tan AP, Tan B, Wright A, Kong JY. Management dilemma
in thoracoamniotic shunt migrations. BMJ Case Rep
2023;16:e255760. Crossref
9. Law BH, Bratu I, Jain V, Landry MA. Refractory tension
pneumothorax as a result of an internally displaced
thoracoamniotic shunt in an infant with a congenital
pulmonary airway malformation. BMJ Case Rep
2016:2016:bcr2016216324. Crossref
10. Chung MY, Leung WC, Tse WT, et al. The use of Somatex
shunt for fetal pleural effusion: a cohort of 8 procedures.
Fetal Diagn Ther 2021;48:440-7. Crossref